Supplemental Digital Content is Available in the Text.
Key Words: ketotifen, drug delivery, CAC
Abstract
Purpose:
A contact lens (CL)-based drug delivery system for therapeutic delivery of the antihistamine ketotifen was tested in 2 parallel, conjunctival allergen challenge-based trials.
Methods:
Both trials employed the same multicenter, randomized, placebo-controlled protocol. Test lenses were etafilcon A with 0.019 mg ketotifen; control lenses were etafilcon A with no added drug. Subjects were randomized into 3 treatment groups. Group 1 received test lens in one eye and control lens in the contralateral eye; the eye chosen to receive test lens was randomly selected in a 1:1 ratio. Group 2 received test lenses bilaterally, and group 3 received control lenses bilaterally. Allergen challenges were conducted on 2 separate visits: following lens insertion, the subjects were challenged at 15 minutes (to test onset) and 12 hours (to test duration). The primary endpoint was ocular itching measured using a 0 to 4 scale with half-unit steps. Secondary endpoints included ciliary, conjunctival, and episcleral hyperemia.
Results:
The mean itching scores were lower for eyes wearing the test lens as compared to those that received control lenses, indicating that the test lens effectively reduced allergic responses. Mean differences in itching were statistically and clinically significant (mean score difference ≥ 1) at both onset and duration for both trials.
Conclusions:
This large-scale assessment (n = 244) is the first demonstration of efficacy for CL delivery of a therapeutic for ocular allergy. Results are comparable to direct topical drug delivery and suggest that the lens/ketotifen combination can provide a means of simultaneous vision correction and treatment for CL wearers with ocular allergies.
Ocular allergy is a pervasive condition that affects up to 20% of the US population, with a similar prevalence worldwide.1–3 Allergic conjunctivitis (AC), the most common type of ocular allergy, is clinically defined as an IgE-mediated hypersensitivity response to exposure of the ocular surface to one or more allergens, including tree or grass pollens, pet dander, or dust mite dander.4 The impact of AC on overall health and quality of life is consistently underestimated,1–3 and although not typically sight-threatening, AC and related conditions underlie significant loss in patient productivity and quality of life.2,3,5,6
Patients who wear contact lenses (CLs) are particularly impacted by AC. For daily wear CL users, it creates a higher threshold for effective hygiene to minimize allergen accumulation on lenses.7 Furthermore, because the primary symptom of AC is itch, patients who naturally (and often, unconsciously) respond to ocular itch with eye-rubbing cause an exacerbation of their allergic symptoms and risk damage to both their ocular surface and their lenses.7,8 Often, patients who prefer CL use for refractive correction revert to spectacle use during allergy season to avoid these complications.
Currently available antiallergic medications are generally compatible with CL use but require that lenses be removed before topical drug application.8,9 This is in part because of concerns regarding interactions between lenses and drop preservatives and also because clinical studies of allergy drops have typically excluded CL users from study populations. Without data to establish compatibility, regulatory guidelines emphasize that drops should not be used while wearing lenses.
Signs and symptoms of AC include hyperemia, watery discharge, chemosis, and itch.4,8 Although all of these conditions are seen in other ocular disorders, ocular itching is often considered pathognomonic for conjunctivitis that is of allergic origin.8,9 The itch response is due to conjunctival mast cell release of histamine and other inflammatory mediators, and thus the therapeutic approaches to AC have focused on use of topical antihistamines such as olopatadine, bepotastine, or ketotifen.9 These second-generation antihistamines are characterized by a rapid, prolonged suppression of allergen-associated itch responses.
Ketotifen exhibits a unique spectrum of activity among the agents used to treat AC.9,10 Like many ocular antihistamines, ketotifen possess both antihistamine and mast-cell stabilizing properties; it is a high-affinity antagonist of H1 receptors9 which acts both as a competitive inhibitor of histamine and as an inhibitor of histamine release. In addition, ketotifen has been shown to attenuate accumulation of mast cells, neutrophils, and eosinophils at the sites of allergen deposition.10 This action prevents the initiation of more chronic allergic responses and contributes to the efficacy of ketotifen in the treatment of AC.
