Abstract
Background.
Corneal neovascularization is considered an important risk factor for allograft rejection after corneal transplantation (keratoplasty). Therefore, the aim of this study was to determine whether preoperative reduction of corneal neovascularization by fine-needle thermal cauterization combined with bevacizumab reduces the incidence of allograft rejection after subsequent high-risk keratoplasty.
Methods.
In this interventional uncontrolled clinical pilot study, 31 eyes of 31 patients with corneal neovascularization in at least one corneal quadrant were included. All eyes were treated by fine-needle thermal cauterization of corneal vessels and subconjunctival injection of bevacizumab. Both treatments were repeated in the cases of visible reperfusion of occluded vessels. Afterward, penetrating keratoplasty was performed. When corneal neovascularization was present on the day of keratoplasty, additional vessel cauterization and injection of bevacizumab was performed. Patients were then followed to determine the incidence of allograft rejection.
Results.
In 18 eyes, vessel cauterization with bevacizumab injection was performed once before keratoplasty, whereas 13 eyes required retreatment before keratoplasty. No complications were observed. In 23 eyes, corneal neovascularization was present on the day of keratoplasty due to reperfusion of previously occluded vessels and simultaneous vessel cauterization with bevacizumab injection was performed. During follow-up (mean: 560 days; range: 59–1095 days), 4 graft rejection episodes in 4 eyes were observed. Estimated probabilities of corneal graft survival were 92.9% after 1 year (number at risk: 23), 78.4% after 2 years (number at risk: 9), and 78.4% after 3 years (number at risk: 3).
Conclusions.
Our initial results indicate that angioregressive treatment of pathological corneal vessels by fine-needle thermal cauterization combined with subconjunctival injection of bevacizumab before high-risk keratoplasty seems to result in graft survival rates comparable to survival rates seen in normal-risk keratoplasty. The findings of our pilot study warrant further controlled clinical trials with longer follow-up in a larger patient cohort.
The cornea is the transparent and avascular front part of the eye. Corneal avascularity is essential for good vision and therefore maintained by several antiangiogenic mechanisms, which are able to buffer minor angiogenic stimuli.1-3 However, in response to severe inflammation, these mechanisms might be overwhelmed, and the cornea can secondarily be invaded by pathological corneal neovessels (corneal neovascularization). Corneal neovascularization reduces corneal transparency when vessels are growing into the optical center and is also considered an important risk factor for allograft rejection after penetrating corneal transplantation (keratoplasty).4 Whereas grafts placed into nonvascularized recipients have a 5-year survival rate of >80%,5,6 the risk of graft rejection is more than doubled when corneal grafting is performed in prevascularized corneas.7-9 Thus, antiangiogenic therapy seems to be feasible, and animal studies have shown that this is a valid approach to improve high-risk corneal graft survival.10-14 Also in the clinical setting, several strategies have been established to reduce corneal neovascularization before surgery, including laser or fine-needle thermal cauterization of pathological corneal neovessels, application of immunomodulatory or anti-vascular endothelial growth factor agents, and others.15-21 However, it is so far unknown whether these strategies are indeed effective in reducing the risk of corneal allograft rejection in the clinical setting. Thus, the aim of this pilot study was to determine the impact of angioregressive treatment of pathological corneal vessels by fine-needle thermal cauterization combined with subconjunctival injection of the vascular endothelial growth factor-blocking antibody bevacizumab on the incidence of allograft rejection after subsequent high-risk keratoplasty.
