Abstract
Physiological systems often display 24hr rhythms that vary with the light/dark cycle. Disruption of circadian physiological rhythms have been linked to the progression of various cardiovascular diseases, and advances in the understanding of these rhythms have led to novel interventions and improved clinical outcomes. Although respiratory function has been known to vary between the light and dark periods, circadian rhythms in breathing have been understudied in clinical conditions. In the current study, we have begun to assess light/dark variations in respiration in chronic heart failure (CHF), a condition associated with abnormal resting and chemoreflex breathing as well as exercise intolerance. CHF was induced using coronary artery ligation and verified using echocardiography. Sham animals underwent a thoracotomy without coronary artery ligation. Tidal volume, respiratory frequency, and minute ventilation were all determined by whole body plethysmography under resting conditions and in response to chemoreflex challenges during the light and dark periods. Light/dark differences in voluntary exercise were assessed using a running wheel. The sham control group showed light/dark differences in resting and chemoreflex breathing, as well as arterial pressure, and these effects were eliminated in the CHF group. Both groups completed more rotations on the running wheel during the dark period compared to during the light period. The data suggest that CHF disrupts cardiovascular and respiratory circadian rhythms.
Keywords: circadian rhythm, chronic heart failure, chronobiology, plethysmography
1.1. Introduction
Many cellular and physiological processes display cyclic patterns with the same periodicity as the 24hr light/dark cycle(Partch et al. 2014), and these circadian rhythms appear to be disrupted in a variety of clinical conditions (Durgan and Young 2010; Ayala et al. 2013). Among the circadian rhythms in physiology, rhythmic changes in cardiovascular function have perhaps been most studied. Research into circadian rhythms in cardiovascular physiology and pathophysiology has led to recent clinical efforts to utilize knowledge of these rhythms in treating cardiovascular diseases (Tsimakouridze et al. 2015), and these efforts have been shown to reduce adverse cardiac remodeling, buffer the morning surge in blood pressure, and reduce the number of adverse cardiovascular events (Tsimakouridze et al. 2015). While advances in chronobiology have improved outcomes for patients with cardiovascular diseases, less is known about circadian rhythms in breathing.
Chronic heart failure (CHF) is a condition characterized by disordered breathing, intermittent hypoxia, exercise intolerance, and chronically elevated sympathetic tone (Morrissey et al. 2011). Patients with reduced ejection fraction CHF do not show circadian rhythms in blood pressure (Komori et al. 2016). Typically, in healthy humans, blood pressure decreases during the night by about 10–20% of the mean daytime values (Floras et al. 1978). CHF patients are more likely than the general population to show abnormal light/dark blood pressure rhythms, and the particular pattern of blood pressure rhythm appears to vary with the type of CHF (Komori et al. 2016). CHF with preserved ejection fraction shows a “riser” pattern in which blood pressure is greater during the sleeping period, and CHF patients with reduced ejection fraction fail to show light/dark variations in blood pressure (Komori et al. 2016). While circadian cardiovascular rhythms have been examined in CHF, no one has yet to assess circadian rhythms in breathing.
Respiratory function has been shown to vary with the light/dark cycle (Mortola 2004). While light/dark variations in breathing have consistently been observed using continuous monitoring of respiration (Mortola and Seifert 2002; Seifert and Mortola 2002), respiratory measurements taken at two discrete time points have produced conflicting results. Light/dark variations in resting breathing were detected in neonate rats (Saiki and Mortola 1995) but not in adult rats (Peever and Stephenson 1997) when observed at two time points. Peever and Stephenson (1997) assessed ventilation in Sprague-Dawley rats at two time-points and found that chemoreflex breathing to hypercapnia increased during the night, but resting breathing showed no change between the light and dark periods. Notably, the respiratory rates for these animals at rest were considerably fast (96±6 bpm during the day and 103±8 during the night), which may have masked differences between the light and dark periods. In our experience, respiratory rates of adult Sprague-Dawley rats are much faster after first placing the animal in the plethysmography chamber, and respiratory rates decline to around 50–70bpm during the day. It is unclear why the respiratory rates in the Peever and Stephenson study remained elevated. In the current study, we assessed resting and chemoreflex breathing using whole body plethysmography at two time points, separated by about 12hrs, in a sham operated control group and a group with CHF induced by coronary artery ligation. Subsequently, light/dark variations in spontaneous exercise were also assessed using a running wheel.