Topical treatments for ocular disease provide significant pharmacological advantages, particularly in terms of delivery of the therapeutic at the site of action and minimization of systemic exposure.8 Despite this, topicals are limited by a number of factors: tear film turnover is high (limiting exposure time), and accurate, reproducible instillation of drops is a challenge for many patients. Use of CLs as a vehicle for the delivery of therapeutic agents to the ocular surface may be a solution to these issues for lens users dealing with allergies11 or other conditions, including glaucoma,12 ocular infection,13 or ocular trauma.14
In this report, we describe the use of a CL-based delivery modality for ketotifen therapy of AC. The studies employed the conjunctival allergen challenge (CAC; Ora, Inc, Andover, MA) model to measure the lens/ketotifen combination efficacy.15,16 The CAC model provides a controlled alternative to traditional environmental allergy trials and allows for reproducible allergic responses that can be used as a reliable test of antiallergic efficacy. In this method, a reproducible allergic response to a quantified, instilled allergen is established in each subject. In subsequent visits, these challenges are repeated following instillation of test compounds such as antihistamines or steroids.15–17 By varying the time between treatment and challenge, both onset and duration of the therapeutic effects can be quantified. The model has been validated over many trials, and CAC-based studies are an established standard for FDA approval of ophthalmic antiallergics16 such as olopatadine, bepotastine, and alcaftadine.
METHODS
Data for this report were collected through 2 separate studies (ClinicalTrials.gov numbers NCT00445874 and NCT00432757 for study 1 and 2, respectively), both multicenter trials conducted using an identical protocol. Institutional review of the protocol, protocol amendments, and informed consent were by IntegReview, Inc (Austin, TX). The studies were conducted in compliance with Good Clinical Practices, including the International Conference on Harmonisation Guidelines, and with the 1996 version of the Declaration of Helsinki. Written informed consent (or assent and parental/guardian permission in subjects under 18) was obtained before any study procedures were conducted. All study-related procedures were conducted by clinically trained researchers who had prior experience in implementation of the CAC methodology.
Inclusion Criteria
For inclusion, all subjects were required to be at least 8 years of age at visit 1 and be able and willing to follow all study instructions and attend all study visits. Subjects under 18 were required to sign an assent form, have a parent or legal guardian sign the informed consent, and be accompanied by a parent/legal guardian for each visit. All subjects 18 and older were required to provide written informed consent and sign the Health Insurance Portability and Accountability Act (HIPAA) form prior to initiation of visit 1 procedures.
All subjects were current, regular soft contact lens wearers (≥6 h/d for at least 5 d/wk in a month prior to enrollment) who had been adequately fitted with lenses by an optometrist or ophthalmologist at visit 1. All had a best-corrected visual acuity (Snellen) of 20/30 or better in each eye at visit 1 with spherocylindrical refraction. All were required to have a correction from +6.00 to −12.00 diopters in each eye and astigmatism of −1.00 diopter or less in each eye.
In addition, the subjects were required to have a positive history of ocular allergies and a positive skin test reaction to at least one of the following allergens: cat hair, cat dander, grass, ragweed, or trees; for each subject, the allergen eliciting the greatest response on skin tests was used for the conjunctival challenge. The subjects were also required to exhibit a positive bilateral CAC reaction after allergen titration at screening visits 1 and 2 and a positive bilateral CAC reaction for at least 2 of 3 time points following the challenge at visit 3.
All study participants also needed to be able and willing to avoid all disallowed medications for the appropriate washout period before visit 1 and during the study; women of childbearing age were required to have a negative urine pregnancy test at enrollment and at study exit, and agreed to use a medically approved form of birth control for the duration of the study.