MATERIALS AND METHODS
In this interventional uncontrolled single-center study, 31 eyes of 31 patients (17 female, 14 male) with corneal neovascularization in at least one corneal quadrant were enrolled. Neovascularization was graded from slit-lamp pictures in a standardized fashion.15 Before keratoplasty, fine-needle thermal cauterization of corneal vessels and subconjunctival injection of bevacizumab (2.5 mg in 0.1 mL) was performed as described previously.19,20 Bevacizumab was injected into the subconjunctival space that was closest to the area(s) with corneal neovascularization. In the cases of reperfusion of the occluded vessels, both treatments were repeated (after 4 wk at the earliest). Afterward, penetrating keratoplasty was performed. When neovascularization was still present on the day of keratoplasty due to reperfusion of previously occluded vessels, additional vessel cauterization and bevacizumab injection was performed. Twenty-five eyes received a non–HLA-matched graft and 6 eyes a HLA-matched graft. Postoperatively, patients were treated with topical prednisolone acetate 1% (5× daily), which was tapered monthly (5× daily for 1 mo, 4× daily for 1 mo, 3× daily for 1 mo, 2× daily for 1 mo, 1× daily for 1 mo), and kept at one drop per day indefinitely, antibiotics (Ofloxacine, 3× daily for 2 wk), and lubricants (5× daily indefinitely). Afterward, patients were followed regularly (at 1, 3, 6, 12, 18, 24, and 36 months after keratoplasty and at any additional visit). The diagnosis of endothelial graft rejection was based on characteristic signs on slit-lamp biomicroscopy, including hyperemia, anterior chamber reaction, keratic precipitates confined to the corneal graft, and graft edema.6 Distribution of time-to-event data was described by the Kaplan-Meier method using SPSS Statistics 25 (IBM Corp., Armonk, NY).
The study was performed in conformance with the tenets of the Declaration of Helsinki and adheres to all German federal and state laws. Written informed consent was obtained from all patients before surgery.
RESULTS
Of the 31 eyes enrolled in this study, 7 had preexisting vessels in 1 corneal quadrant, 5 in 2 quadrants, 5 in 3 quadrants, and 14 in 4 quadrants. Underlying causes for corneal opacification and neovascularization were herpetic keratitis (n = 15), bacterial keratitis (n = 6), chemical burn (n = 4), perforating ocular trauma (n = 4), and acanthamoeba keratitis (n = 2). Twelve eyes had a history of a previous graft (penetrating keratoplasty). In 18 eyes, vessel cauterization with bevacizumab injection was performed once before keratoplasty (Figure 1A), whereas 13 eyes required retreatment before keratoplasty due to reperfusion of occluded vessels (9 eyes: 2×; 4 eyes: ≥3×). No intra- or postoperative complications were observed. The mean time between (last) cauterization with bevacizumab application and keratoplasty was 309 days (in eyes that did not receive simultaneous keratoplasty and vessel cauterization; range 27–794 days, n = 8, Figure 1B). In 23 eyes, neovascularization was still present on the day of keratoplasty due to reperfusion of previously occluded vessels (10 of 18 eyes that were once treated with vessel cauterization and bevacizumab before keratoplasty and 13 of 13 eyes that were treated more than once before keratoplasty) and corneal vessel cauterization with bevacizumab application was performed simultaneously. During follow-up (mean follow-up time: 560 days; range: 59–1095 days), 27 eyes showed no signs of corneal graft rejection (Figure 1C). In the remaining 4 eyes, 4 graft rejection episodes were observed (Figure 2). The time between keratoplasty and diagnosis of rejection was 68, 338, 512, and 661 days, respectively. All rejection episodes occurred in eyes where vessel cauterization with bevacizumab application had been performed simultaneously to keratoplasty due to reperfusion of previously occluded vessels. One of the 4 eyes with graft rejection had a history of a previous graft. This eye was the only case in the HLA-matched group with graft rejection; the remaining 3 graft rejections were observed in the non–HLA-matched group. One of the 4 eyes with graft rejection (non–HLA-matched graft) had neovascularization in two corneal quadrants, whereas the remaining 3 eyes (2 non–HLA-matched grafts, 1 HLA-matched graft) had neovascularization in all 4 quadrants before cauterization and bevacizumab injection. One of the 4 rejection episodes (non–HLA-matched graft) resulted in graft failure and this eye required a regraft, whereas in 3 cases (2 non–HLA-matched grafts, 1 HLA-matched graft) graft failure could be avoided by intensified treatment with glucocorticosteroids (characteristics of eyes with graft rejection are shown in the Table 1). Based on these findings, the estimated probabilities of corneal graft survival after keratoplasty were 92.9% after 1 year (number at risk: 23), 78.4% after 2 years (number at risk: 9), and 78.4% after 3 years (number at risk: 3) for all eyes (non–HLA-matched and HLA-matched grafts) and 95.8% after 1 year (number at risk: 18), 75.8% after 2 years (number at risk: 6), and 75.8% after 3 years (number at risk: 2) for eyes with non–HLA-matched grafts (the Kaplan-Meier estimates of cumulative graft survival are shown in Figure 3).