1.2. Methods
1.2.1. Animals
Adult male Sprague Dawley rats (300–450g) were used in this study. Animals were held under a 12:12 light/dark cycle with lights on at 6am. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center.
1.2.2. Whole body plethysmography
Tidal volume (VT), respiratory frequency (fR), and minute ventilation (VE) were all determined by whole body plethysmography. VT was measured using a differential pressure transducer (Kent Scientific) and amplifier (MP45, Validyne Engineering Corporation) prior to recording through a Power Lab System (AD Instruments) and Lab Chart v8.1.5. Rats were given at least 45 min to acclimate to the chamber, and then exposed to a series of gas challenges (10% O2 with 2% CO2, 5% CO2, 90% O2, 5% CO2 with 90% O2) with 3–5 min of recovery time in between in each challenge. Chemoreflex breathing was assessed during the day (8am-2pm) and during the night (8pm-2am). VT was normalized to body weight (kg). Chemoreflex sensitivity was quantified by subtracting VE during a given challenge (10%O2, 5% CO2, and 90%O2 with 5%CO2) from baseline VE.
1.2.3. Coronary artery ligation
Heart failure was induced via coronary artery ligation (n=5) as previously described (Del Rio et al. 2013). Sham animals underwent thoracotomy without coronary artery ligation (n=5). Six weeks following surgery, heart failure was verified using echocardiography (Vevo 770; Visualsonics). Two-dimensional short-axis view of the left ventricle was used to assess end-diastolic and end-systolic diameter as well as end-diastolic and end-systolic volume of the left ventricle. Animals with an ejection fraction <45% were considered to be in heart failure.
1.2.4. Scurry wheel
Voluntary exercise was assessed using Scurry Wheel (Lafayette Instruments, Model 80859S) which consists of a running wheel (inner diameter 35.56cm; width 10.92cm) inside a cage (40.64 × 50.80 × 20.96 cm). The scurry wheel was connected to an 8 channel Powerlab (AD Instruments), and wheel rotations were recorded in LabChart v8.1. To reduce the effects of the novel environment on behavior, each animal was given 24 hrs to acclimate to the cage. Animals had ad libitum access to food and water during the scurry wheel experiment. One heart failure animal died before completing the scurry wheel, reducing the n for the CHF group to 4.
1.2.5. Blood pressure
To assess light/dark variations in blood pressure, a group of animals were implanted with blood pressure telemetry (Datasciences International, either PA-C40 or HD-S10) in the left femoral artery. Surgical implantation occurred under 2–5% isoflurane balanced with oxygen, and animals were given at least 2 wks to recover. Following recovery from telemetry implantation, these animals underwent either coronary artery ligation (n=3) or a sham surgery (n=4), as described above. At least two weeks after the thoracotomy, these animals were placed in the cage with the running wheel, as described above, and blood pressure was recorded along with running wheel rotations. Segments of the blood pressure recordings in which the animal remained at rest for an extended period of time (>25 min) were selected to assess light/dark variations in blood pressure.
1.2.6. Statistics
All data are presented as mean ± SEM, unless otherwise noted. One-way or two-way ANOVAs with Fisher’s LSD used for pairwise comparisons were used to analyze the data. p<0.05 was considered statistically significant.
1.3. Results
1.3.1. Echocardiographic data
Bodyweights between the sham (366 ± 12) and CHF (339 ± 7) groups did not differ (p=0.08). Differences were seen in cardiac structure and function (Table 1). The CHF group had greater end-diastolic (p<0.01) and end-systolic (p<0.01) volumes, greater end-diastolic (p=0.02) and end-systolic (p<0.01) diameters, and lower ejection fraction (p<0.0001) and fractional shortening (p<0.0001). Two animals that were infarcted had ejection fractions greater than 45%, so they were excluded from the study.
Table 1.
