Abstract
Purpose:
Chest wall (CW) pain and rib fractures are frequently diagnosed following stereotactic body radiotherapy (SBRT) for malignant lung tumors. We hypothesize that multiple risk factors including bone mineral density (BMD) are associated with CW toxicity and that CW pain and rib fractures often evolve into chronic clinical problems.
Methods:
118 lung tumors treated with SBRT in 100 patients with a minimum follow up of 2 years were retrospectively analyzed. The incidence, clinical course, and related demographic, clinical and dosimetric factors of CW pain and rib fractures were analyzed. In addition, BMD was assessed, and the radiographic appearance of radiation-induced rib fractures and their healing process was characterized.
Results:
Median follow up was 49 months (range 24-106). CW pain developed in 33/118 treatments (28%) after on average 12.5 months (0-50) and was more common in women (p=0.04). Mean duration of CW pain was 25 months (2-63), and 36% of patients never had resolution of CW pain. 34 of 118 (29%) treatments resulted in rib fractures at a mean time of 22 months (3-46) and were more common in women, African Americans, upper/middle lobe tumors, and patients with lower BMD (p<0.05). Mean duration of rib fractures was 25 months (5-41), and only 16 (47%) rib fractures healed. Shorter PTV-CW distance resulted in higher risk for both rib fractures and CW pain (p=0.01). 67% of fractures developed surrounding soft tissue fibrosis and 62% (21/34) developed heterotopic ossification. Diabetes, BMI, and steroid use were not associated with CW pain or rib fracture.
Conclusions:
Several factors were associated with higher risk of SBRT-related CW toxicity. Optimal chest wall sparing (e.g. VMAT, lower dose per fraction) should be considered in this patient group without compromising tumor control. SBRT-induced rib fractures commonly heal abnormally and result in potential chronic CW pain.
Introduction
Stereotactic Body Radiotherapy (SBRT) has been established as an effective treatment option in medically inoperable patients with Stage I-II non-small cell lung cancer, but is becoming increasingly popular even in operable patients.1 While the long term efficacy of SBRT is currently being studied,2 long term toxicity of SBRT is less well described. In particular, information on chest wall (CW) toxicity with long term follow up is lacking.
Chest wall toxicity resulting in rib fracture or pain has been described since thoracic SBRT was first investigated.1,3 The pathophysiology of radiation-induced rib fractures or CW pain has been postulated to be related to cortical thinning.4 There has also been evidence to support vascular injury leading to myofilament disruption, protein breakdown and endothelial injury.5 Compared to traumatic rib fractures with a short healing phase of <4 months,6 the pattern of symptoms and radiographic changes following radiation-induced rib fractures is less well described. Despite a growing body of evidence that CW pain and radiation-induced rib fractures are prevalent toxicities with a reported incidence between 1.5 and 33% for CW pain and between 1.6 and 42.4% for rib fractures, the duration of these side effects has not been well established in the long term setting.7,8,9,10 In particular, little is known about the characteristics of the healing process after radiation-induced rib fractures. In the present study therefore incidence and duration of CW pain and rib fractures after SBRT are investigated, and the long term radiographic healing process is characterized.
In addition, several risk factors of CW toxicity have been reported. These factors were predominantly related to patient gender and CW dose with varying findings.7,8 In the present study, demographic and dosimetric risk factors as well as comorbidities, medications and also bone mineral density (BMD) are investigated for their association with CW toxicity.
Through the present analysis we aim to provide clinicians with a better understanding of risk factors for CW toxicity and long term clinical outcomes. This information can be critical for patient counseling and might affect radiation treatment planning through improved CW sparing. In addition, awareness of the wide range of healing processes following radiation-related rib fractures on radiographic imaging is important both for radiation oncologists and radiologists to differentiate these findings from similar appearing CW pathologies such as bone metastases.
Methods
Patient Characteristics
After institutional IRB approval, we retrospectively analyzed patients who received SBRT between 2008 and 2015 for primary or metastatic lung tumors. Only patients with a minimum clinical and radiographic follow up of 24 months were included. Patients who received multiple courses of SBRT were allowed provided there was no overlap between fields. Follow up consisted of clinic visits with Chest computed tomography (CT) imaging every 3 months for years 1-2, every 6 months for years 3-5, and yearly after this. Patient characteristics are listed in Table 1.
