Abstract
Objective:
In kidney diseases, uncontrolled blood pressure, inflammation, oxidative stress, imbalanced immunity response, and metabolic dysfunction were associated with the progressive deterioration of renal function. Short-chain fatty acids (SCFAs), as a group of metabolites fermented by gut microbiota exerted regulatory effects on kidney diseases through their activation of trans-membrane G protein-coupled receptors and their inhibition of histone acetylation. In this review article, we updated recent research advances that provided an opportunity to explore our understanding in physiology and function of SCFAs in kidney disease.
Data sources:
We performed a comprehensive search in both PubMed and Embase using “short-chain fatty acids” and “kidney” with no restrictions on publication date.
Study selection:
After reading through the title and abstract for early screening, the full text of relevant studies was identified and reviewed to summarize the roles of SCFAs in kidney diseases.
Results:
Though controversial, growing evidence suggested SCFAs appeared to have a complex but yet poorly understood communications with cellular and molecular processes that affected kidney function and responses to injury. From recent studies, SCFAs influenced multiple aspects of renal physiology including inflammation and immunity, fibrosis, blood pressure, and energy metabolism.
Conclusions:
The roles of intestinal SCFAs in kidney diseases were exciting regions in recent years; however, clinical trials and animal experiments in kidney diseases were still lacked. Thus, more research would be needed to obtain better understanding of SCFAs’ potential effects in kidney diseases.
Keywords: Gut microbiome, Short-chain fatty acid, Kidney disease, Gut-kidney axis
Introduction
The human intestinal tract harbors a diverse and complex microbial community, which contributes to gut homeostasis and the host's health. In recent years, metabolites produced by gut microbiota, particularly short-chain fatty acids (SCFAs), are generally proven to improve health.[1,2] SCFAs are straight chain saturated fatty acids which are consisted of less than six carbon atoms including acetate (two carbons), propionate (three carbons), butyrate (four carbons), and valproic acid (five carbons). These SCFAs are produced by anaerobic bacteria in the distal small intestine and the colon after carbohydrates fermentation and also could be absorbed in the bloodstream.[3] The decreased levels of SCFAs were usually related with the happen of illnesses, including inflammatory bowel disease, obesity, diabetes mellitus, multiple sclerosis, colon cancer, as well as kidney diseases.[3–8]
The functions of SCFAs are mainly related to their activation of trans-membrane G protein-coupled receptors (GPCRs) such as Gpr41, Gpr43, and olfactory receptor 78 and their inhibitory effects of histone acetylation (HDAC).[9] SCFAs also exerted anti-bacterial, anti-inflammatory, anti-oxidative, anti-diabetic, and anti-cancer effects in numerous studies.[8] However, the mechanisms of SCFAs in the gut-kidney axis have not yet to be fully explored. The review provides an overview of gut microbiota-derived SCFAs in kidney disease and the involved signal pathway.
Effects of SCFAs in kidney diseases
Inflammation
SCFAs modulated inflammation both in intestinal and extra-intestinal environments and possessed multifarious effects against inflammatory bowel disease and allergic airway disease by decreasing inflammatory response.[10,11] SCFAs also could regulate renal dysfunction both in acute kidney injury (AKI) and chronic kidney disease (CKD) via their anti-inflammatory properties.
In AKI animal models, no matter contrast-induced or ischemia/reperfusion-induced kidney injury, SCFAs administrations significantly improved acute renal dysfunction.[1,12] The key mechanism of SCFAs against AKI was suggested to be the reduction of inflammatory cytokines and chemokines locally and systemically by decreasing nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) cell signal pathway.[1,12] Acetate could also inhibit HDAC activity of T cells to influence Toll-like receptor 4 (TLR4)-induced NAPDH oxidase 2 (NOX2)/reactive oxygen species (ROS) signaling and thus to show anti-inflammatory effect.[13] It was also interesting to note that SCFAs[14] or SCFA-producing bacteria[15] might be benefited in the ischemia/reperfusion injury models of other tissues, implying that the underlying mechanism may be common across tissues.