Exclusion Criteria
Subjects were excluded from the study if they had any known contraindications to any study materials, if they had any active ocular infection, or if they had a history of any other ocular condition that, in the investigators' opinion, could impact the subject's health or the study parameters. This included blepharitis, pterygium, narrow angle glaucoma, dry eye, or a history of herpetic ocular infection. Subjects with a history of corneal surgery or those who had any ocular surgery in the previous 6 months were excluded, as were those with baseline corneal staining ≥3 (on a 0–4 scale) at visit 1 or baseline ocular redness scores ≥2 (on a 0–4 scale) at any visit. Use of disallowed medications during the washout periods or over the duration of the study was also cause for exclusion, as was pregnancy or a positive pregnancy urine test in the course of the study.
Study Chronology
Each of the 2 studies consisted of 5 visits, each separated by 1 to 2 weeks, over a 6-week period. The visits were scheduled to fall within a 6-day window (±3 days):
Visit 1 (day –28): Subject screening, enrollment, informed consent/HIPAA, allergen titration, and etafilcon A CL fitting. A total of 120 to 125 subjects were enrolled for each study.
Visit 2 (day –21): Repeat (confirm) allergen challenge with subjects wearing etafilcon A CL.
Visit 3 (day –14): Repeat allergen challenge with subjects wearing etafilcon A CL.
Visit 4 (day 0): The subjects were randomized into 3 treatment groups: 1) test lens in one eye, placebo lens in the other eye, 2) test lenses in both eyes, and 3) placebo lenses in both eyes. There were approximately 40 subjects in each group. After 12 hours, the subjects underwent allergen challenge, and scores for ocular itching and redness were collected.
Visit 5 (day +14): The subjects received the same randomized treatment assignment as at visit 4 and underwent allergen challenge 15 minutes after lens insertion. Scores for ocular itching and redness were collected as at visit 4.