Table 1:
Patient, Tumor, and Radiotherapy Characteristics* (n=100 patients/118 lesions)
| Characteristic | Number/Median (range) |
|---|---|
| Age (years) | 67 (44-91) |
| Sex | 59 female 41 male |
| Race | 42 African American 58 Caucasian |
| Smoking Status | 58 quit within 1 year 30 yes 12 no |
| Location of Tumor | 72 upper lobe 6 middle lobe 40 lower lobe |
| Diabetes | 18 yes 82 no |
| Karnofsky Performance Status (%) | 80 (50-100) |
| Body Mass Index | 27 (14.3-47) |
| Smoking (pack years) | 45 (0-240) |
| Bone Mineral Density (HU) | 149.7 (38-388) |
| Statin Use | 49 yes 51 no |
| Inhaled Corticosteroids | 55 yes 45 no |
| Systemic Steroids | 9 yes 91 no |
| ACEi Use | 34 yes 66 no |
| NSAID Use | 47 yes 53 no |
| Primary Tumor or Metastasis | 18 metastases 100 primary lesions |
| Biologically Effective Dose (BED) of Prescription (Gy, α/β=10 Gy) | 105.6 (48-151.2) |
| Equivalent Prescription Dose in 2Gy per Fraction (Gy, α/β=10 Gy) | 144 (54–226.8) |
| Planning Target Volume (PTV) (cc) | 23 (3.9-292) |
| Treatment Technique | 101 IMRT 17 VMAT |
| PTV-Chest Wall Distance (cm) | 0 (0-6) |
| V10Gy (cc) | 328.6 (30.2-820.5) |
| V20Gy (cc) | 72.8 (0-427.4) |
| V30Gy (cc) | 17.6 (0-266.1) |
| V40Gy (cc) | 4.9 (0-62.6) |
| CW Mean Dose (Gy) | 5.5 (2.72-34.2) |
| CW Max Dose (Gy) | 51.6 (19.9-103.2) |
| CW D30cc (Gy) | 26.3 (11.8-45.2) |
HU: Hounsfield units; cc: cubic centimeters; IMRT: Intensity Modulated Radiation Therapy; VMAT: Volumetric Modulated Arc Therapy; ACEi: Angiotensin converting enzyme inhibitor; NSAID: Non-steroidal anti-inflammatory drug; CW: Chest Wall
All except location, treatment technique, dosimetric data, PTV, BED and primary versus metastasis are based on patient number, not individual lesions.
Stereotactic Body Radiotherapy
All patients were treated supine in a customized vacuum bag for immobilization with arms raised using a wingboard device. Simulations were performed using 4-D CT technique (Brilliance CT, Philips, Andover, MA), using individual sets of 10 respiration phases as well as maximum intensity projections (MIP) to contour target volumes and organs at risk (OAR) (Pinnacle v9.6, Philips, Andover, MA). Internal tumor motion was accounted for during delineation of the internal gross tumor volume (iGTV) by both manually going through each respiratory phase and the MIP on default lung window settings. OARs were contoured on the 30% phase using default lung and mediastinal windows. 5mm uniform expansions were added on the iGTV to create the planning target volume (PTV). All treatments used 6-MV photon beams with 7-10 field IMRT or VMAT and were planned with heterogeneity corrections. Daily cone-beam CT allowed accurate setup to the tumor. Treatments were delivered every other day over the course of 1-3 weeks. The most common fractionation schemes were 48Gy/4fx and 40Gy/5fx in 90 and 14 treatments, respectively. Other fractionations ranged from 30Gy/5fx to 60Gy/5fx.
Late Radiation Induced Chest Wall Injury - Clinical Analysis
Initial data gathering was performed by chart review of consultation and follow up notes. Clinical factors documented included medications (ACE-Inhibitors, Aspirin, anti-diabetic medication, NSAIDs, inhaled and systemic corticosteroids, and statins), medical comorbidities (smoking history, BMI, diabetes mellitus (DM), connective tissue disease), Karnofsky Performance Status, tumor site, and demographic factors (age, sex, race, weight). We then recorded presence of CW pain, time from radiation treatment to onset, duration, pharmacologic requirements, and whether pain interfered with activities of daily living (ADLs). Chest wall pain was uniformly graded using CTCAE v4.0 in which grade 1 pain is mild, grade 2 is moderate and limits ADLs, and grade 3 is severe and limits self care.
Radiographic Analysis
All follow up CT images were reviewed by a radiation oncologist and a radiologist. Rib fractures which were outside of the radiation fields were not included. Onset and duration of rib fractures were recorded. Rib fracture healing was defined by the formation of a fused fracture line without any subsequent development of irregular sclerosis, or other abnormal morphological changes to surrounding tissues. Furthermore, in a chronological fashion, the healing process of each rib fracture was described including the formation of soft tissue fibrosis and heterotopic ossification.
Bone Mineral Density
Bone mineral density was analyzed as a risk factor for CW toxicity using reliable methods verified by Marinova et al.11 Using a circular 1cm paint brush the bone marrow of the T12 vertebral body was contoured on the center vertical 3mm slice on pre-radiation, non-contrast CT scans using default bone window. The average Hounsfield units (HU) were measured for this area excluding any cortex.
