Abstract
Previous work from our lab revealed that adult female rats have increased levels of myelin basic protein (MBP), a marker for myelination, in the orbitofrontal cortex (OFC) as compared to adult males. The goal of the present study was to determine the role of gonadal hormones, acting either in adulthood or at puberty, in the development of an adult sex difference in OFC levels of MBP. In an initial experiment, we replicated our previous results demonstrating that gonadally intact female rats have increased levels of MBP in the OFC as compared to males. In a second experiment, gonadectomy in adulthood did not alter MBP levels in rats of either sex. In a third experiment, gonadectomy immediately prior to pubertal onset resulted in significant reduction of levels of MBP in adult females but not males. This reduction eliminated the sex difference in adult MBP levels in the OFC. These results reveal puberty to be an organizational time point for a sex difference in the OFC of adult rats in levels of a marker of myelination. This neuroanatomical difference may contribute to observed sex differences in OFC-associated behaviors including in inhibitory control.
Keywords: sex difference, gonadal hormone, orbitofrontal cortex, myelin basic protein, inhibitory control, impulsivity
Introduction
The orbitofrontal cortex (OFC), located on the ventral portion of the prefrontal cortex, plays an important role in executive function, including in inhibition of cue responses and impulsive behaviors in both humans and rodent models (Brass and von Cramon 2002; Cardinal 2006; Dove et al. 2000). The OFC exerts effects by modulating activity of downstream targets involved in motor control and decision making (Crews and Boettiger 2009; Galvan et al. 2006; Peper et al. 2013; Perry et al. 2011; Schilman et al. 2008). Disruption of OFC activity by lesion results in increased levels of impulsivity on both serial (Chudasama et al. 2003) and stop-signal (Eagle et al. 2008) reaction time tasks. Understanding the structural differences within the OFC between populations and developmental time points for their derivations will contribute to understanding mechanisms underlying differences in impulsive behavior between groups.
Results of research from our lab reveal a sex difference in levels of markers for connectivity within the OFC (Bayless and Daniel 2015). Specifically, adult female rats exhibit greater levels of myelin basic protein (MBP), an abundant protein involved in myelin structure (Boggs 2006), than males. MBP is a structurally significant myelin protein in the central nervous system represented in four isoforms: 14 kDa, 17 kDa, 18 kDa, and 21 kDa. The functions of the different isoforms are still under investigation, but evidence suggests importance in membrane structure and adhesion (isoforms 14 kDa and 18 kDa) and oligodendrocyte development, survivability and stability (isoforms 17 kDa and 21 kDa) (Harauz and Boggs 2013). In mammals, increased myelin levels can indicate either faster or more efficient neuronal communication via increased neuronal connections or an increase in axonal diameter (Sherman and Brophy 2005). An observed structural sex difference in myelin proteins within the adult rat OFC may be related to observed sex differences in OFC-dependent behavior with females having increased inhibitory control than males (Bayless et al. 2012, 2013; Trent and Davies 2012; Weafer et al. 2014).
The mechanisms underlying sex differences in markers of OFC myelination are unknown. However, gonadal hormones act throughout the lifespan to mediate sex differences in biology and behavior in both human and rodent populations. The actions of hormones have been traditionally characterized as activational or organizational. Activational hormone effects are typically observed in the adult animal, are transient in nature, and derive from effects of exposure to circulating hormones. Examples of activational hormone fluctuations include estrus cycling in mammals (Walker et al. 2001) and seasonal bird behavior including nesting, mating, and migrating (Wingfield and Farner 1993). In addition to activational effects, gonadal hormones may also organize the brain in a more permanent manner through organizational effects. Organizational effects are relatively permanent in nature and derive from actions of hormones during sensitive periods of development. The canonical theory of sexual differentiation of the brain and its influence on sex differences in behavior illustrates the importance of exposure to steroid hormones during an early critical period of perinatal development (for review, see Wallen and Baum 2002).