Cardiac Function
| Condition | BW (g) | LVEDD (mm) | LVESD (mm) | LVEDV (µL) | LVESV (µL) | SV (µL) | EF (%) | FS (%) |
|---|---|---|---|---|---|---|---|---|
| Sham | 441 ± 27.8 | 0.83 ± 0.06 | 0.56 ± 0.05 | 132 ± 25 | 44 ± 8 | 88 ± 17 | 66.8 ± 3.55 | 31.8 ± 3.24 |
| CHF | 484 ± 16.9 | 1.21 ± 0.07 * | 1.03 ± 0.07 * | 353 ± 51 * | 228 ± 39 * | 123 ± 12 * | 36.4 ± 2.65 * | 15.5 ± 1.26 * |
statistically different from sham; sham, n=5; chf n=5
1.3.2. Circadian rhythms in resting and chemoreflex breathing
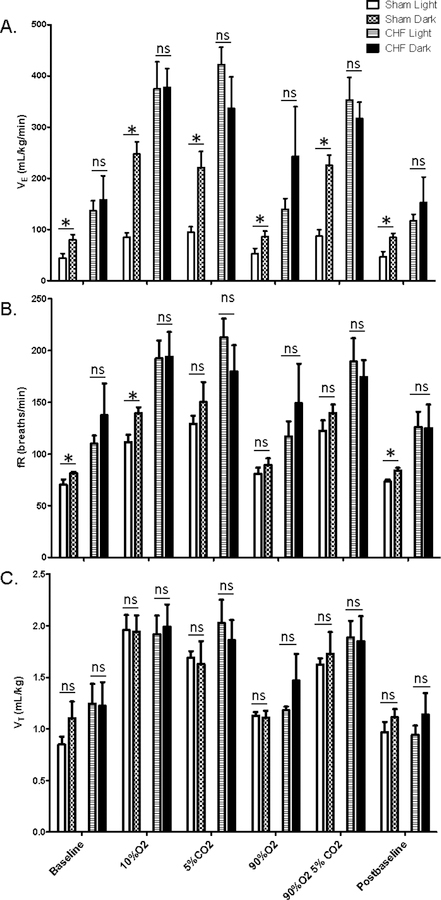
Resting and chemoreflex breathing was assessed using whole-body plethysmography at two discreet time points, separated by approximately 12 hrs. The sham-operated control group displayed light/dark variations in VE when breathing room air under baseline (p=0.040) and post-baseline (p=0.038) conditions (Fig 1A). The sham group also showed light/dark differences in VE during chemoreflex breathing, specifically hypoxia (p<0.001), hypercapnia (p<0.001), and hyperoxia with hypercapnia (p<0.001)(Fig 1A). In contrast, chronic heart failure eliminated circadian rhythms in resting breathing under the baseline (p=0.57) and post-baseline (p=0.32) conditions as well as during chemoreflex breathing (hypoxia p=0.87, hypercapnia p=0.07, and hyperoxia with hypercapnia p=0.47)(Fig 1A). There was also a main effect of heart failure status (p<0.001) with the CHF group displaying greater VE than the sham control group (Fig 1A).
Fig 1. Light/Dark variations in resting and chemoreflex breathing.
Minute ventilation (VE), respiratory frequency (fR), and tidal volume (VT) were measured during the light and dark periods. Data are presented as mean ± SEM. *statistically different; ns, not significant; p<0.05
Analysis of fR data showed a main effect of heart failure status (p<0.001) with the CHF showing greater respiratory rates and a main effect of time (p=0.02) with respiratory rates increasing during the dark (Fig 1B). The sham group showed light/dark differences in fR under resting conditions (baseline: p=0.02; post-baseline: p=0.04) and in response to hypoxia (p=0.01), but not in response to hypercapnia (p=0.54), hyperoxia (p=0.22), or hyperoxia with hypercapnia (p=0.15)(Fig 1B). Respiratory rate did not differ between light and dark periods in the CHF group (p=0.16)(Fig 1B).
CHF animals had greater VT than did sham animals (p<0.05), but no main effect of time (p=0.06) or interaction (p=0.48) was seen (Fig 1C).
1.3.3. Circadian rhythms in chemoreflex sensitivity
Chemoreflex sensitivity was also assessed in sham and CHF animals during the light and dark periods. The data showed a statistically significant time x condition interaction (p=0.005), with the sham group displaying greater sensitivity during the dark compared to during the light period (p=0.01) while the CHF group showed no difference in chemoreflex sensitivity between the day and the night (p=0.12) (Fig 2).
Fig 2. Chemoreflex sensitivity.