Dosimetric Analysis
To analyze the effect of various dosimetric parameters on the development of CW toxicity, CW volume was defined as a 3cm ring around the bilateral lung tissue covering the whole length of the lungs. The following data were collected: Volume of CW receiving ≤10 Gy(V10Gy), 20Gy(V20Gy), 30Gy(V30Gy), and 40Gy(V40Gy), maximum CW dose to 0.03cc(CWmax), mean CW dose(CWmean), and minimum dose to the 30cc of chest wall receiving the highest radiation doses(D30cc). Each of these dosimetric values was converted to equivalent dose in 2Gy/fraction(EQD2) to normalize between different fractionation schemes. PTV volumes and the distance between the closest edge of PTV and CW were recorded. Biologically Effective Dose (BED, α/β=10Gy) was also calculated for tumor.
Statistical Analysis
Multivariate analysis of risk factors for CW toxicity was performed using logistic regression with all baseline demographic, clinical and dosimetric parameters as covariates. Stepwise selection was used to build the models with significantly associated risk factors. Separate models were used for rib fracture and CW pain and the odds ratio was estimated from the final model. Rib fracture duration and associated risk factors were analyzed using time to event analysis with Cox proportional hazards model within the subpopulation having rib fracture. The same Cox model approach was used to analyze CW pain duration. Logistic regression was used to build the model for soft tissue fibrosis and heterotopic ossification. Receiver operating characteristic (ROC) analysis was performed to assess discriminating thresholds of each risk factor. All analyses used the statistical software SAS v9.4.
Results
Between 2008 and 2015, a total of 100 patients (59 female, 41 male) with at least 24 months of follow up received treatment to 118 lesions in separate lobes. Median follow up was 49 months (24-106).
Chest Wall Pain
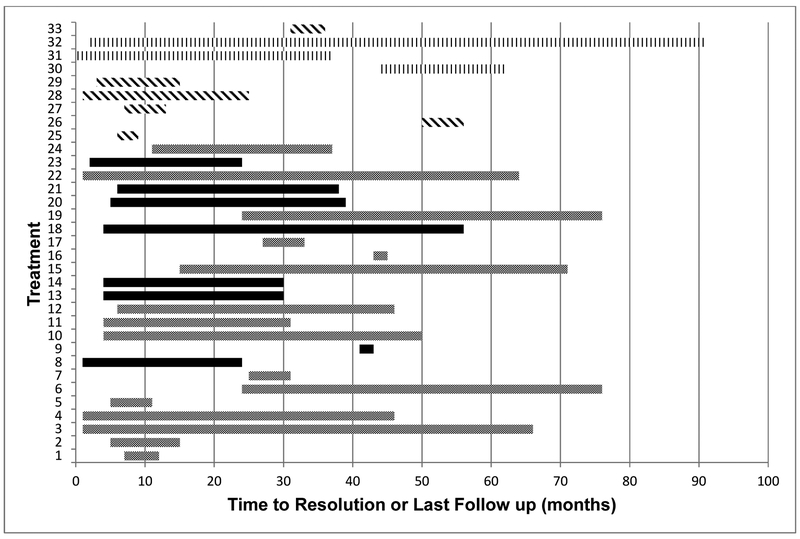
CW pain was observed in 33 of 118 treatments (28%), in 32% of patients. Mean time to development of CW pain was 12.5 months (0-50). 10 treatments resulted in grade 1, 15 treatments in grade 2, and 8 treatments in grade 3 pain. There was no reported grade ≥4 CW pain. Female patients and treatments with higher mean CW dose, or closer PTV-CW distance were at higher risk for CW pain (see Table 2 for OR). A PTV-CW distance of 0.9cm predicted for a 20% rate of chest wall pain. Neither medications nor other clinical parameters were correlated with the development of CW pain. Figure 1 shows the timeline of development, duration, and status of healing of CW pain in each of the 34 treatments.