In addition to the neonatal period, puberty has more recently been identified as a critical period of brain organization (for review see Schulz et al. 2009). Puberty is a period of development marked by a large surge in gonadotropin releasing hormone (GnRH) (Watanabe and Terasawa 1989). This surge in turn leads to a rise in the production of steroid hormones including testosterone, and the pituitary gonadotropins follicular stimulating hormone (FSH) and luteneizing hormone (LH) (Watanabe and Terasawa 1989). The pubertal period has been linked to permanent changes in brain morphology including in levels of grey and white matter (Giedd 2004; Knickmeyer et al. 2010). In adult female rats, peripubertal gonadectomy has been shown to influence the number of neurons of the visual cortex. Gonadectomized females were shown to exhibit greater neuron count than intact females in adulthood (Nunez, Sodhi, and Juraska 2002). To our knowledge, it has yet to be explored as to what degree either adult circulating gonadal hormones or exposure to gonadal hormones during puberty contribute to sex differences in myelin levels within the prefrontal cortex.
The goal of the present study was to test the hypothesis that gonadal hormone exposure, either during adulthood or at puberty, mediates a sex difference in adult levels of myelin protein within the OFC. To test this hypothesis we conducted three experiments. In Experiment 1, we confirmed a previously observed sex difference (Bayless and Daniel 2015) in adult levels of myelin basic protein (MBP). In Experiment 2, we examined the role of adult circulating gonadal hormone exposure in mediating a sex difference in OFC MBP levels via activational hormone effects. In Experiment 3, we examined the role of pubertal gonadal hormone exposure in mediating an adult sex difference in OFC MBP levels via organizational hormone effects. Together, results of these experiments identify pubertal gonadal hormone exposure as mediator of the development of an adult sex difference in MBP levels in the OFC.
Experimental Procedures
Experiment 1: Impact of biological sex on myelin basic protein levels in the OFC of adult male and female rats
Animals
Five female and five male Long-Evans hooded rats, approximately 60 days old, were purchased from Envigo, Inc. (Indianapolis, IN, USA). Animal care was in accordance with the guidelines set by the National Institute of Health Guide for the Care and Use of Laboratory Animals (1996). All procedures were approved by the Institutional Animal Care and Use Committee of Tulane University. Rats were housed in a temperature controlled vivarium under a 12 hour light/dark cycle (lights on at 7:00am) and were fed and watered ad libitum. See Figure 1 for overview of experimental timeline.
Fig. 1.
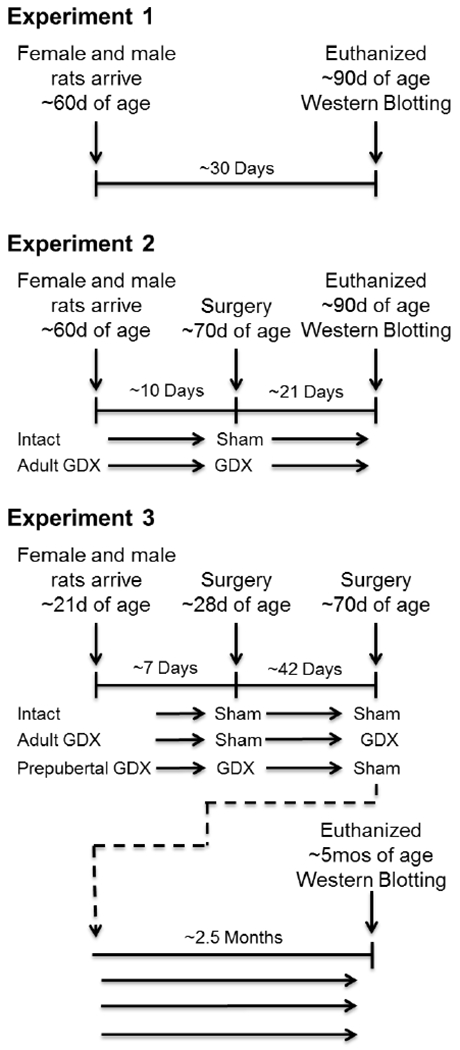
Summary of experimental protocols for Experiments 1–3. GDX, gonadectomy.
