Abstract
Background.
Type 2 diabetes mellitus (T2DM) is prevalent in patients undergoing heart transplant, and in those without preexisting T2DM, posttransplant diabetes mellitus may develop. Both T2DM and posttransplant diabetes mellitus have been associated with increased morbidity and mortality following heart transplantation. Empagliflozin is an effective glucose-lowering therapy that reduces the incidence of major cardiovascular events in patients with T2DM. The safety and efficacy of empagliflozin in transplant patients with diabetes mellitus has yet to be established.
Methods.
Clinical outcomes were retrospectively examined in 22 heart transplant recipients treated with empagliflozin and compared with those of 79 heart transplant patients with diabetes mellitus receiving alternative glucose-lowering therapies.
Results.
Three adverse events were recorded in empagliflozin-treated patients, leading to treatment discontinuation in 1. There were no genitourinary infections. Treatment with empagliflozin for 12 months was associated with reductions in weight, body mass index, glycated hemoglobin, and frusemide dose that were not seen in the control group. There were no large changes observed in blood pressure (systolic or diastolic) or renal function (serum urea, creatinine, or estimated glomerular filtration rate) after 12 months of treatment with empagliflozin or alternative glucose-lowering therapies.
Conclusions.
Empagliflozin appears safe and effective in the management of selected patients with diabetes mellitus following heart transplantation.
Type 2 diabetes mellitus (T2DM) is prevalent in patients undergoing cardiac transplantation. In those without diabetes mellitus before transplant, many will develop abnormalities of glucose metabolism in the posttransplant period. Both preexisting T2DM and posttransplant diabetes mellitus (PTDM) are prognostically significant following heart transplant and are associated with increased morbidity and mortality compared with other transplant recipients with normal glucose metabolism.1 In the modern era, survival following heart transplantation has improved, primarily because of improvements in immunosuppressive and anti-infective medication availability and tolerability.2 However, the diabetogenic effects of these agents have contributed to increased rates of PTDM, and the optimal management of diabetes mellitus in the posttransplant period remains ill defined, particularly with regard to the use of newer oral glucose-lowering agents such as the sodium glucose cotransporter 2 (SGLT2) inhibitors.3
Empagliflozin is one such SGLT2 inhibitor. It exerts a glucose-lowering effect via induction of glycosuria through inhibition of SGLT2 channels in the proximal renal tubule.4 In a large randomized trial of patients with T2DM and established cardiovascular disease and/or risk factors, empagliflozin was associated with significant reductions in major adverse cardiovascular events, hospitalizations for heart failure, and all-cause mortality.5 Additional studies have reported further benefits with the use of empagliflozin, including reduction in glycated hemoglobin (HbA1c) levels, weight, blood pressure, arterial stiffness and vascular resistance, albuminuria, visceral adiposity, and plasma urate levels.6-8 The primary aim of this study was to investigate the safety of empagliflozin in the postheart transplant diabetic population with a particular focus on the incidence of genitourinary infections in an immunosuppressed patient population. Secondary aims of this study were to describe long-term effectiveness of empagliflozin in the postheart transplant diabetic population with regard to HbA1c, weight, body mass index (BMI), blood pressure, kidney function, and diuretic usage.
MATERIALS AND METHODS
Study Population
One hundred and one consecutive heart transplant recipients with either T2DM predating transplant or PTDM were reviewed in the heart transplant follow-up clinic between January 1, 2015, and August 14, 2017. Results of outcomes after at least 3 months of empagliflozin treatment have been reported previously by our group.9
Study Design
We conducted a retrospective single-center observational study of patients attending the heart transplant clinic at a single center. It is routine for heart transplant recipients to attend clinic at least biannually, with more frequent visits for patients in the first 2 years posttransplantation or among those with transplant-related complications. Before transplantation, patients were not routinely screened for diabetes mellitus. However, patients undergo multiple random plasma glucose samples before transplantation, which would identify most cases of impaired glucose tolerance and overt T2DM. Following transplantation, blood glucose levels were monitored 4 times daily in hospital, and if they remained elevated at the time of discharge, patients were taught to self-monitor glucose levels and followed up in a dedicated transplant-endocrine clinic. Eligible participants were selected using clinic attendance records to confirm heart transplant and diabetes mellitus status. T2DM was defined in accordance with the American Diabetes Society consensus guidelines,10 and PTDM was defined by the diagnostic criteria outlined in the most recent international guidelines.11 Only patients with follow-up after a minimum period of 12 months of empagliflozin therapy or other diabetes mellitus treatment were included in this analysis. The decision to commence empagliflozin was made by the treating endocrinologist or cardiologist. Treatment was altered throughout the study period as clinically indicated. The study protocol was approved by the human research ethics committee at St. Vincent’s Hospital, Sydney. The reference number for this study is LNR/16/SVH/215.