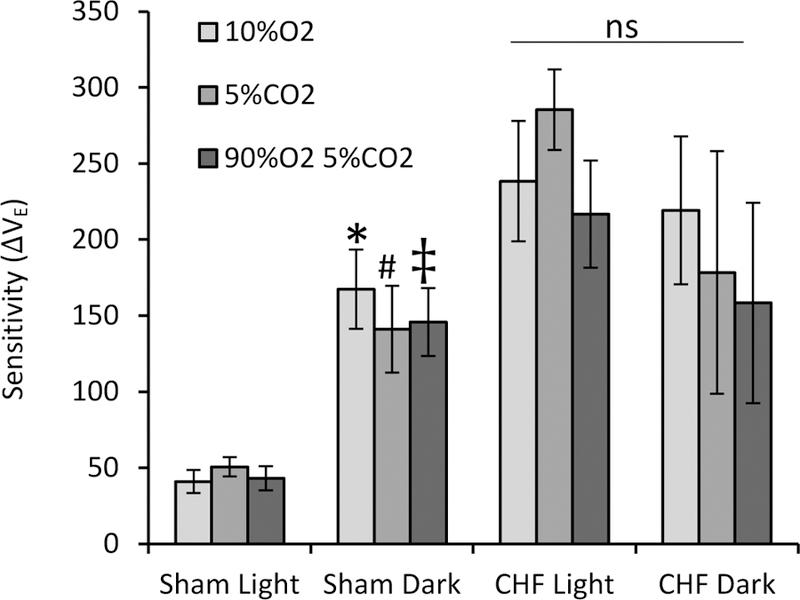
Light/dark variations in chemoreflex sensitivity by subtracting the VE during baseline breathing for each group from each chemoreflex challenge.* statistically different from sham light 10% O2; # statistically different from sham light 5% CO2; ‡ statistically different from sham light 90%O2 5%CO2; ns, not significant
1.3.4. Circadian rhythms in voluntary exercise
Voluntary locomotor activity was assessed using a running wheel, following a 24hr period in which each animal acclimated to the novel cage containing the running wheel (Fig 3). The total number of rotations were determined for the light and for the dark periods. Wheel running showed a main effect of time (p<0.001) with no main effect of heart failure status (p=0.69) or interaction (p=0.73). The sham group ran 128 ± 14 rotations during the light period and 582 ± 241 rotations during the dark period (p<0.05). The CHF group also ran more during the dark period (425 ± 60 rotations) compared to during the light period (124 ± 42 rotations) (p<0.05). However, there was no difference the sham and CHF groups.
Fig 3. Voluntary exercise.
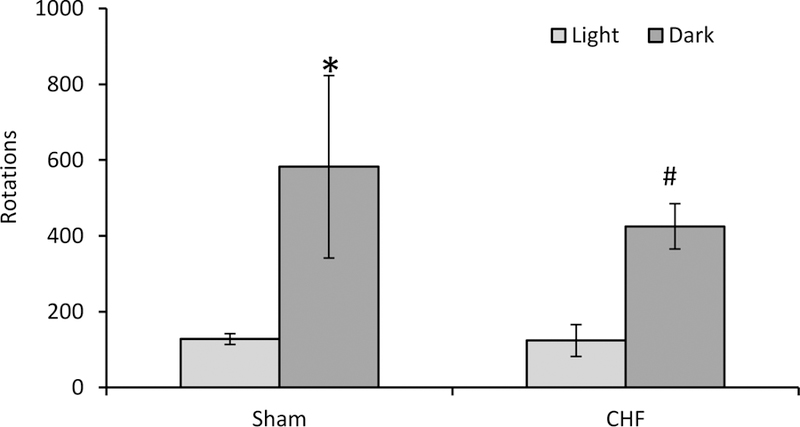
The number of running wheel rotations was recorded for the sham (n=5) and CHF (n=4) groups during the light and dark periods. *statistically significant from sham light; # statistically significant from CHF light.
1.3.4. Circadian rhythms in blood pressure
Circadian rhythms in blood pressure and voluntary exercise were assessed in rats with chronically implanted telemetry. Mean arterial pressure (MAP) differed between the light and dark periods in the sham (light, 102 ± 2.13 mmHg; dark, 111 ± 3.49 mmHg; p=0.03) but not in the CHF group (light, 97 ± 3.80 mmHg; dark, 85 ± 1.36 mmHg; p>0.05). Similarly, systolic pressure was greater during the dark compared to the light in the sham group (light, 128 ± 1.14 mmHg; dark, 136 ± 1.62 mmHg; p=0.03) but not the CHF group (light, 119 ± 8.96 mmHg; dark, 123 ± 9.42 mmHg; p>0.05). Diastolic pressure did not differ between the light and dark periods in either the sham (light, 95 ± 4.86 mmHg; dark, 93 ± 4.6 mmHg; p=0.75) or CHF group (light, 85 ± 1.36 mmHg; dark, 88 ± 2.93 mmHg; p=0.82). The sham and CHF groups did not differ from each other in MAP (p=0.14), systolic pressure (p=0.21), or diastolic pressure (p=0.22).