As to CKD, after supplementation of dietary fiber or xylooligosaccharide (XOS), the increased cecal SCFAs improved intestinal epithelial tight junctions, inflammation, and were positively correlated with CKD clinical manifestations both in rats and in patients with end-stage renal disease (ESRD).[16–18] Not only the dietary fiber showed the benefits of reducing inflammation, propionic acid supplementation to hemodialysis patients, also alleviated pro-inflammatory parameters.[8] The subjects with propionic acid supplementation revealed a significant decline of parameters C-reactive protein, interleukin (IL)-2, IL-17, IL-6, and interferon-gamma, while a significant increase of anti-inflammatory cytokine IL-10. The reduction of inflammatory response was also associated with a lowering of ferritin and a significant increase in hemoglobin, which might be further related to mortality.[19,20] Four weeks after the end of the treatment phase, all improved parameters deteriorated again, which emphasized the effects of propionic acid in improving inflammation in hemodialysis patients.[8]
Surprisingly, not all the treatment of SCFAs showed anti-inflammatory effects. Treatment of the mice by valproic acid failed to block the accumulation of monocytes, macrophages and neutrophils in the angiotensin II (Ang II)-treated rats.[21] After chronically increasing oral doses of SCFAs to higher than physiologic levels in mice, Th1 and Th17 cells were observed to generate in the ureteropelvic junction and proximal part of the ureter, which further caused kidney inflammation and hydronephrosis.[22] Besides acetate-induced renal disease, mice fed a high-fiber diet increased gut butyrate and were more susceptibility to infection with Escherichia coli,[23] as well as enhanced Gb3 level in the gut and kidney, which resulted in severe kidney damage.[24]
The controversial of SCFAs on inflammation were possibly due to the complication of different types of SCFAs. It was mentioned previously that SCFA propionate was negatively correlated with blood urine nitrogen and serum creatinine in adenine-induced nephropathy, while butyrate showed the strong positive correlation.[16] The controversial effects of different types of SCFAs on renal physiology could be definitely observed. Further investigation focusing on SCFAs, especially different types of SCFAs, in regulation inflammation would be necessary.
Immunity
The generation of SCFAs was confirmed to influence innate immunity and adaptive immunity. Defensin alpha 5 (Defa5), as a microbicidal and cytotoxic peptide highly expressed in the Paneth cells of the ileum and involved in host defense of pathogen bacteria[25] was observed to increase in CKD mice. The XOS supplementation potentially increased cecal SCFAs levels, and further inhibited Defa5 to promote an optimal gut microbiota profile in CKD mice and enhance the host's resistance to infection.[16]
Besides enhancing intestinal innate immunity, SCFAs have also been indicated to induce immunologic responses by regulating immune cell production, differentiation, and function.[26] Previous studies reported that SCFAs could induce Tregs production[27,28] by cellular bioenergetic metabolism,[29] indirectly affect T-cell differentiation patterns via a broadly immunosuppressive or tolerogenic effect on antigen-presenting cells,[29,30] and inhibit HDAC activity in T cells to ameliorate sepsis-induced AKI.[13,30,31]
Although SCFAs mostly regulated the immune system to show the protective effects, the activity of SCFAs in immune and epithelial cells also led to damage, if not properly regulated.[28] Thus, the function of SCFAs still seemed to be inconsistent and complex, and further studies could provide mechanistic insights into how gut microbiota-derived metabolites contribute to immune inflammation.
Antioxidant
Kidney diseases are usually associated with the higher levels of ROS, which could damage proteins, DNA, lipids, and other macromolecules in the body.[31] After supplementation of propionic acid to hemodialysis patients, the parameters of oxidative stress, malondialdehyde, and glutathione peroxidase activity were reduced.[8]
Besides the clinical studies, considerable studies were still in experimental phases. It was shown that SCFA acetate and ethyl acetate extract of Zingiber zerumbet rhizome ameliorated sepsis-induced AKI and paracetamol-induced nephrotoxicity through its anti-oxidant properties.[13,32] Not only acetate but also butyrate was verified to protect kidney injury via enhancing superoxide dismutase, catalase activity, as well as reducing glutathione.[33]
The benefits of SCFAs inhibiting ROS generation, no matter in glomerular mesangial cells induced by high glucose and lipopolysaccharide (LPS)[33] or human kidney epithelial HK-2 cells after hypoxia,[1] was reported to be modulated through Gpr43 activity as previous studies.[34,35] However, in sepsis-induced AKI experimental model, the suppression of NOX2/ROS signaling pathway in T cells after acetate administration was due to the inhibition against HDAC activity and not due to Gpr43 activation.[13] These suggested that the signal pathways responded to SCFAs were complicated in different cells and animals, which needed further investigations to clarify the issue.