Data Collection
Data were collected through review of hospital records, specialist letters, and internal/external pathology results. Demographic data (age, sex, comorbidities, diabetes mellitus status, diabetes mellitus duration, and transplant date) were confirmed via medical record. Adverse effects that could be reasonably attributed to empagliflozin were documented.
Outcome Measures
The primary outcome was the prevalence of adverse effects attributable to empagliflozin, with genitourinary infection as the key variable of interest. Genitourinary and perineal infections were detected clinically in patients presenting with symptoms and signs and confirmed microbiologically. Routine screening for asymptomatic genitourinary infection was not performed. The secondary outcomes compared the effect of treatment with empagliflozin at 12 months with that of non-empagliflozin-based diabetes mellitus management on body weight, BMI, blood pressure, HbA1c, diuretic usage (furosemide dose), and renal function.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median ± interquartile range. Categorical data were expressed as frequencies and percentages of the overall cohort. Wilcoxon signed-rank tests were used to compare changes in body weight, BMI, systolic and diastolic blood pressures, HbA1c, insulin dose, furosemide dose, urea, serum creatinine, and estimated glomerular filtration rate (eGFR) before empagliflozin, with subsequent measurements occurring at least 12 months following drug initiation. This procedure was repeated for patients receiving any other form of diabetes mellitus treatment without empagliflozin. All analyses were performed using SPSS software version 24.0 (SPSS Inc., Chicago, IL). All reported values are 2-sided with statistical significance defined as P < 0.05.