FIGURE 1.
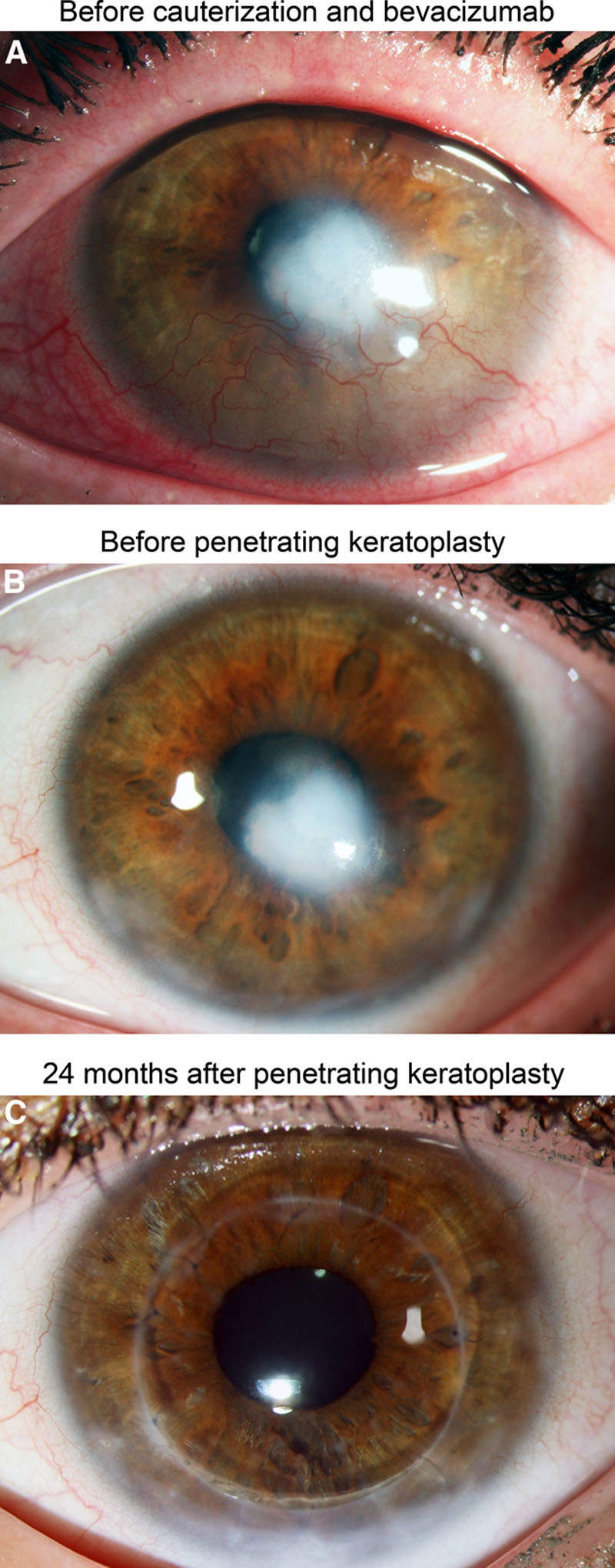
Clinical follow-up of a patient with corneal neovascularization treated with fine-needle vessel cauterization and bevacizumab application before penetrating keratoplasty. In this eye, no graft rejection was observed. A, Herpetic corneal scar with corneal neovascularization in the lower corneal quadrants before fine-needle vessel cauterization and bevacizumab application. B, Corneal scar without neovascularization 6 mo after fine-needle cauterization and bevacizumab application (1 day before penetrating keratoplasty). C, Clear cornea without any signs of allograft rejection 24 mo after penetrating keratoplasty.
FIGURE 2.