Euthanasia, tissue dissection, and processing
Rats were anesthetized at ~90 days of age by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (7 mg/kg) and killed by decapitation. Brains were removed, quick-frozen on dry ice, and stored at −80°C. The OFC was dissected as described previously (Bayless and Daniel 2015). Briefly, coronal sections containing the OFC were cut in a cryostat at −20°C using coordinates from Paxinos and Watson (1998) (OFC: AP +4.2 mm to +2.7 mm). Using a scalpel and visual cues from natural boundaries, the OFC was dissected at −20°C (see Fig 2 for dissection details). Care was taken to avoid underlying white matter. Tissue samples from both hemispheres were pooled for each animal and stored at −80°C until processing. Tissue was homogenized in 20 μl/mg lysis buffer containing 1mM EGTA, 1mM EDTA, 20 mM Tris, 1 mM sodium pyrophosphate tetrabasic decahydrate, 4 mM 4-nitrophenyl phosphate disodium salt hexahydrate, 0.1 μM microcystin, and 1% protease inhibitor cocktail (Sigma-Aldrich). Samples were then centrifuged for 15 min at 1000 × g at 4°C, protein concentration of supernatants was determined (Bradford Protein Assay Kit; Pierce, Rockford, IL), and each sample was diluted 1:1 with Laemmli Sample Buffer (Bio- Rad; Hercules, CA), mixed with 350 mM D,L-dithiothreitol, boiled for 5 min, and stored at −80°C.
Fig. 2.
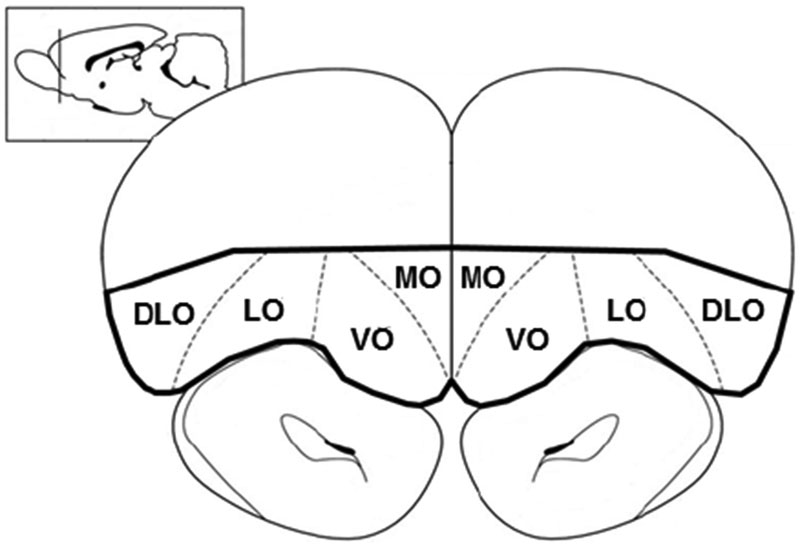
Graphical representation of region dissected out for Western Blotting, bolded in black outline. Coronal sections containing the orbitofrontal cortex (OFC) were cut in a cryostat at −20°C using coordinates from Paxinos and Watson (1998) (OFC: AP +4.2 mm to +2.7 mm). Using a scalpel and visual cues from natural boundaries, the OFC was dissected at −20°C. DLO, dorsolateral orbitofrontal cortex; LO, lateral orbitofrontal cortex; MO, medial orbitofrontal cortex, VO, ventral orbitofrontal cortex.
Protein of interest
Western blotting procedures were used to assess protein levels of myelin basic protein (MBP) in the orbitofrontal cortex (OFC). MBP consists of four major isoforms with molecular masses of 21.5, 18.5, 17.0, and 14.0 kDa (Akiyama et al. 2002) and represents one of the most abundant myelin proteins within the brain (Boggs 2006).