RESULTS
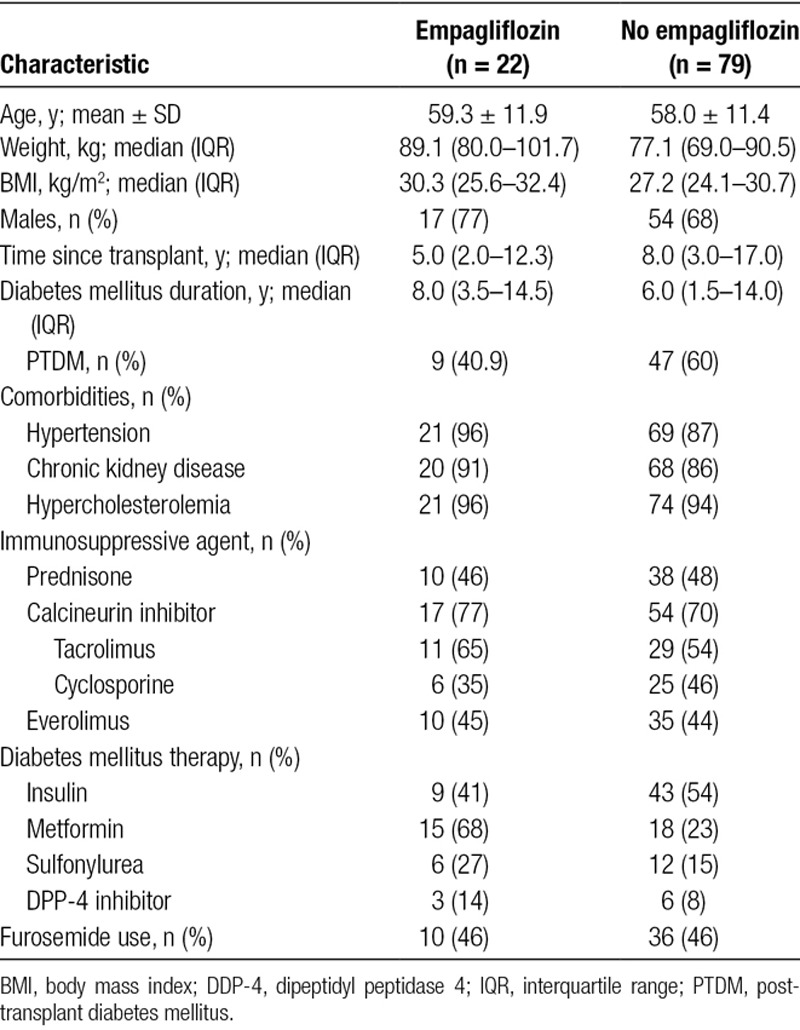
Throughout the study period, 337 heart transplant recipients attended clinic for routine follow-up. The mean age was 56.3 ± 14.4 years, with an average time posttransplant of 10.1 years. The cohort was predominantly male (71%), and T2DM or PTDM was present in 101 patients (30%), with an average diabetes mellitus duration of 8.2 years. There were 22 patients treated with empagliflozin as a component of their diabetes mellitus therapy. Empagliflozin use was not randomized, and dose was determined by the prescribing physician, with 5 patients commencing treatment before transplantation. In the empagliflozin cohort, 12 patients received 10 mg daily, and 10 received 25 mg daily. Pretreatment and posttreatment data, recorded 12 months after beginning empagliflozin therapy, were available for 20 of 22 patients. Twelve-month follow-up data were available for 77 of 79 patients treated with non-empagliflozin-based glucose-lowering treatments. Baseline characteristics are presented in Table 1. Groups were similar at baseline apart from metformin use, with 68% (15/22) of the empagliflozin group coprescribed metformin compared with 23% (18/79) in the control group.
TABLE 1.
Baseline characteristics
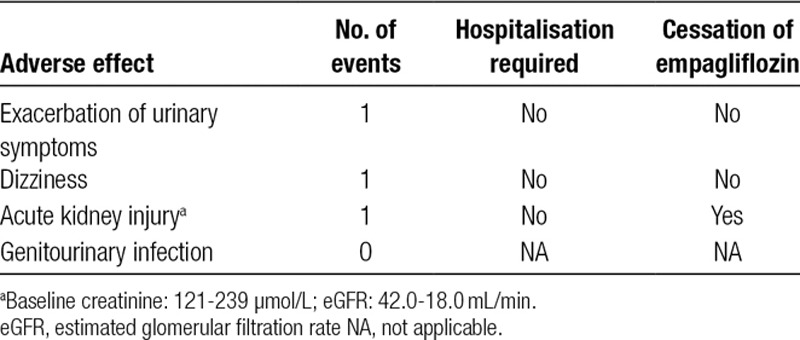
Across 384 collective months of empagliflozin treatment in 22 patients, there were 3 adverse effects potentially attributable to treatment with empagliflozin. Adverse effects are noted in Table 2. Interestingly, there were no genitourinary tract infections documented in the empagliflozin-treated group compared with 9 urinary infections in heart transplant recipients treated using non-empagliflozin-based diabetes mellitus medications.
TABLE 2.