Table 2:
Odds Ratios and Hazards Ratios of Statistically Significant Variables on Multivariate Analysis
| Rib Fracture Incidence |
Duration of Rib Fracture |
Chest Wall Pain | Duration of Chest Wall Pain |
|
|---|---|---|---|---|
| Female Gender | OR 11.2 (p=0.005) | OR 2.97 (p=0.04) | ||
| African American | OR 9.7 (p=0.005) | HR 0.06 (p=0.006) | ||
| Older Age | HR 1.1 (p=0.005) | |||
| Higher Mean CW Dose | OR 1.1 (p=0.02) | |||
| Larger PTV-CW Distance | OR 0.1 (p=0.01) | OR 0.38 (p=0.01) | ||
| PTV Volume | HR 0.97 (p=0.02) | |||
| Upper/Middle Lobe Tumors | OR 5.5 | HR 0.1 P=0.03) | ||
| Higher Bone Mineral Density | OR 0.95 (p<0.0001) | HR 1.04 (p=0.01) | ||
| Higher CW V40Gy | HR 0.61 (p=0.001) |
OR: Odds Ratio; HR: Hazards Ratio; PTV: Planning Target Volume; CW: Chest wall
Figure 1:
Time of onset and duration of CW pain
Each bar represents a treatment resulting in CW Pain. Grey: females/CW pain resolved; Black: females/not resolved on last follow up; Diagonal Lines: males/resolved; Vertical Lines: males/not resolved on last follow up.
Rib Fracture
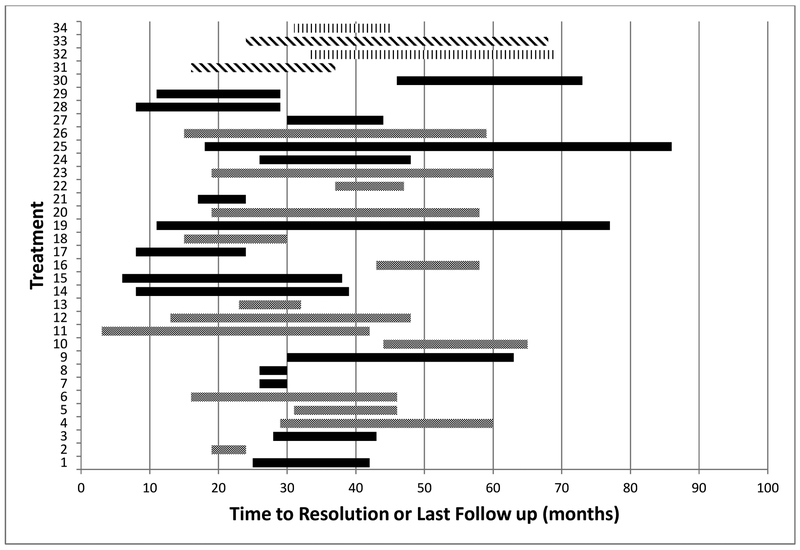
A total of 34 rib fractures (in 32% of patients) were detected out of 118 treatments (29%). The earliest rib fracture was seen 3 months after SBRT and the latest time point at which any rib fracture developed was 46 months. Mean time to development of rib fracture was 22 months. 59% (20/34) of treatments resulting in rib fractures also caused concomitant CW pain, while 14 rib fractures were asymptomatic. Figure 2 shows the timeline of development, duration, and status of healing of rib fractures in each of the 34 treatments. Displacement of ribs was seen in 23/34 fractures (68%) at the time of diagnosis.
Figure 2:
Time of onset and duration of rib fracture
Each bar represents a treatment resulting in a rib fracture. Grey: females/healed; Black: females/not healed on last follow up; Vertical Blue: males/healed; Diagonal Blue: males/not healed on last follow up.
Rib fractures were significantly more common in females, patients with lower bone mineral density and African Americans. A PTV-CW distance of 0.45cm and a BMD of 159.6HU each, respectively, predicted for a 20% rate of rib fracture. No dosimetric, clinical or demographic parameters were found to be significantly correlated with development of rib fractures otherwise.
Healing Process – Chest Wall Pain
Mean duration of chest wall pain was 25 months (2-63) with 12 patients (36%) having ongoing pain at last follow up. Opioids were required to manage chest pain in 21 patients while over-the-counter pain medications were required in an additional 6 patients for pain control. Older patients, patients taking statins, and those with larger PTV volumes had significantly longer duration of CW pain (see Table 2 for HR).