Electrophoresis and Western Blotting
For total protein samples obtained from each rat, 25 μg of total protein were loaded and separated at 200 V on 18% SDS-PAGE gels (Bio-Rad) for 60 min in order to probe for myelin basic protein levels. Proteins were transferred to nitrocellulose membranes at 100V for 60 min. Membranes were blocked with 5% nonfat dry milk in 0.1% Tween/1 X Tris-buffered saline (TTBS) at room temperature for 60 min. Following this, membranes were cut to separate the section containing the proteins of interest from the section containing the loading control β-actin. Membranes were then incubated with primary antibodies for MBP (mouse monoclonal; 1:5000, Abcam, AB78156), or β-actin (mouse monoclonal; 1:15,000; Santa Cruz) overnight at 4°C in 1% nonfat dry milk-TTBS. Blots were washed three times for 15 min each with TTBS and incubated with 5% nonfat dry milk containing goat antimouse IgG (MBP, 1:10,000; β-actin, 1:10,000; Santa Cruz) conjugated to horseradish peroxidase for 1.5 h at room temperature. Blots were washed again three times for 15 min each and incubated for 1 min with the chemiluminescent substrate Pierce ECL western blotting substrate (Fisher Scientific) and exposed to film (Kodak Biomax MR) for varying durations to capture optimal signal intensity. Films were imaged using MCID Core imaging software (InterFocus Imaging Ltd., Cambridge, England), and optical density x area was measured for bands of interest. All values were represented as a percentage relative to β-actin for each sample.
Statistical analyses
A log base 10 transformation of data was completed using SPSS statistical software to normalize data and reduce skew. Optical density x area values as a percentage of β-actin were analyzed using a 2-way repeated measures ANOVA with sex as the between-subjects factor and isoform as the within-subjects factor. Results are expressed as Log10 of the relative amount of myelin basic protein as a percentage of β-actin. Following transformation of data, Levene’s Test of Equality of Error Variances and Shapiro-Wilk Test were performed to test for equal variance and normal distribution respectively.
Experiment 2: Impact of gonadal hormones acting in adulthood on myelin basic protein levels in the OFC of adult male and female rats
Animals
Sixteen female and sixteen male Long-Evans hooded rats, approximately 60 days old, were purchased from Envigo, Inc. Male and female rats were randomly assigned to one of two groups: adult gonadectomy or gonadally intact. See Figure 1 for overview of experimental timeline.
Gonadectomy procedures
Half of male rats and half of female rats were gonadectomized and the remainder received sham surgery at approximately 70 days of age. Surgery was performed while under anesthesia induced by injections of ketamine (100mg/kg, ip; Bristole Laboratories; Syracuse, NY) and xylazine (7mg/kg, ip; Miles Laboratories; Shawnee, KS). Buprenorphine (0.025 mg/kg, Buprenex) was administered subcutaneously as an analgesic and eye ointment applied to protect from eye desiccation during surgery.
Gonadectomy efficacy and vaginal cytology
To confirm endocrine status, daily vaginal smears were collected by lavage, via medicine dropper, from gonadally intact adult females for ten days prior to euthanasia. Gonadectomized females and all males received sham lavages, during which a small amount of water was placed on the genitals using a medicine dropper. At the time rats were killed, right uterine horns of females and ischiocavemosa muscle weight of males were extracted and weighed to confirm gonadectomy status.
Euthanasia, tissue dissection, processing, and Western Blotting
Rats were euthanized at ~90 days of age. Each intact female was yoked to a gonadectomized female, a gonadectomized male, and an intact male. Rats of each yoked group were euthanized when intact females were at proestrus, a time at which estradiol levels are at their peak and vaginal cytology is characterized by large nucleated epithelial cells (Becker et al. 2005). Euthanasia, tissue dissection and processing and Western Blotting were as described in Experiment 1.
Statistical analyses
A log base 10 transformation of data was completed. Optical density x area values as a percentage of β-actin were analyzed using a 3-way repeated measures ANOVA with surgery and sex as the between-subject factors and isoform as the within-subjects factor. Fisher’s LSD post hoc testing was applied as appropriate.
Experiment 3: Impact of gonadal hormones acting at puberty on myelin basic protein levels in the OFC of adult male and female rats
Animals
Eighteen female and eighteen male Long-Evans hooded rats, approximately 21 days old, were purchased from Envigo, Inc. Male and female rats were randomly assigned to one of three groups: pre-pubertal gonadectomy, adult gonadectomy, or gonadally intact. See Figure 1 for overview of experimental timeline.