Adverse effects recorded in patients treated with empagliflozin over 12 mo
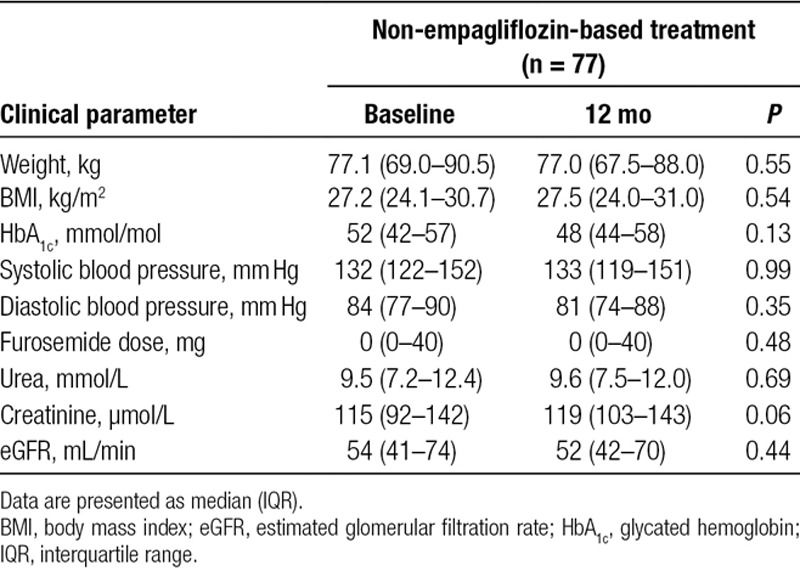
Following 12 months of treatment with empagliflozin, there was a significant reduction in median body weight of 2.0 kg (P = 0.003; mean reduction 4.7 ± 5.7), and a corresponding median decrease in BMI of 1.3 kg/m2 (P = 0.004; mean reduction 1.6 ± 2.0). Weight reduction was concurrent with a significant decrease in median furosemide dose (P = 0.02). In contrast, the control group did not experience significant reductions in weight, BMI, or furosemide dosage. Systolic and diastolic blood pressures were not significantly different following 12 months of empagliflozin treatment, which was also the case in participants treated with non-empagliflozin-based therapies. Glycemic control improved following 12 months of treatment with empagliflozin, with a mean reduction in HbA1c of 6.6 mmol/mol. Although statistically nonsignificant, glycemic control in non-empagliflozin-treated subjects deteriorated at 12 months, with an average HbA1c increase of 3.7 mmol/mol, yielding a difference of 10.3 mmol/mol. Both empagliflozin and control groups experienced nonsignificant changes in renal function based on biochemical parameters. Pre- and postresults are presented in Table 3 for patients treated with empagliflozin and in Table 4 for patients treated with non-empagliflozin-based diabetes mellitus medications.
TABLE 3.
Metabolic and hemodynamic outcomes in heart transplant recipients with diabetes mellitus before and after 12 mo of treatment with empagliflozin
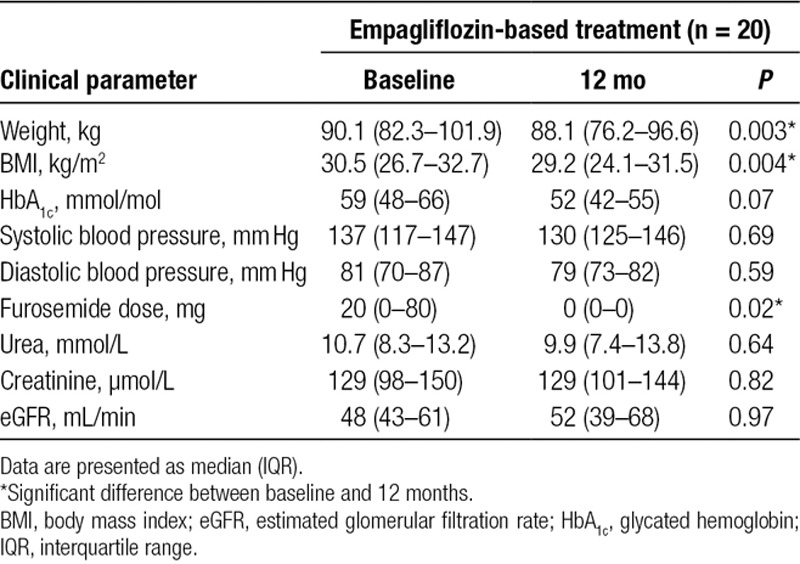
TABLE 4.
Metabolic and hemodynamic outcomes in heart transplant recipients with diabetes mellitus before and after 12 mo of treatment with non-empagliflozin-based therapies
DISCUSSION
Diabetes mellitus remains a significant issue in the management of patients following cardiac transplantation. As long-term survival continues to improve, so too will the incidence of PTDM. The role of novel antihyperglycemic agents, such as SGLT2 inhibitors, remains to be established. Given their beneficial effects in the nontransplant T2DM population, they may have potential to improve outcomes in heart transplant recipients with diabetes mellitus as well. The primary outcome of this study was to examine the safety of empagliflozin in heart transplant recipients with T2DM and/or PTDM. Overall, empagliflozin was well tolerated, with only 3 adverse effects potentially attributable to treatment. Following 384 cumulative months of empagliflozin therapy, only 1 patient experienced an adverse event, which required permanent discontinuation of the drug.