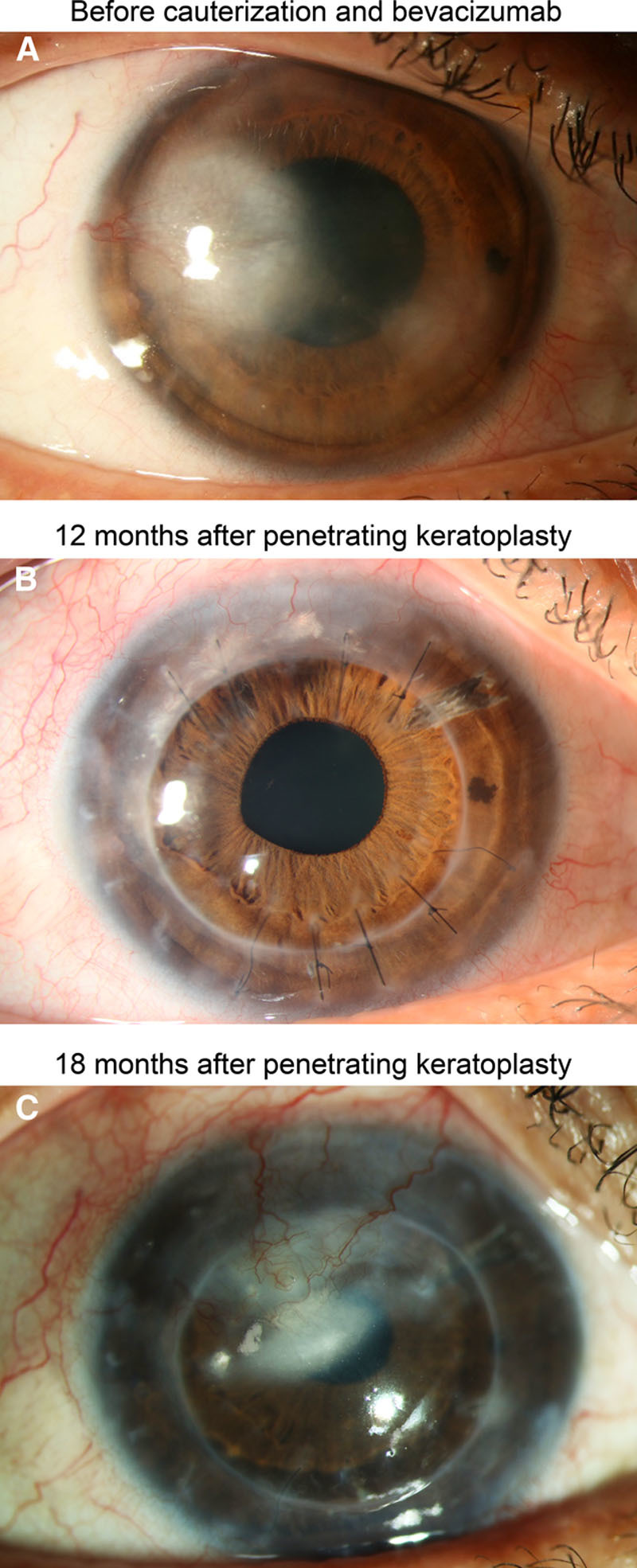
Clinical follow-up of a patient with graft rejection. A, Herpetic corneal scar with corneal neovascularization before fine-needle vessel cauterization and bevacizumab application. B, Clear cornea without signs of allograft rejection 12 months after penetrating keratoplasty. C, Corneal opacification and neovascularization due to allograft rejection 18 mo after penetrating keratoplasty.
TABLE 1.
Characteristics of eyes with corneal allograft rejection
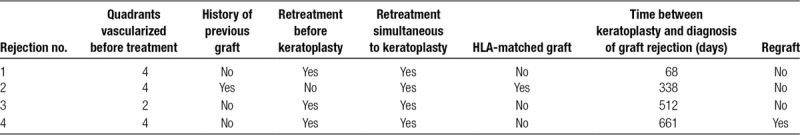
FIGURE 3.