Antifibrosis
Renal immune-inflammatory processes play key roles in kidney injury leading to chronic glomerulosclerosis and interstitial fibrosis.[33] With the protective effects of SCFAs on immune inflammation, the direct supplementations of acetate or butyrate were both reported to attenuate glomerular and tubulointerstitial fibrosis as well as collagen deposition in the DOCA-salt mice[36] and juvenile diabetic rats,[37] respectively. XOS supplementation, which increased SCFA-producing bacteria was active against fibrosis in adenine-induced kidney damage[16] with the decrease of M2 macrophage, known to play an important role in renal fibrosis.[16,38]
Several signaling pathways were involved in the elimination of renal fibrosis. Valproic acid lowered HDAC activity and suppressed the phosphorylation of ERK, which further inhibited the proliferation of pericytes to block Ang II-induced fibrosis.[21] Increased concentration of butyrate in proximal tubular epithelial cells could prevent transforming growth factor beta 1 (TGF-β1) generation and thus to diminish renal fibrosis.[21,39] However, in porcine kidney fibroblast, the increase of sodium butyrate was along with the markedly enhanced Wilms tumor 1 (WT1), which was involved in cell proliferation and development.[40,41]
Taken these together, anti-fibrosis properties of SCFAs in kidney was mainly through preventing TGF-β1 signaling pathway, inhibiting HDAC activity, and reducing phosphorylation of ERK. Whether SCFAs inhibited cell proliferation or influenced related signal pathways to show anti-fibrosis effects needed further studies to figure out.
Blood pressure
Growing evidences suggested that SCFAs were involved in blood pressure modulation.[42–45] In kidney disease, the systolic blood pressure of hemodialysis patients decreased 10% after supplementation of sodium propionate, while the diastolic pressure was unchanged.[8] Several SCFA receptors were expressed in human kidneys. Olfr78 as an olfactory receptor expressed on the afferent arteriole (juxtaglomerular apparatus) was reported to increase blood pressure through mediating renin secretion and subsequent vasoconstriction.[44] Other SCFA receptors such as Gpr41 were expressed in the renal vasculature (especially smooth-muscle cells of the small resistance vessels) with controversial effects on regulating blood pressure.[45–47] Gpr41, which was reported to have hypotensive effects decreased in the kidney of mice treated with Ang II[48] and null mice for Gpr41 were shown hypertensive.[44] Administration of propionate to Gpr41-deficient mice induced blood pressure elevation, also suggesting that Gpr41 is needed to counterbalance the pressor response to SCFAs.[49]
With respect to how SCFA receptors might be regulated in the kidney, previous study identified miRNAs altered in the hypertensive kidney that target and potentially bind to SCFA receptors.[48,50] It was interesting to note that miR-329 and miR-132 were up-regulated in the hypertensive kidney whereas miR-129 was down-regulated. Actually, miR-329 and miR-132 could target Gpr41 and Gpr43, respectively, while miR-129 was predicted to target Olfr78.[48,50] These data point toward a role for miRNA regulation of SCFA receptors in hypertensive kidney[48]; however, the regulation of these receptors was still not clear.
Metabolism
It was reported previously that the improved inflammation and oxidative stress were associated with the amelioration of impaired glucose metabolism. SCFAs are also involved in the regulation of metabolism, including body weight control, insulin sensitivity,[51] glucose homeostasis,[52] cholesterol synthesis,[53] and retardation of progressive CKD.[54]
In patients with ESRD, insulin resistance accelerated muscle protein degradation,[55] atherosclerosis development, and cardiovascular mortality.[56] Therefore, the improved glucose homeostasis may delay above-mentioned complications. In hemodialysis patients, after propionic acid supplementation, a decline of fasting insulin level and an improvement of the HOMA index (homeostasis model assessment) was observed.[8] Interestingly, fasting glucose and HbA1c remained unchanged. It was hypothesized that the unchanged HbA1c level was correlated with the longer survival of erythrocytes, which was shortened in hemodialysis patients, due to propionic acid's anti-inflammatory ability.[8] However, few studies focused on the effects of SCFAs on metabolism in kidney diseases.