Healing Process- Rib Fractures
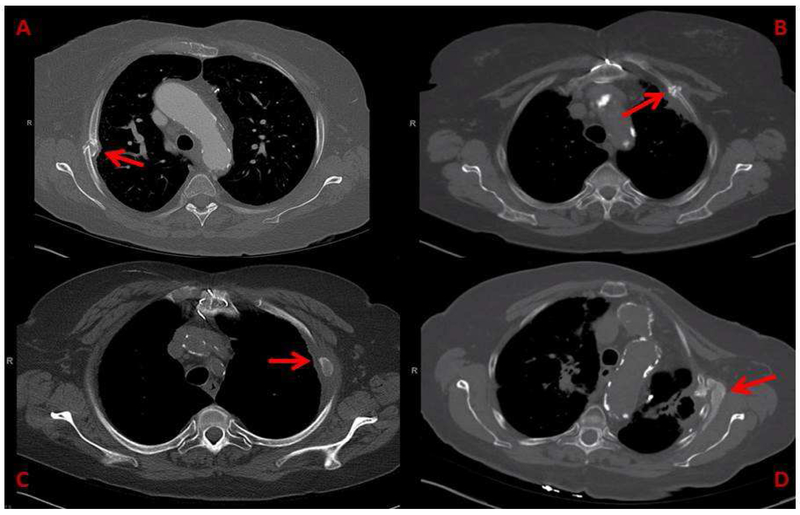
Radiographic healing of rib fractures was found in only 16 of 34 rib fractures. Mean duration of the rib fracture healing process was 25 months (5-41) and notably 18 rib fractures did not heal on last available imaging. Treatments to middle and upper lobe tumors, those in African American patients as well as patients with higher bone mineral density or higher chest wall V40Gy had significantly longer time to healing (see Table 2 for HR). With respect to the healing process, 67% (23/34) of fractures developed visible surrounding soft tissue fibrosis and 62% (21/34) developed heterotopic ossification. Development of soft tissue fibrosis was significantly higher in treatments with larger PTV volumes (OR 1.1; 95%CI: 1.0-1.15; p=0.04). Figure 3 depicts examples of abnormal and disorganized healing processes of radiation-induced rib fractures.
Figure 3:
Abnormal Healing Processes following SBRT-induced rib fracture
A: SBRT-induced rib fracture with chronic displacement; B: Disorganized callous formation with non-union following SBRT-induced rib fracture at 35 months; C: Bony island formation with soft tissue fibrosis in a SBRT-induced rib fracture at 75 months; D: Extensive and dysmorphic heterotopic ossification and soft tissue fibrosis surrounding a SBRT-induced rib fracture at 15 months.
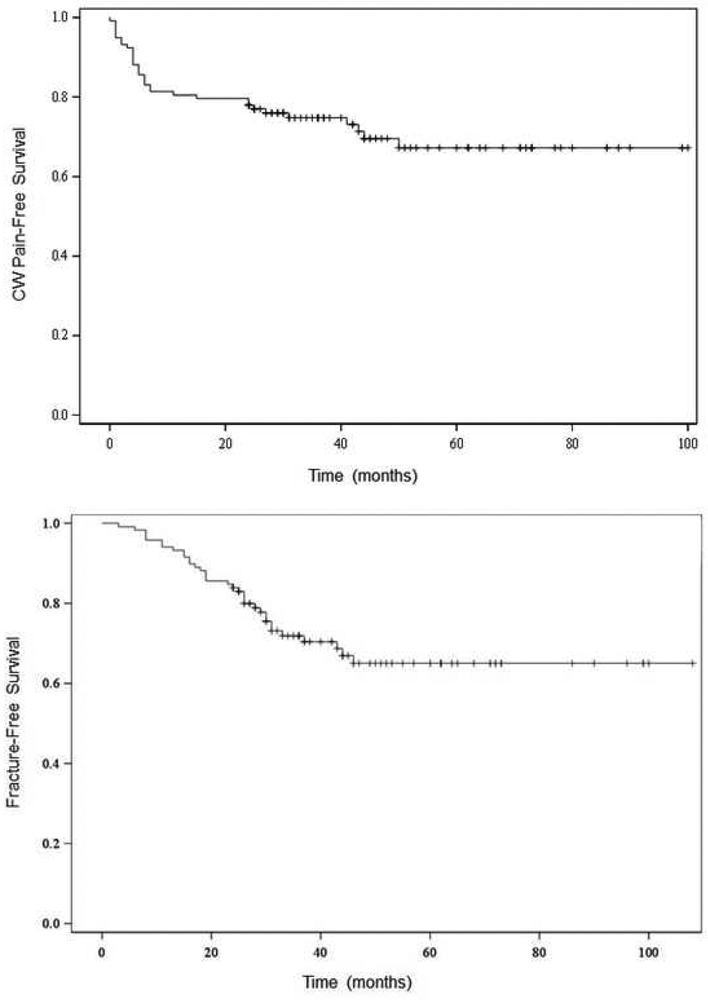
Toxicity-Free Survival
In our patients, 3- and 5-year CW pain-free survival was 75% and 67%, respectively (Figure 4a). 3- and 5-year rib fracture-free survival was 72% and 65%, respectively (Figure 4b).
Figure 4:
Toxicity Free Survival Kaplan Meier Curves
A: CW pain-free survival at 5 years: 67%; B: Rib Fracture-free survival at 5 years: 65%; Each step represents development of Toxicity and “+” represents censorship for death or lost to follow up
Discussion
Incidence of CW Pain and Rib Fractures
Our data confirm previous studies showing that while SBRT is a safe technique, it has a significant potential to cause CW pain and rib fractures. This was originally discovered in early Phase I and II trials evaluating the efficacy of SBRT when chest wall pain and/or rib fractures were seen in a substantial portion of patients.1,12 A summary table of similar studies examining CW toxicity can be found in the Appendix.