Ocular itch was scored by the subjects at 3, 5, and 7 minutes after CAC at visits 4 and 5; scoring employed a 0 to 4 scale (using 0.5 increments) where 0 = no itch and 4 = an incapacitating itch with an irresistible urge to rub. Secondary measurements were scored by the investigator at 7, 15, and 20 minutes after CAC; these included ciliary, conjunctival, and episcleral redness using a 0 to 4 scale (using 0.5 increments) where 0 = none and 4 = severe.
Safety assessments conducted at all study visits include slit-lamp biomicroscopy (with corneal fluorescein staining), visual acuity, undilated fundoscopy, and cataloging of any adverse events (AEs).
Statistical Methods
For the efficacy analyses, the unit for comparison between the test and control lens treatments was the eye. The primary efficacy endpoint, ocular itching, was analyzed using a 2 sample t test for both visit 4 and for visit 5. Demographic characteristics were compared using analysis of variance for continuous measures and χ2 tests for categorical measures. Data from the 2 studies are presented as 2 independent measures of the same treatment protocol.
RESULTS
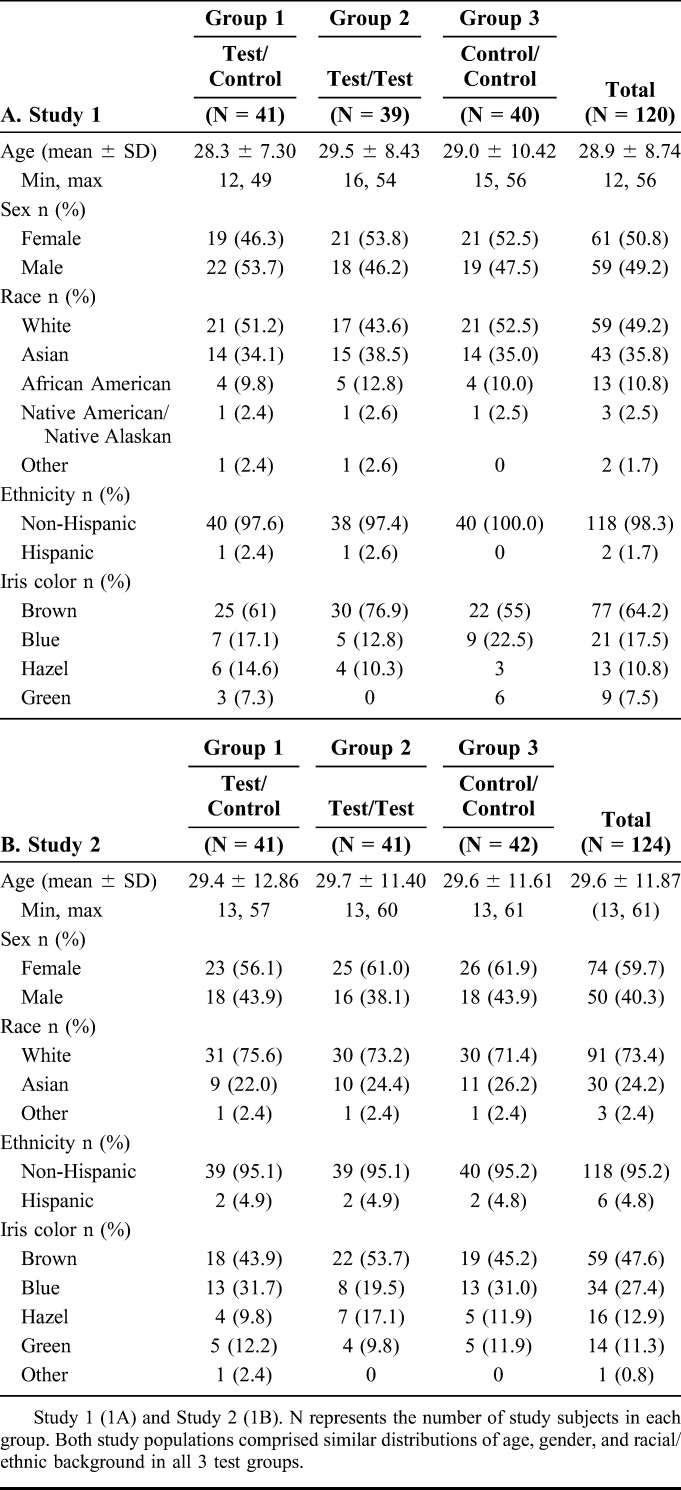
In the 2 studies, a total of 244 subjects were enrolled in the intent-to treat populations. A summary of the demographic characteristics of each population is provided in Table 1, A and B. In both studies, comparison of factors including gender, race, ethnicity, and iris color established that none of the groups showed significantly demographic differences. Subject ages ranged from 12 to 61 years of age, with a mean age of 29.3. Overall, the subject population was 61.5% white (150/244), 29.9% Asian (73/244), and 55.3% female (135/244).
TABLE 1.
Demographics of Intent-to Treat Population
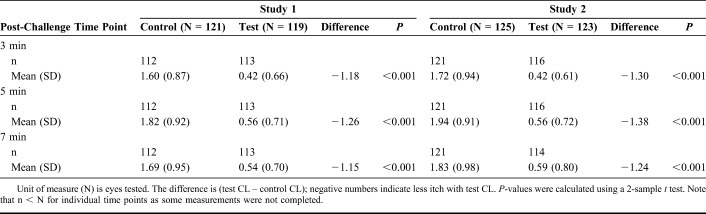
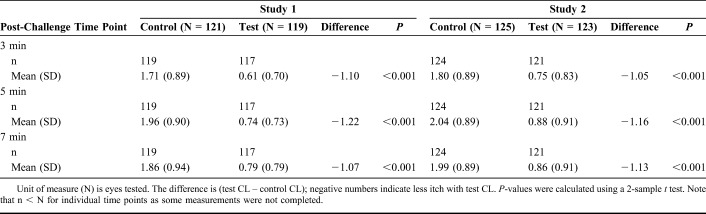
Primary efficacy measures focused on the onset of ketotifen action (15 minutes after lenses are inserted; Table 2) and the duration of action at 12 hours after lens insertion (Table 3). These data are depicted graphically in Figure 1. In both cases, the mean itch scores for eyes with ketotifen-containing lenses were significantly lower (P < 0.001 for all measures) at all time points in both studies. In the CAC, clinically significant changes in itch scores are designated as those where the difference between test and control is greater than or equal to 1; this threshold was met at all time points in both studies.