In summary, the roles of the intestinal SCFAs in kidney disease were exciting regions, which influenced several aspects of kidney including inflammation and immunity, fibrosis and proliferation, blood pressure, and energy metabolism in Figure 1. Clinical trials and animal experiments related SCFAs in kidney diseases were still rare and preliminary. Thus, more research will be needed to obtain better understanding of their potential effects in kidney diseases.
Figure 1.
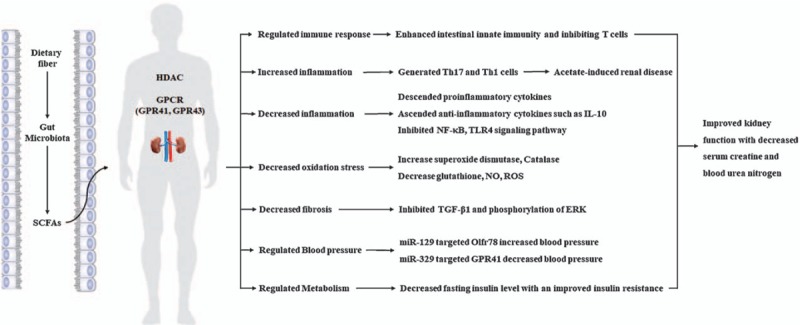
Schematic roles of short-chain fatty acids (SCFAs) in kidney diseases. In kidney, SCFAs regulated immune responses, decreased inflammation, enhanced anti-oxidant, reduced kidney fibrosis, as well as modulated blood pressure and metabolism mainly related to their activation of G protein-coupled receptors (GPCRs) and the inhibition of histone acetylation (HDAC). All of these benefits from SCFAs improved kidney function with the decreased levels of serum creatinine and blood urea nitrogen in both acute kidney jury and chronic kidney disease. However, SCFAs also promoted acetate-induced renal disease under certain condition as the negative outcomes. IL-10: Interleukin 10; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B; ROS: Reactive oxygen species; TGF-β1: Transforming growth factor beta 1; TLR4: Toll-like receptor 4.
Funding
This study is supported by a grant from the National Key Research and Development Program of China (No. 2016YFC1305403).
Conflicts of interest
None.
Footnotes
How to cite this article: Li LZ, Tao SB, Ma L, Fu P. Roles of short-chain fatty acids in kidney diseases. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000228
References
- 1.Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJF, de Almeida DC, et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J Am Soc Nephrol 2015; 26:1877–1888. doi: 10.1681/Asn.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018; 23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Puddu A, Sanguineti R, Montecucco F, Viviani GL. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm 2014; 2014:162021.doi: 10.1155/2014/162021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992; 103:51–56. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]
- 5.Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain 2018; 141:1900–1916. doi: 10.1093/brain/awy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Ma L, Fu P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug Des Devel Ther 2017; 11:3531–3542. doi: 10.2147/DDDT.S150825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010; 328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marzocco S, Fazeli G, Di Micco L, Autore G, Adesso S, Dal Piaz F, et al. Supplementation of short-chain fatty acid, sodium propionate, in patients on maintenance hemodialysis: beneficial effects on inflammatory parameters and gut-derived uremic toxins, a pilot study (PLAN study). J Clin Med 2018; 7: doi: 10.3390/jcm7100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin MY, de Zoete MR, van Putten JP, Strijbis K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front Immunol 2015; 6:554.doi: 10.3389/fimmu.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aurélien T, Gollwitzer ES, Koshika Y, Sichelstiel AK, Norbert S, Catherine NB, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20:159.doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 11.Galvez J, Rodrãguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res 2010; 49: doi: 10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- 12.Machado RA, Constantino LDS, Tomasi CD, Rojas HA, Vuolo FS, Vitto MF, et al. Sodium butyrate decreases the activation of NF-(B reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol Dial Transplant 2012; 27:3136–3140. doi: 10.1093/ndt/gfr807. [DOI] [PubMed] [Google Scholar]
- 13.Al-Harbi NO, Nadeem A, Ahmad SF, Alotaibi MR, AlAsmari AF, Alanazi WA, et al. Short chain fatty acid, acetate ameliorates sepsis-induced acute kidney injury by inhibition of NADPH oxidase signaling in T cells. Int Immunopharmacol 2018; 58:24–31. doi: 10.1016/j.intimp.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar-Nascimento JE, Salomão AB, Nochi RJ, Jr, Nascimento M, Neves Jde S. Intraluminal injection of short chain fatty acids diminishes intestinal mucosa injury in experimental ischemia-reperfusion. Acta Cir Bras 2006; 21:21–25. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Zhang W, Zuo L, Zhu W, Wang B, Li Q, Li J. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br J Nutr 2013; 109:1990–1998. doi: 10.1017/S0007114512004308. [DOI] [PubMed] [Google Scholar]