CW pain was reported following 28% of treatments and a majority (64%) of these patients had an underlying rib fracture. The rate of CW pain varies in the literature, ranging from 1.5% to 33%,7,8 though required follow up is minimal and long term clinical follow up is often short. Similar to rib fracture development in our cohort, time to onset of CW pain is variable and can occur late. One patient developed CW pain immediately after radiation ended while the longest interval to development was 50 months following SBRT.
Several reports provided cross-sectional incidence of rib fractures after SBRT. Asai et al. reported on 116 patients, of whom 24% developed rib fractures after a median time of 22 months (9-42).7 A prospective study by Nambu et al. showed 23.7% of 177 patients with a minimum 6 month follow up and a median follow up of 23 months (6-95) developed rib fractures at 4-58 months from treatment.13 With a minimum 24 month and median 49 month follow up in our study, our aim was to contribute information on particularly the duration and long term healing process.
While the incidence of rib fractures with 29% appears comparable with published data cited in the Appendix, other reports quote rib fracture rates as low as 0.5% to 2%.14,15 We hypothesize that this might be attributed to longer follow up and detailed radiologic review of each follow up CT scan in our study, as not all rib fractures are clinically symptomatic (21/34 caused pain in our study) and therefore might go unnoticed. Given the sometimes long time to development of rib fractures, diagnosis might be missed and the incidence might appear artificially low in reports with short follow up.
Factors related to CW Toxicity
Certain clinical factors have been found to increase the risk for rib fractures or CW pain. In our patient population, we have found that patients with lower bone mineral density are at significantly higher risk of developing a rib fracture; this has not been previously described. Additionally, lower BMD was also associated with prolonged time to healing of rib fractures. African Americans also had higher rates of rib fractures compared with Caucasians which is somewhat counterintuitive given that, in the general population, African Americans have been found to have higher BMD and favorable bone microarchitecture.16
Previous reports are conflicted regarding gender differences in the incidence of rib fractures (see Appendix). In our cohort, women were at a significantly higher risk for rib fracture as were those with lower BMD though independence could not be established in this study. Other factors such as BMI and DM were both found to be correlated with risk of CW pain in one study but these conditions were not found to be relevant in our cohort.17 Since lower BMD appears as a risk factor in our study, bisphosphonate use might be considered to protect against radiation induced cortical thinning.
Healing Process
Unlike post-traumatic rib fractures that are usually acute with a lower risk of chronic pain,18 radiation-induced fractures can result in prolonged symptoms. Chronic pain from traumatic rib fractures also tends to be manageable without the use of opioids19 and these rib fractures most commonly heal appropriately with union and require minimal intervention20,21. With our longer-term results and radiographic evaluation, we show that radiation-induced rib fractures frequently do not heal, even with prolonged follow up, and that chronic pain is common.
The radiographic difference between SBRT-induced rib fractures and traumatic rib fractures has not been well studied. Furthermore, there has previously been very little information available on radiographic changes during healing of radiation-induced rib fractures. Kim et al. reported on 126 lesions treated with SBRT and graded rib fractures based on the degree of healing.8 Fractures were graded as a simple healed line fracture (Gr1), dislocation ≥½ of rib diameter (Gr2), or associated soft tissue fibrosis (Gr3); in this study only 6 months of follow up were required, and median follow up was 22 months. In this report the majority of rib fractures healed with simple healed line fracture (52%), 31% developed soft tissue fibrosis, and displacement was not reported. In our study, the majority of rib fractures were displaced at time of diagnosis (68%) and developed subsequent soft tissue fibrosis (67%) and/or heterotopic ossification (62%). This is in contrast to traumatic rib fractures which typically heal with union and callous but minimal to no associated fibrosis or heterotopic ossification.22 Differentiation of abnormal rib fracture healing following radiotherapy, likely due to osteoradionecrosis, from bone metastasis may be difficult. Understanding of the healing characteristics after radiation-induced rib fracture and appropriate repeat imaging or biopsy will lead to the correct diagnosis.