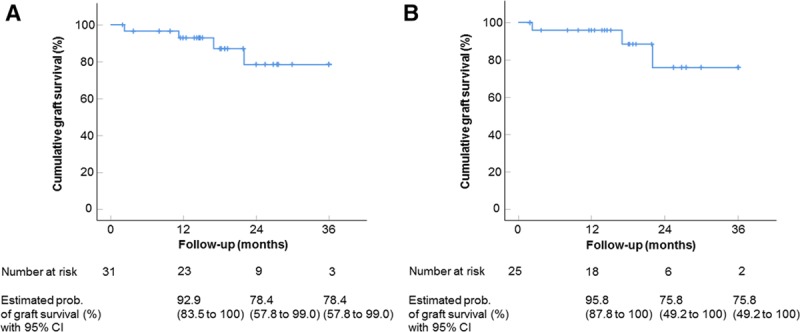
Impact of pretransplant fine-needle corneal vessel coagulation combined with subconjunctival bevacizumab injection on corneal allograft survival after high-risk penetrating keratoplasty. Kaplan-Meier curves depicting the estimated probabilities of rejection-free corneal graft survival after penetrating keratoplasty. Estimated probabilities of rejection-free corneal graft survival were 92.9% after 1 y (number at risk: 23), 78.4% after 2 y (number at risk: 9), and 78.4% after 3 y (number at risk: 3) for all study eyes (non–HLA-matched and HLA-matched keratoplasties) (A) and 95.8% after 1 y (number at risk: 18), 75.8% after 2 y (number at risk: 6), and 75.8 after 3 y (number at risk: 2) for eyes only with non–HLA-matched keratoplasties (B). Mean follow-up time: 560 days; range: 59–1095 days. Vertical dashes indicate censored observations. CI, confidence interval.
DISCUSSION
Penetrating keratoplasty in nonvascularized corneas has a 5-year survival rate of >80%.5,6 However, when keratoplasty is performed in prevascularized corneas, the risk of graft rejection is more than doubled.7-9 Experimental studies indicate that pathological corneal vessels ease the connection of the graft with the secondary lymphoid organs, leading to accelerated sensitization against donor antigens and augmented access of immune effector cells to the graft.2 Thus, successful antiangiogenic treatment strategies for the preconditioning of these high-risk eyes before keratoplasty have been developed in animal models.10,11,13 However, clinical studies analyzing whether preoperative reduction of corneal neovascularization is indeed effective in reducing the risk of corneal allograft rejection are missing. The initial results of our pilot study indicate that thermal fine-needle cauterization of pathological corneal vessels combined with subconjunctival injection of bevacizumab before or during high-risk penetrating keratoplasty results in rejection-free graft survival rates of >90% after 1 year and almost 80% after 2 and 3 years.
In the largest report on fine-needle cauterization of corneal neovascularization, Trikha and colleagues22 have shown that this treatment is safe with a very low rate of complications, but retreatments might be required. We also did not note any complication during vessel cauterization and bevacizumab injection, but 23 of 31 eyes required at least one retreatment, which is slightly higher than the retreatment rate reported by Trikha and colleagues.22
Limitations of our study are the heterogeneity of the study patients (in terms of vascularized quadrants before treatment and in terms of HLA matching), lack of a control group, and the relatively small patient number. Thus, only comparison to historical controls is possible. Williams and colleagues5 have previously reported on the largest cohort followed after keratoplasty and have determined graft survival rates in nonvascularized versus vascularized recipients. Graft survival rates were 96% after 1 year, 87% after 3 years, and 62% after 9 years in recipients without corneal neovascularization, and 90% after 1 year, 70% after 3 years, and 36% after 9 years in recipients with corneal neovascularization, indicating that survival rates in these high-risk eyes continuously decrease during follow-up. In our study, rejection-free survival rates were 96% after 1 year and 76% after 3 years (for non–HLA-matched keratoplasties), indicating an improvement of graft survival when compared with the cohort reported in the study by Williams and colleagues, although the follow-up time in our study is rather short and data on longer follow-up in our patients are not yet available. We therefore cannot rule out that graft rejections might occur at later stages. Thus, further controlled clinical trials with longer follow-up in a larger population are necessary to confirm the results of our pilot study. Beyond that, studies will have to show whether, for example, additional postoperative antiangiogenic therapy might further increase corneal graft survival, as already demonstrated in the experimental setting.10
In summary, our initial results indicate that pretransplant angioregressive treatment of pathological corneal neovessels by fine-needle thermal cauterization combined with subconjunctival injection of bevacizumab is a well-tolerated and safe approach and seems to be effective in reducing the risk of allograft rejection after high-risk penetrating keratoplasty. Nonetheless, future approaches should further optimize the outcome after high-risk grafting by even further reducing the immune reaction risk. In addition, a prospective randomized study is needed.
Footnotes
Published online 25 April, 2019.
D.H. and C.C. participated in research design, the writing of the article, and performance of the research. V.N.H.L. participated in the performance of the research. M.H. participated in data analysis. S.S. participated in the performance of the research. S.R. participated in the performance of the research. B.O.B. participated in the performance of the research.