1.4. Discussion
Respiratory function has been shown to have a circadian periodicity and vary with the light/dark cycle(Mortola 2004). A previous study that acquired respiratory measurements at discrete time points did not find light/dark differences in resting breathing in adult Sprague-Dawley rats (Peever and Stephenson 1997); however, we assessed breathing during the light and dark periods, and we detected light/dark variations in VE in both resting and chemoreflex breathing as well as light/dark differences in chemoreflex sensitivity in the control group. The elevated respiratory rates (~90 bpm) of the former study may have masked light/dark variations in resting breathing. In contrast, the control group of the current study showed respiratory rates of 70±3.87 bpm during the light period and 81 ± 3.73 bpm during the dark. The current study demonstrates that measurements of respiratory function taken at discrete time points can be used to detect light/dark variations in breathing in healthy adult rats. Theses findings are consistent with previous studies that found greater HVR (Seifert and Mortola 2002) and HCVR (Peever and Stephenson 1997) during the dark in rats.
In addition to finding circadian rhythms in resting and chemoreflex breathing in healthy adult rats, we also assessed these rhythms in CHF rats with reduced ejection. To our knowledge, this is the first study to assess circadian rhythms in respiration in CHF. Light/dark variations in respiration and arterial pressure appear to be abolished in CHF with reduced ejection fraction. Our study corroborates previous work on blood pressure rhythms in human heart failure patients (Komori et al. 2016) and mice with myocardial infarctions (Mousa et al. 2014). The current study expands previous findings in showing that CHF also eliminates respiratory circadian rhythms. Although CHF rats did not show a circadian rhythm in breathing, chemoreflex sensitivity, or blood pressure, the CHF group showed light/dark variation in voluntary exercise. These results suggest that CHF impairs circadian rhythmicity of autonomic function with respect to neural control of breathing and cardiovascular function before significantly impacting circadian variations in behavioral locomotor activity.
Also, surprisingly, the CHF group exercised the same amount as the sham control group. The reason for this finding is unclear. Exercise intolerance in CHF appears to be mediated largely by skeletal muscle myopathy (Piepoli and Crisafulli 2014). Thus, it is conceivable that while ejection fraction was decreased and respiratory function was abnormal, skeletal muscle myopathy was not sufficiently advanced to impair locomotor activity.
The mechanism underlying the respiratory circadian rhythm in the control group and the lack of light/dark variations in the CHF group have not been determined but 24hr variations in carotid body chemoreceptor sensitivity is a promising target. A series of ex vivo experiments in the rat carotid body suggest that carotid body sensitivity varies between the light and dark periods (Chen et al. 2005; Tjong et al. 2006). The firing rate of the rat carotid body increases significantly in response to hypoxia increases in the presence of melatonin (Chen et al. 2005; Tjong et al. 2006), a hormone that is released during the night in both humans (Grivas and Savvidou 2007) and rats (Lynch et al. 1984). Altered carotid body function may explain the lack of light/dark variations in breathing in CHF. While the responsiveness of the carotid body in the presence of melatonin may promote light/dark variations in healthy controls, carotid body function is exaggerated at rest and in response to hypoxia and hypercapnia in CHF(Andrade et al. 2015). The already heightened activity of these cells during the light and dark periods may mask light/dark differences in resting and chemoreflex breathing. Future studies should assess the role of melatonin in modulating resting and chemoreflex breathing during the light and dark periods in healthy controls and explore how this process may be altered in CHF.
Acknowledgements
The authors would like to acknowledge Mary Ann Zink and Kaye Talbitzer for their technical assistance in completing this project.
This work was supported by NIH PO1-HL062222-17.