Dosimetric Factors
Several reports focused on evaluating dosimetric quantities which may predict for CW toxicity, see also Appendix. One dual-institutional study found a volume threshold of 30cm3 before observing severe pain and/or rib fracture.10 Bongers et al. reviewed 500 patients with 530 tumors and found that treatment volumes were larger and distance to CW shorter in those patients with CW pain and rib fractures.9 Other studies have focused on D2cc and D70cc and these have been incorporated into international guidelines.23,24,25 Another analysis on 476 patients found Dmax<225Gy to be correlated with lower risk of rib fractures.26
In our study, higher CW V40Gy resulted in prolonged time to healing of rib fractures, and larger PTV and prescription doses resulted in prolonged healing of CW pain. Higher mean CW dose and closer distance from CW to tumor resulted in higher risk of chest wall pain, as shown in Table 2.9,13
Short Comings
Although this is a retrospective study with its known limitations, review of CT imaging for all patients provided an objective measure of CW toxicity, in addition to the more subjective diagnosis of CW pain. Also, larger patient numbers and inclusion of other parameters might allow identification of additional significant predictors of CW toxicity or may identify factors, such as treatment duration or fraction size which were not investigated in our patient population. In addition, the distribution of medical comorbidities or demographic factors in our cohort may not be applicable to other populations.
Conclusion
In conclusion, CW toxicity is a frequent consequence of thoracic SBRT which can lead to prolonged CW pain and slow or non-healing rib fractures. Subsequently, long term opioid use is common in this population. Radiographically, radiation-induced rib fractures commonly have associated soft tissue fibrosis and/or heterotopic ossification. Identifying these changes is important in differentiating between radiation induced bony injury and other differential diagnoses such as bone metastases. These observations should be recognized when discussing thoracic SBRT with patients, particularly with women, African Americans, and osteopenic patients. During treatment planning, consideration should be given to avoid large volumes with high doses in the chest wall. The use of highly conformal techniques (VMAT) and increasing the number of fractions should be considered,5,28 without compromising target coverage.9 Simultaneous integrated boost delivering higher doses to actual tumor while maintaining lower dose at the PTV margin can be considered for good local control without increasing toxicity.31
Supplementary Material
Acknowledgements:
We would like to thank Justin Sperlazza, MD for his assistance on this project. Services in support of the research project were generated by the VCU Massey Cancer Center Biostatistics Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Timmerman R, Paulus R, Galvin J et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010. March 17;303(11):1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol 2018. May. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritz P, Kraus HJ, Blaschke T, et al. Stereotactic, high single dose irradiation of stage I non-small cell lung cancer (NSCLC) using four-dimensional CT scans for treatment planning. Lung Cancer 2008; 60:193–199. [DOI] [PubMed] [Google Scholar]
- 4.Okoukoni C, Lynch SK, McTyre ER, Randolph DM, et al. A cortical thickness and radiation dose mapping approach identifies early thinning of ribs after stereotactic body radiation therapy. Radiother Oncol 2016. June; 119(3):449–53. doi: 10.1016/j.radonc.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 5.Fain R III, Thomas CR Jr, and Nabavizadeh N. Shoulder Pain After Definitive Lung Radiotherapy. JAMA Oncology. 2016. April; 2(4): 537–8. [DOI] [PubMed] [Google Scholar]
- 6.Kerr-Valentic MA, Arthur M, Mullins RJ, et al. Rib fracture pain and disability: can we do better? J Trauma. 2003. June; 54(6): 1058–1063. [DOI] [PubMed] [Google Scholar]
- 7.Asai K, Shioyama Y, Nakamura K, et al. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy: risk factors and dose-volume relationship. Int J Radiat Oncol Biol Phys 2012. November; 84(3): 768–73. doi: 10.1016/j.ijrobp.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Kim SS, Song SY, Kwak J, et al. Clinical prognostic factors and grading system for rib fracture following stereotactic body radiation therapy (SBRT) in patients with peripheral lung tumors. Lung Cancer 2013. February; 79(2):161–6. 10.1016/j.lungcan.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Bongers EM, Haasbeek CJ, Lagerwaard FJ, et al. Incidence and Risk Factors for Chest Wall Toxicity after Risk-Adapted Stereotactic Radiothearpy for Early-Stage Lung Cancer. Journal of Thoracic Oncology. 2011. December; 6: 2052–2057. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010. March;76(3):796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Marinova M et al. Use of routine thoracic and abdominal computed tomography scans for assessing bone mineral density and detecting osteoporosis. Current Medical Research and Opinion. 2015; 31(10): 1871–1881. doi: 10.1185/03007995.2015.1074892. [DOI] [PubMed] [Google Scholar]