Gonadectomy procedures
Six male rats and six female rats were gonadectomized at 28 days of age and prior to the onset of puberty. Other rats received sham surgery at 28 days of age. All females were examined for vaginal opening prior to pubertal gonadectomy to confirm puberty onset had yet to begin. At 70 days of age, another six male rats and six female rats were gonadectomized, and the other rats received sham surgery. The remaining six males and females remained gonadally intact. Gonadectomy efficacy and vaginal cytology procedures as described in Experiment 2.
Euthanasia, tissue dissection, and Western Blotting
Gonadally intact females were yoked to one subject from each of the other five groups as described under Experiment 2. Rats were euthanized at -five months of age. Rats were aged an additional two months prior to euthanasia as compared to Experiments 1 and 2 to determine if length of gonadal hormone deprivation following adult gonadectomy impacts levels of MBP in the OFC. During this time, rats were used to pilot a behavioral task. Tissue dissection, electrophoresis and western blotting were as described in Experiment 1.
Statistical analyses
A log base 10 transformation of data was completed.. Optical density × area values as a percentage of β-actin were analyzed using a 3-way repeated measures ANOVA with surgery and sex as the between-subject factors and isoform as the within-subjects factor. Fisher’s LSD post hoc testing was applied as appropriate.
Results
Experiment 1: Impact of biological sex on myelin basic protein levels in the OFC of adult male and female rats
As illustrated in Figure 3, gonadally intact adult female rats exhibited greater levels of MBP in the OFC than did males. Western blots for MBP revealed four isoform bands of MBP-like immunoreactivity at approximately 21.5, 18.5, 17.0, and 14.0 kDa. Results revealed a significant main effect of sex (F(1,8) = 6.349, p =.036). Results also revealed a significant main effect of isoform (F(3,24) = 267.575, p < .001). Post-hoc analyses revealed that levels of all four isoforms were significantly different from one another (p < .05). There was no significant interaction between sex and isoform. No significant effect of sex on β-actin loading control was found. Levene’s Test demonstrated data to exhibit equal variance and Shapiro-Wilk’s Test demonstrated data to be normally distributed.
Fig. 3.
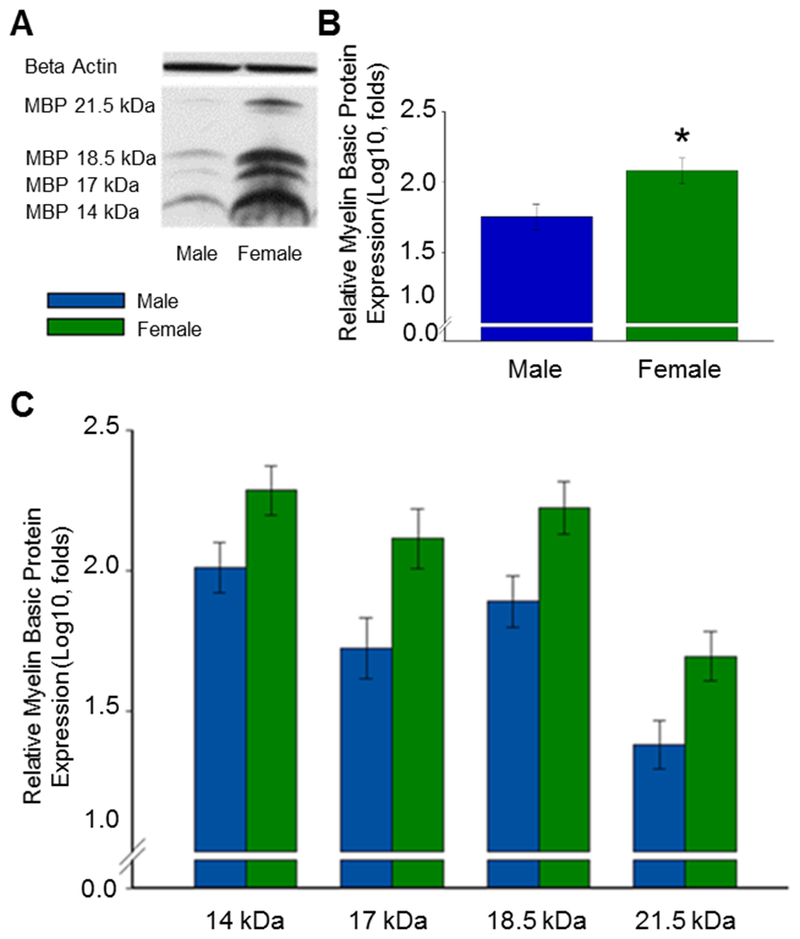
Effect of sex on levels of myelin basic protein (MBP) in the orbitofrontal cortex (OFC) of gonadally intact adult male and female rats. (A) Representative western blot images for MBP and the loading control β-actin. MBP is expressed in four isoforms at approximately 21.5kDa, 18.5kDa, 17.0 kDa, and 14.0 kDa. (B) MBP isoforms isolated. Log10 transformation of mean density × area (±SEM) expressed relative to control β-actin. (C) MBP isoforms averaged. Overall main effect of sex: *p = .036.