References
- Andrade DC, Lucero C, Toledo C, Madrid C, Marcus NJ, Schultz HD, Del Rio R (2015) Relevance of the Carotid Body Chemoreflex in the Progression of Heart Failure. Biomed Res Int 2015:467597 10.1155/2015/467597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala DE, Moya A, Crespo JJ, Castineira C, Dominguez-Sardina M, Gomara S, Sineiro E, Mojon A, Fontao MJ, Hermida RC, Hygia Project I (2013) Circadian pattern of ambulatory blood pressure in hypertensive patients with and without type 2 diabetes. Chronobiol Int 30 (1–2):99–115. 10.3109/07420528.2012.701489 [DOI] [PubMed] [Google Scholar]
- Chen Y, Tjong YW, Ip SF, Tipoe GL, Fung ML (2005) Melatonin enhances the hypoxic response of rat carotid body chemoreceptor. J Pineal Res 38 (3):157–163. 10.1111/j.1600-079X.2004.00187.x [DOI] [PubMed] [Google Scholar]
- Del Rio R, Marcus NJ, Schultz HD (2013) Inhibition of hydrogen sulfide restores normal breathing stability and improves autonomic control during experimental heart failure. J Appl Physiol 114 (9):1141–1150. 10.1152/japplphysiol.01503.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Young ME (2010) The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res 106 (4):647–658. 10.1161/circresaha.109.209957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floras JS, Jones JV, Johnston JA, Brooks DE, Hassan MO, Sleight P (1978) Arousal and the circadian rhythm of blood pressure. Clin Sci Mol Med Suppl 4:395s–397s [DOI] [PubMed] [Google Scholar]
- Grivas TB, Savvidou OD (2007) Melatonin the “light of night” in human biology and adolescent idiopathic scoliosis. Scoliosis 2:6 10.1186/1748-7161-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Eguchi K, Saito T, Hoshide S, Kario K (2016) Riser Pattern: Another Determinant of Heart Failure With Preserved Ejection Fraction. J Clin Hypertens (Greenwich) 18 (10):994–999. 10.1111/jch.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HJ, Deng MH, Wurtman RJ (1984) Light intensities required to suppress nocturnal melatonin secretion in albino and pigmented rats. Life Sci 35 (8):841–847 [DOI] [PubMed] [Google Scholar]
- Morrissey RP, Czer L, Shah PK (2011) Chronic Heart Failure. Am J Cardiovasc Drugs 11 (3):153–171. 10.2165/11592090-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Mortola JP (2004) Breathing around the clock: an overview of the circadian pattern of respiration. Eur J Appl Physiol 91 (2–3):119–129. 10.1007/s00421-003-0978-0 [DOI] [PubMed] [Google Scholar]
- Mortola JP, Seifert EL (2002) Circadian patterns of breathing. Respir Physiol Neurobiol 131 (1–2):91–100 [DOI] [PubMed] [Google Scholar]
- Mousa TM, Schiller AM, Zucker IH (2014) Disruption of cardiovascular circadian rhythms in mice post myocardial infarction: relationship with central angiotensin II receptor expression. Physiol Rep 2 (11). 10.14814/phy2.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partch CL, Green CB, Takahashi JS (2014) Molecular architecture of the mammalian circadian clock. Trends in Cell Biol 24 (2):90–99. 10.1016/j.tcb.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever JH, Stephenson R (1997) Day-night differences in the respiratory response to hypercapnia in awake adult rats. Respir Physiol 109 (3):241–248 [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Crisafulli A (2014) Pathophysiology of human heart failure: importance of skeletal muscle myopathy and reflexes. Exp Physiol 99 (4):609–615. 10.1113/expphysiol.2013.074310 [DOI] [PubMed] [Google Scholar]
- Saiki C, Mortola JP (1995) Hypoxia abolishes the morning-night differences of metabolism and ventilation in 6-day-old rats. Can J Physiol Pharmacol 73 (1):159–164 [DOI] [PubMed] [Google Scholar]
- Seifert EL, Mortola JP (2002) Circadian pattern of ventilation during prolonged hypoxia in conscious rats. Respir Physiol Neurobiol 133 (1–2):23–34 [DOI] [PubMed] [Google Scholar]
- Tjong YW, Chen Y, Liong EC, Tipoe GL, Fung ML (2006) Chronic hypoxia modulates the function and expression of melatonin receptors in the rat carotid body. J Pineal Res 40 (2):125–134. 10.1111/j.1600-079X.2005.00286.x [DOI] [PubMed] [Google Scholar]
- Tsimakouridze EV, Alibhai FJ, Martino TA (2015) Therapeutic applications of circadian rhythms for the cardiovascular system. Front Pharmacol 6:77 10.3389/fphar.2015.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]