TABLE 2.
Itch Scores in Response to CAC After 15 Minutes of CL Wear
TABLE 3.
Itch Scores in Response to CAC After 12 Hours of CL Wear
FIGURE 1.
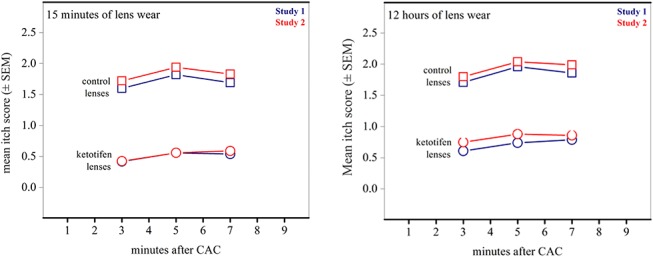
Itch scores following allergen challenge. Mean itch scores at onset of lens wear (15 minutes) and after prolonged lens use (12 hours) both show decreases of > 1 unit compared to control lens scores. SEM values range between 0.067 and 0.92 for all points (within markers).
Ciliary, conjunctival, and episcleral redness were secondary endpoints in both studies (see Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/ICO/A765). For each of these endpoints, at least 2 of 3 mean scores for eyes with test lenses reached statistical significance compared to control lenses (P < 0.05). However, none of the differences between eyes with test and control CLs in these redness scores reached the threshold for clinical significance (mean difference between scores ≥ 0.5 and at least 1 measure ≥ 1.0).
Between the 2 studies, there were 24 ocular AEs in a total of 488 subject eyes (4.9%). The majority of these were mild in severity and not considered study related (see Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/ICO/A765). Two ocular AEs in study 2 were judged severe; these involved an increased lacrimation (reported by one subject in both eyes). In addition, one subject in study 2 opted out of the study due to a pregnancy.
Visual acuity assessments were similar for control- and test-CL groups over the course of both studies. Small changes, within normal variation, were reported in the CL-corrected and best-corrected visual acuity of subject eyes in both studies; this observation indicated that the addition of ketotifen to CLs had no adverse effects on visual acuity (see Supplemental Table 3, Supplemental Digital Content 1, http://links.lww.com/ICO/A765).
DISCUSSION
The goal of these clinical trials was to test whether addition of an antiallergic to a soft CL could be used as an approach to treat AC in CL wearers who also suffer from ocular allergies. Over the last decade, there has been considerable interest in extending the function of the CL beyond that of vision correction alone.12 Efforts to utilize CLs as vehicles to deliver therapeutics has been suggested as an approach to address limitations in both efficacy and compliance of current therapies, and research into this approach is ongoing.12–14
CL drug delivery provides several advantages over direct topical ophthalmic application. Combining vision correction and therapeutic treatment for allergy increases compliance for both conditions by simplifying overall management. Delivery of therapeutics with CLs takes advantage of the aqueous layer created between the lens and the cornea, where a mixture of tears and the instilled therapeutic is compartmentalized and partially shielded from the tear film turnover effects of the blinking reflex.12 Drugs released into or otherwise confined within this layer may have a longer precorneal residence time.12 The long duration of ketotifen action in these studies is consistent with a prolonged residence time of drug on the ocular surface. Thus, by combining a CL and a drug such as ketotifen, allergy sufferers who wear lenses can receive reliable daily allergy relief that lasts for as long as they typically wear the CLs, while avoiding potential adverse effects of preservatives commonly found in topical ocular preparations.