- 12.Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a Phase I study in medically inoperable Stage I non-small cell lung cancer. Chest 2003; 124: 1946–1955. 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 13.Nambu A, Onishi H, Aoki S, et al. Rib fracture after stereotactic radiotherapy for primary lung cancer: prevalence, degree of clinical symptoms, and risk factors. BMC Cancer 2013. February; 13(1): 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008. March; 70(3): 685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 15.Ricardi U, Frezza G, Filippi AR, et al. Stereotactic Ablative Radiotherapy for stage I histologically proven non-small cell lung cancer: an Italian multicenter observational study. Lung Cancer 2014. June; 84(3): 248–253. 10.1016/j.lungcan.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Popp KL, Hughes JM, Martinez-Betancourt A, et al. Bone mass, microarchitecture and strength are influenced by race/ethnicity in young adult men and women. Bone 2017. October; 103:200–208. [DOI] [PubMed] [Google Scholar]
- 17.Welsh J, Thomas J, Shah D, et al. Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2011. September; 81(1):91–6. doi: 10.1016/j.ijrobp.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordy S, Fabricant L, Ham B, et al. The contribution of rib fractures to chronic pain and disability. Am J Surg 2014. May; 207(5):659–62. doi: 10.1016/j.amjsurg.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Shelat VG, Eileen S, John L, et al. Chronic pain and its impact on quality of life following a traumatic rib fracture. Eur J Trauma Emerg Surg 2012. October; 38:451–455. [DOI] [PubMed] [Google Scholar]
- 20.Ng AB, Giannoudis PV, Bismil Q, et al. Operative stabilization of painful non-united multiple rib fractures. Injury. 2001. October; 32(8): 637–639. [DOI] [PubMed] [Google Scholar]
- 21.Richardson JD, Franklin GA, Heffley S, et al. Operative fixation of chest wall fractures: an underused procedure? Am Surg 2007. June; 73(6):591–6. [PubMed] [Google Scholar]
- 22.Marasco S, Liew S, Edwards E, et al. Analysis of bone healing in flail chest injury: do we need to fix both fractures per rib? J Trauma Acute Care Surg. 2014. September: 77(3): 452–8. doi : 10.1097/TA.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 23.De Ruysscher D, Faivre-Finn C, Moeller D et al. European Organization for Research and Treatment of Cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiotherapy and Oncology 2017; 124:1–10. 10.1016/j.radonc.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 24.Kimsey F; McKay J, Gefter J, et al. Dose-Response Model for Chest Wall Tolerance of Stereotactic Body Radiation Therapy. Semin Radiat Oncol 2016; 26:129–34. 10.1016/j.semradonc.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 25.Murray L, Karakaya E, Hinsley S, et al. Lung stereotactic ablative radiotherapy (SABR): dosimetric considerations for chest wall toxicity. Br J Radiol 2016; 89: (1058) 20150628. doi: 10.1259/bjr.20150628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stam B, van der Bijl E, Peulen H, et al. Dose–effect analysis of radiation induced rib fractures after thoracic SBRT. Radiother Oncol 2017; 123(2): 176–181. 10.1016/j.radonc.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 27.Coroller TP, Mak RH, Lewis JH, et al. Low Incidence of Chest Wall Pain with a Risk-Adapted Lung Stereotactic Body Radiation Therapy Approach Using Three or Five Fractions Based on Chest Wall Dosimetry. PLoS One 2014. 9(4):e94859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu AS, Maxim PG, Loo BW, et al. Chest wall dose reduction using noncoplanar volumetric modulated arc radiation therapy for lung stereotactic ablative radiation therapy. Practical Radiation Oncology 2018. August: 8(4):e199–207. 10.1016/j.prro.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 29.Aoki M, Hatayama Y, Kawaguchi H, et al. Clinical outcome of stereotactic body radiotherapy for primary and oligometastatic lung tumors: a single institutional study with almost uniform dose with different five treatment schedules. Radiation Oncology 2016; 11: 5. doi: 10.1186/s13014-016-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibault I, Chiang A, Erler D, et al. Predictors of Chest Wall Toxicity after Lung Stereotactic Ablative Radiotherapy J Clinical Oncology 201628: 28–35. 10.1016/j.clon.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 31.Andolino DL, Forquer JA, Henderson MA, et al. Chest Wall Toxicity after Stereotactic Body Radiotherapy for Malignant Lesions of the Lung and Liver. Int J Radiat Oncol Biol Phys 2011; 80(3):692–697. doi: 10.1016/j.ijrobp.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Creach KM, El Naqa I, Bradley JD, et al. Dosimetric predictors of chest wall pain after lung stereotactic body radiotherapy. Radiother Oncol 2012; 104(1): 23–27. 10.1016/j.radonc.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 33.Zhao L, Zou S, Balter P, Shen C, et al. Planning Target Volume D95 and Mean Dose Should Be Considered for Optimal Local Control for Stereotactic Ablative Radiation Therapy. Int J Radiat Oncol Biol Phys 2016. July 15;95(4):1226–35. doi: 10.1016/j.ijrobp.2016.01.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.