Genitourinary and perineal infections are recognized potential adverse effects of treatment with SGLT2 inhibitors. Given the immunosuppressed profile of the posttransplant patient, there was theoretically increased risk of genitourinary infection with empagliflozin use in this setting. However, across 384 cumulative months of treatment, no recorded cases of genitourinary infection were documented among the 22 patients treated with empagliflozin. We acknowledge that antimicrobial prophylaxis is a fundamental component of posttransplant care, but the choice of antibiotics is not directed at preventing genitourinary infection, so it is unlikely that anti-infective prophylaxis mitigated the risk of genitourinary infection in heart transplant recipients treated with empagliflozin relative to those treated without.
There have been multiple reports of euglycemic diabetic ketoacidosis (DKA) occurring in patients receiving SGLT2 inhibitor therapy.12 Although rare, ketoacidosis is a life-threatening emergency, and it is reassuring that no instances were observed in our treated patients. Screening, as in the nontransplant setting, is not routinely performed and requires vigilance on the part of treating physicians to consider ketonemia in patients with unexplained metabolic acidosis and raised anion gap. Ketoacidosis is usually clinically apparent, and given the posttransplant patient is closely monitored, we feel it is unlikely that cases were missed. However, as screening was not routinely performed, it is possible that mild cases may have been missed, and larger studies are required to establish if DKA risk is higher in transplant patients compared with non-transplant patients with T2DM, where euglycemic DKA is very rare.
Posttransplantation treatment regimens are complex and intricately balanced, as patients require a range of immunosuppressive and anti-infective therapies in addition to the management of comorbidities frequently seen in this population, such as hypertension and hypercholesterolemia. Although there is presently minimal data available regarding the use of SGLT2 inhibitors in transplant patients, a pharmacokinetic study did not demonstrate any meaningful interaction between canagliflozin and cyclosporine.13 In our cohort, there were no apparent drug-drug interactions involving empagliflozin, although this was not specifically assessed. Metabolism of empagliflozin is via simple hepatic glucuronidation and would not be expected to interfere with P450 or P-glycoprotein pathways via which calcineurin inhibitors and mTOR agents are metabolized. From a safety perspective, the results of this study suggest that empagliflozin is suitable for use in the postcardiac transplant setting. Nonetheless, in patients with a history of genitourinary infections or preexisting lower urinary conditions, we would advise caution in the use of SGLT2 inhibitors.
The secondary outcome of this study was to examine efficacy of empagliflozin therapy in heart transplant recipients with diabetes mellitus. In our studied patient population, empagliflozin resulted in significant reductions in body weight and BMI that were not observed in patients treated using glucose-lowering therapies that did not include empagliflozin. Trial data from nontransplant T2DM patients treated with empagliflozin have demonstrated significant reductions in blood pressure by week 16,5 which was maintained throughout the study period. In our cohort, empagliflozin did not appreciably change systolic or diastolic blood pressures after 12 months of treatment. This may be an effect of the small sample size of our cohort, although interestingly, patients treated with empagliflozin were more likely to have ceased or to be on a reduced dose of furosemide after 12 months of treatment compared with patients not treated with empagliflozin, where no significant changes in blood pressure or diuretic use were observed.
We observed a mean reduction in HbA1c of 6.6 mmol/mol in the empagliflozin group. Although not statistically significant, it is clinically relevant given that patients treated with other non-empagliflozin-based glucose-lowering treatments on average experienced a mean increase in HbA1c of 3.7 mmol/mol despite similar degrees of follow-up and endocrinology involvement. These data suggest empagliflozin was efficacious for improving glycemic control in heart transplant patients with diabetes mellitus relative to treatment using non-empagliflozin-based diabetes mellitus therapies and is consistent with the published data in this area.5,8
The prevalence of chronic kidney disease in the posttransplant setting has been reported as 25%, 51%, and 68% at 1, 5, and 10 years, respectively, among surviving recipients.14 This is largely due to the modern immunosuppressive regimens and widespread use of nephrotoxic calcineurin inhibitors.15 In our study, renal function in both empagliflozin and control groups remained stable over the 12-month observation period. This is in keeping with nontransplant data from a T2DM population, where empagliflozin in addition to standard care resulted in slower progression of kidney disease and significantly lowered the risk of multiple other clinically relevant renal outcomes when compared with placebo.16 A longer duration of observation will be needed to determine whether the SGLT2 inhibitors are also renoprotective in patients with diabetes mellitus following heart transplantation.