The authors declare no conflicts of interest.
D.H. was supported by German Research Foundation (DFG) FOR2240 “(Lymph) angiogenesis and Cellular Immunity in Inflammatory Diseases of the Eye” (HO 5556/1-1) and Center for Molecular Medicine Cologne (CMMC B3; CMMC CAP 11). C.C. was supported by German Research Foundation (DFG) FOR2240 “(Lymph) angiogenesis and Cellular Immunity in Inflammatory Diseases of the Eye” (Cu 47/9-1; www.for2240.de); Center for Molecular Medicine Cologne (CMMC B3); and EU Arrest Blindness.
REFERENCES
- 1.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50. [DOI] [PubMed] [Google Scholar]
- 3.Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103:11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann BO, Laaser K, Cursiefen C, et al. A method to confirm correct orientation of descemet membrane during descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2010;149:922. [DOI] [PubMed] [Google Scholar]
- 5.Williams KA, Keane MC, Galettis RA, et al. The Australian Corneal Graft Registry 2015 report. 2015. Available at http://hdl.handle.net/2328/35402. Accessed February 18, 2019.
- 6.Nguyen NX, Seitz B, Martus P, et al. Long-term topical steroid treatment improves graft survival following normal-risk penetrating keratoplasty. Am J Ophthalmol. 2007;144:318. [DOI] [PubMed] [Google Scholar]
- 7.Alldredge OC, Krachmer JH. Clinical types of corneal transplant rejection. Their manifestations, frequency, preoperative correlates, and treatment. Arch Ophthalmol. 1981;99:599. [DOI] [PubMed] [Google Scholar]
- 8.Price MO, Thompson RW, Jr, Price FW., Jr Risk factors for various causes of failure in initial corneal grafts. Arch Ophthalmol. 2003;121:1087. [DOI] [PubMed] [Google Scholar]
- 9.Sellami D, Abid S, Bouaouaja G, et al. Epidemiology and risk factors for corneal graft rejection. Transplant Proc. 2007;39:2609. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich T, Bock F, Yuen D, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le VNH, Schneider AC, Scholz R, et al. Fine needle-diathermy regresses pathological corneal (lymph)angiogenesis and promotes high-risk corneal transplant survival. Sci Rep. 2018;8:5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bock F, Maruyama K, Regenfuss B, et al. Novel anti(lymph)angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89. [DOI] [PubMed] [Google Scholar]
- 14.Hos D, Schlereth SL, Bock F, et al. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: what can we learn from the eye? Semin Cell Dev Biol. 2015;38:117. [DOI] [PubMed] [Google Scholar]
- 15.Cursiefen C, Viaud E, Bock F, et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: the I-CAN study. Ophthalmology. 2014;121:1683. [DOI] [PubMed] [Google Scholar]
- 16.Sheppard JD, Jr, Epstein RJ, Lattanzio FA, Jr, et al. Argon laser photodynamic therapy of human corneal neovascularization after intravenous administration of dihematoporphyrin ether. Am J Ophthalmol. 2006;141:524. [DOI] [PubMed] [Google Scholar]
- 17.Heiligenhaus A, Steuhl KP. Treatment of HSV-1 stromal keratitis with topical cyclosporin A: a pilot study. Graefes Arch Clin Exp Ophthalmol. 1999;237:435. [DOI] [PubMed] [Google Scholar]
- 18.Gerten G. Bevacizumab (avastin) and argon laser to treat neovascularization in corneal transplant surgery. Cornea. 2008;27:1195. [DOI] [PubMed] [Google Scholar]
- 19.Koenig Y, Bock F, Kruse FE, et al. Angioregressive pretreatment of mature corneal blood vessels before keratoplasty: fine-needle vessel coagulation combined with anti-VEGFs. Cornea. 2012;31:887. [DOI] [PubMed] [Google Scholar]
- 20.Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41:2148. [PubMed] [Google Scholar]
- 21.Goto S. Q-switched Nd:YAG laser treatment for corneal neovascularization. Jpn J Ophthalmol. 1992;36:291. [PubMed] [Google Scholar]
- 22.Trikha S, Parikh S, Osmond C, et al. Long-term outcomes of fine needle diathermy for established corneal neovascularisation. Br J Ophthalmol. 2014;98:454. [DOI] [PubMed] [Google Scholar]