In these 2 clinical studies, addition of ketotifen to CLs achieved a clinically and statistically significant reduction in mean ocular itching scores, both at 15 minutes and 12 hours after lens insertion. There is no evidence from either study that the incorporation of the drug into the lens solution has any structural, optical, or refractive effect on the lenses. The 12-hour time point equals the longest duration of clinical efficacy demonstrated by ketotifen in a CAC-based study17,18; this suggests that the CL-based delivery does not interfere with the established prolonged duration of ketotifen action.18 At the same time, the lenses provided effective refractive error correction. Overall, the combination lens was well tolerated. Collectively, these results support the use of lenses with ketotifen for the prevention of ocular itching associated with AC in patients who use CLs for vision correction.
Supplementary Material
Footnotes
These studies funded by Johnson & Johnson Vision.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
REFERENCES
- 1.Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2011;11:471–476. [DOI] [PubMed] [Google Scholar]
- 2.Blaiss MS. Allergic rhino-conjunctivitis: burden of disease. Allergy Asthma Proc. 2007;28:393–397. [DOI] [PubMed] [Google Scholar]
- 3.Gomes PJ. Trends in prevalence and treatment of ocular allergy. Curr Opin Allergy Clin Immunol. 2014;14:451–456. [DOI] [PubMed] [Google Scholar]
- 4.Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am. 2008;28:43–58. [DOI] [PubMed] [Google Scholar]
- 5.Smith AF, Pitt AD, Rodruiguez AE, et al. The economic and quality of life impact of seasonal allergic conjunctivitis in a Spanish setting. Ophthalmic Epidemiol. 2005;12:233–242. [DOI] [PubMed] [Google Scholar]
- 6.Pitt AD, Smith AF, Lindsell L, et al. Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol. 2004;11:17–33. [DOI] [PubMed] [Google Scholar]
- 7.Butrus SI, Abelson MB. Contact lenses and the allergic patient. Int Ophthalmol Clin. 1986;26:73–81. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman S, Smith LM, Gomes PJ. Ocular itch associated with allergic conjunctivitis: latest evidence and clinical management. Ther Adv Chronic Dis. 2016;7:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abelson MB, McLaughlin JT, Gomes PJ. Antihistamines in ocular allergy: are they all created equal? Curr Allergy Asthma Rep. 2011;11:205–211. [DOI] [PubMed] [Google Scholar]
- 10.Grant SM, Goa KL, Fitton A, et al. Ketotifen. A review of its pharmacodynamics and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs. 1990;40:412–448. [DOI] [PubMed] [Google Scholar]
- 11.Soluri A, Hui A, Jones L. Delivery of ketotifen fumarate by commercial contact lens materials. Optom Vis Sci. 2012;89:1140–1149. [DOI] [PubMed] [Google Scholar]
- 12.Jones LW, Byrne M, Ciolino JB, et al. Revolutionary future uses of contact lenses. Optom Vis Sci. 2016;93:325–327. [DOI] [PubMed] [Google Scholar]
- 13.Phan CM, Weber S, Mueller J, et al. A rapid extraction method to quantify drug uptake in contact lenses. Transl Vis Sci Technol. 2018;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz CL, Morck DW. Contact lenses as a drug delivery device for epidermal growth factor in the treatment of ocular wounds. Clin Exp Optom. 2010;93:61–65. [DOI] [PubMed] [Google Scholar]
- 15.Abelson MB, Loeffler O. Conjunctival allergen challenge: models in the investigation of ocular allergy. Curr Allergy Asthma Rep. 2003;3:363–368. [DOI] [PubMed] [Google Scholar]
- 16.Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge. A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol. 1990;108:84–88. [DOI] [PubMed] [Google Scholar]
- 17.Greiner JV, Mundorf T, Dubiner H, et al. Efficacy and safety of ketotifen fumarate 0.025% in the conjunctival antigen challenge model of ocular allergic conjunctivitis. Am J Ophthalmol. 2003;136:1097–1105. [DOI] [PubMed] [Google Scholar]
- 18.Berdy GJ, Spangler DL, Bensch G, et al. A comparison of the relative efficacy and clinical performance of olopatadine hydrochloride 0.1% ophthalmic solution and ketotifen fumarate 0.025% ophthalmic solution in the conjunctival antigen challenge model. Clin Ther. 2000;22:826–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.