Strengths of our study are the long-term follow-up (12 months) of transplant patients treated with a new glucose-lowering agent (empagliflozin). This builds on our previous published data of empagliflozin use in heart transplant recipients and improves confidence that use is safe and may impart significant metabolic benefits in a posttransplant population. The most significant limitations of this study relate to its retrospective and nonrandomized design. The decision to commence treatment with empagliflozin was made by the treating endocrinologist or cardiologist. Heart transplant recipients treated with empagliflozin were also more likely to be treated with metformin. The possibility that benefits in terms of weight loss and glycemic control may have been related to metformin rather than empagliflozin (or the combination of the 2) cannot be excluded. Our observations would ideally be confirmed in a prospective randomized trial.
CONCLUSIONS
In conclusion, this study has demonstrated that empagliflozin can be safely used as a long-term option for the management of selected patients with diabetes mellitus following heart transplantation. On the basis of our cohort study results, we suggest that heart transplantation should not act as an absolute contraindication to SGLT2 inhibitor therapy.
Footnotes
Published online 25 April, 2019.
The authors declare no funding or conflicts of interest.
M.C.C. participated in research design, data collection, and data analysis and prepared the first draft of the manuscript. C.A.M. participated in research design, data collection, and data analysis; edited the first draft; and prepared all subsequent revisions of the manuscript. J.R.G. participated in research design, data analysis, and editing of manuscript. C.H., A.J., and E.K. participated in research analysis and editing of the manuscript. P.S.M. supervised the study, participated in research design, data analysis, and editing of the manuscript and all subsequent revisions.
REFERENCES
- 1.Galindo RJ, Wallia A. Hyperglycemia and diabetes mellitus following organ transplantation. Curr Diab Rep. 2016;16:14. [DOI] [PubMed] [Google Scholar]
- 2.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252. [DOI] [PubMed] [Google Scholar]
- 3.Montori VM, Basu A, Erwin PJ, et al. Posttransplantation diabetes. Diabetes Care. 2002;25:583. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh RK, Ghosh SM, Chawla S, et al. SGLT2 inhibitors: a new emerging therapeutic class in the treatment of type 2 diabetes mellitus. J Clin Pharmacol. 2012;52:457. [DOI] [PubMed] [Google Scholar]
- 5.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117. [DOI] [PubMed] [Google Scholar]
- 6.Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369. [DOI] [PubMed] [Google Scholar]
- 7.Chilton R, Tikkanen I, Cannon C, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diab Obes Metab. 2015;17:1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridderstråle M, Andersen KR, Zeller C, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691. [DOI] [PubMed] [Google Scholar]
- 9.Muir CA, Greenfield JR, MacDonald PS. Empagliflozin in the management of diabetes mellitus after cardiac transplantation. J Heart Lung Transplant. 2017;36:914. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl 1):S8. [DOI] [PubMed] [Google Scholar]
- 11.Sharif A, Hecking M, De Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14:1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacs M, Tonks KT, Greenfield JR. Euglycaemic diabetic ketoacidosis in patients using sodium-glucose co-transporter 2 inhibitors. Intern Med J. 2017;47:701. [DOI] [PubMed] [Google Scholar]
- 13.Wallia A, Illuri V, Molitch ME. Diabetes care after transplant: definitions, risk factors, and clinical management. Med Clin North Am. 2016;100:535. [DOI] [PubMed] [Google Scholar]
- 14.Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017;Focus Theme: Allograft ischaemic time. J Heart Lung Transplant. 2017;1037:1046 [DOI] [PubMed] [Google Scholar]
- 15.Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant. 2008;22:1. [DOI] [PubMed] [Google Scholar]
- 16.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323. [DOI] [PubMed] [Google Scholar]