- 16.Yang J, Li Q, Henning SM, Zhong J, Hsu M, Lee R, et al. Effects of prebiotic fiber xylooligosaccharide in adenine-induced nephropathy in mice. Mol Nutr Food Res 2018; e1800014.doi: 10.1002/mnfr.201800014. [DOI] [PubMed] [Google Scholar]
- 17.Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 2014; 9:e114881.doi: 10.1371/journal.pone.0114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 2014; 39:230–237. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karaboyas A, Morgenstern H, Pisoni RL, Zee J, Vanholder R, Jacobson SH, et al. Association between serum ferritin and mortality: findings from the USA, Japan and European Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 2018; 33:2234–2244. doi: 10.1093/ndt/gfy190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant 2004; 19:141–149. doi: 10.1093/ndt/gfg493. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Gao F, Tang Y, Xiao J, Li C, Ouyang Y, et al. Valproic acid regulates Ang II-induced pericyte-myofibroblast trans-differentiation via MAPK/ERK pathway. Am J Transl Res 2018; 10:1976–1989. doi: 10.1493/ajtr0073105. [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, Goergen CJ, HogenEsch H, Kim CH. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J Immunol 2016; 196:2388–2400. doi: 10.4049/jimmunol.1502046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zumbrun SD, Melton-Celsa AR, Smith MA, Gilbreath JJ, Merrell DS, O’Brien AD. Dietary choice affects Shiga toxin-producing Escherichia coli (STEC) O157:H7 colonization and disease. Proc Natl Acad Sci U S A 2013; 110:E2126–E2133. doi: 10.4161/gmic.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zumbrun SD, Melton-Celsa AR, O’Brien AD. When a healthy diet turns deadly. Gut Microbes 2014; 5:40–43. doi: 10.4161/gmic.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomas G. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 2003; 3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 26.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2014; 500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015; 8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw 2014; 14:277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frikeche J, Simon T, Brissot E, Gregoire M, Gaugler B, Mohty M. Impact of valproic acid on dendritic cells function. Immunobiology 2012; 217:704–710. doi: 10.1016/j.imbio.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Berndt BE, Zhang M, Owyang SY, Cole TS, Wang TW, Luther J, et al. Butyrate increases IL-23 production by stimulated dendritic cells. Am J Physiol Gastrointest Liver Physiol 2012; 303:G1384–G1392. doi: 10.1152/ajpgi.00540.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liakopoulos V, Roumeliotis S, Gorny X, Eleftheriadis T, Mertens PR. Oxidative stress in patients undergoing peritoneal dialysis: a current review of the literature. Oxid Med Cell Longev 2017; 2017:1–14. doi: 10.1155/2017/3494867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdul Hamid Z, Budin SB, Wen Jie N, Hamid A, Husain K, Mohamed J. Nephroprotective effects of Zingiber zerumbet Smith ethyl acetate extract against paracetamol-induced nephrotoxicity and oxidative stress in rats. J Zhejiang Univ Sci B 2012; 13:176–185. doi: 10.1631/jzus.B1100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W, Zhou L, Guo H, Xu Y, Xu Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 2017; 68:20–30. doi: 10.1016/j.metabol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Vieira AT, Macia L, Galvao I, Martins FS, Canesso MCC, Amaral FA, et al. A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol 2015; 67:1646–1656. doi: 10.1002/art.39107. [DOI] [PubMed] [Google Scholar]