Experiment 2: Impact of gonadal hormones acting in adulthood on myelin basic protein levels in the OFC of adult male and female rats
As illustrated in Figure 4, gonadectomy in adulthood had no effect on levels of MBP in the OFC of adult male and female rats. Western blots for MBP revealed four isoform bands of MBP-like immunoreactivity at approximately 21.5, 18.5, 17.0, and 14.0 kDa. A significant main effect of sex (F(1,28) = 4.941, p = .034) revealed adult females to have greater levels of MBP in the OFC as compared to males across surgical conditions. Results also revealed a significant main effect of isoform (F(3,84) = 147.272, p < .001). Post-hoc analyses revealed that all levels of all isoforms were significantly different from each other (p < .05). No significant main effect of surgery was found. No significant interactions were found. There were no significant effects on levels of β-actin loading control. Levene’s Test demonstrated data to exhibit equal variance and Shapiro-Wilk’s Test demonstrated data to be normally distributed.
Fig. 4.
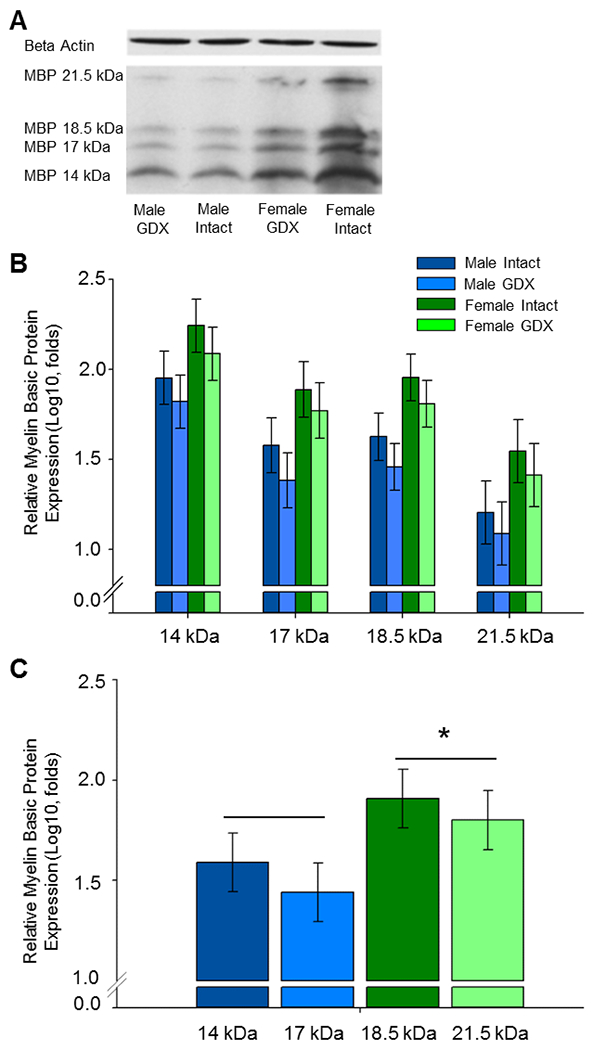
Effects of sex and adult gonadectomy on levels of myelin basic protein (MBP) in the adult orbitofrontal cortex (OFC). Male and female rats were gonadectomized at 70 days of age (GDX) or underwent sham surgery (Intact) and were killed at ~90 days of age. (A) Representative western blot images for MBP and the loading control β-actin. MBP is expressed in four isoforms at approximately 21.5kDa, 18.5kDa, 17.0 kDa, and 14.0 kDa. (B) MBP isoforms isolated. Log10 transformation of mean density × area (±SEM) expressed relative to control β-actin. (C) MBP isoforms averaged. Main effect of sex; *p < .034 vs males. GDX, gonadectomy.