- 35.Nadeem A, Ahmad SF, Al-Harbi N, El-Sherbeeny AM, Al-Harbi MM, Almukhlafi TS. GPR43 activation enhances psoriasis-like inflammation through epidermal upregulation of IL-6 and dual oxidase 2 signaling in a murine model. Cell Signal 2017; 33:59–68. doi: 10.1016/j.cellsig.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Marques FZ, Nelson EM, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High fibre diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in DOCA-salt hypertensive mice. Circulation 2016; 135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 37.Sabbir K, Gopabandhu J. Sodium butyrate, a HDAC inhibitor ameliorates eNOS, iNOS and TGF-β1-induced fibrogenesis, apoptosis and DNA damage in the kidney of juvenile diabetic rats. Food Chem Toxicol 2014; 73:127–139. doi: 10.1016/j.fct.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Kim MG, Kim SC, Ko YS, Lee HY, Jo SK, Cho W. The role of M2 macrophages in the progression of chronic kidney disease following acute kidney injury. PLoS One 2015; 10:e0143961.doi: 10.1371/journal.pone.0143961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto N, Riley S, Fraser D, Al-Assaf S, Ishimura E, Wolever T, et al. Butyrate modulates TGF- β 1 generation and function: potential renal benefit for Acacia(sen) SUPERGUM™ (gum arabic)? Kidney Int 2006; 69:257–265. doi: 10.1038/sj.ki.5000028. [DOI] [PubMed] [Google Scholar]
- 40.Miguel NR, Daniel AH. Wilms’ tumour: connecting tumorigenesis and organ development in the kidney. Nat Rev Cancer 2005; 5:699–712. doi: 10.1038/nrc1696. [DOI] [PubMed] [Google Scholar]
- 41.Tan W, Chen Y, An P, Wang A, Chu M, Shi L, et al. Sodium butyrate-induced histone hyperacetylation up-regulating WT1 expression in porcine kidney fibroblasts. Biotechnol Lett 2015; 37:1195–1202. doi: 10.1007/s10529-015-1794-4. [DOI] [PubMed] [Google Scholar]
- 42.Karbach SH, Schonfelder T, Brandao I, Wilms E, Hormann N, Jackel S, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc 2016; 5:e003698.doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014; 64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 44.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics 2016; 48:826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 2014; 5:202–207. doi: 10.4161/gmic.27492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pluznick JL. Gut microbiota in renal physiology: focus on short-chain fatty acids and their receptors. Kidney Int 2016; 90:1191–1198. doi: 10.1016/j.kint.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pluznick JL. Microbial short-chain fatty acids and blood pressure regulation. Curr Hypertens Rep 2017; 19:25.doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber GJ, Foster J, Pushpakumar SB, Sen U. Altered microRNA regulation of short chain fatty acid receptors in the hypertensive kidney is normalized with hydrogen sulfide supplementation. Pharmacol Res 2018; 134:157–165. doi: 10.1016/j.phrs.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pevsner-Fischer M, Blacher E, Tatirovsky E, Ben-Dov IZ, Elinav E. The gut microbiome and hypertension. Curr Opin Nephrol Hypertens 2017; 26:1–8. doi: 10.1097/MNH.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 50.Weber GJ, Pushpakumar SB, Sen U. Hydrogen sulfide alleviates hypertensive kidney dysfunction through an epigenetic mechanism. Am J Physiol Heart Circ Physiol 2017; 312:H874–H885. doi: 10.1152/ajpheart.00637.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015; 11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 52.Pingitore A, Chambers ES, Hill T, Maldonado IR, Liu B, Bewick G, et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab 2017; 19:257–265. doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]
- 53.Hara H, Haga S, Aoyama Y, Kiriyama S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J Nutr 1999; 129:942–948. doi: 10.1093/jn/129.5.942. [DOI] [PubMed] [Google Scholar]
- 54.Ali R, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 2013; 25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiaonan W, Zhaoyong H, Junping H, Jie D, William EM. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006; 147:4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 56.Kayo S, Tetsuo S, Masanori E, Hideki T, Hidenori K, Eiji I, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol 2002; 13:1894–1900. doi: 10.1097/01.ASN.0000019900.87535.43. [DOI] [PubMed] [Google Scholar]