Experiment 3: Impact of gonadal hormones acting at puberty on myelin basic protein levels in the OFC of adult male and female rats
As illustrated in Figure 5, gonadectomy prior to puberty eliminated an observed sex difference in adult MBP levels in the OFC. Furthermore, the extended period of hormone deprivation following gonadectomy in adulthood used in this experiment as compared to Experiment 2 (see Fig 1 for experimental timelines) did not impact results. Western blots for MBP revealed four isoform bands of MBP-like immunoreactivity at approximately 21.5, 18.5, 17.0, and 14.0 kDa. A significant main effect of sex revealed females to overall exhibit greater levels of MBP in the OFC than males (F(1,30) = 8.281, p = .007). A significant main effect of surgery was found (F(1,30) = 5.452, p =.01). Post hoc testing revealed that adult rats that had experienced gonadectomy prior to puberty had significantly decreased levels of MBP as compared to both rats that underwent gonadectomy in adulthood and to the gonadally intact controls (p < 05). Further, the significant main effect of surgery was primarily driven by prepubertal gonadectomy in females as a planned comparisons via post-hoc testing using Bonferroni adjusted alpha levels of .025 per test (.05/2) revealed that MBP levels in females that underwent pre-pubertal gonadectomy were significantly less than intact females whereas levels in males that underwent pre-pubertal gonadectomy were not significantly different than intact males. Results also revealed a main effect of isoform (F(3,90) = 45.393, p < .001). Post-hoc analyses revealed that levels of all four isoforms were significantly different from one another (p < .05). There were no significant interactive effects nor effects on levels of β -actin loading control. Levene’s Test demonstrated data to exhibit equal variance and Shapiro-Wilk’s Test demonstrated data to be normally distributed.
Fig. 5.
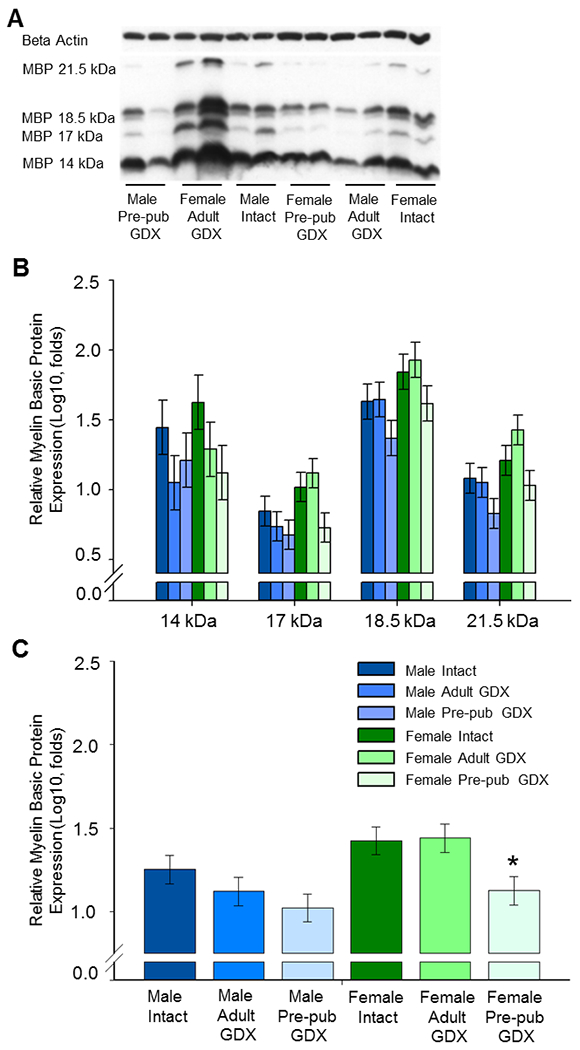
Western blot data showing the effect of sex, adult gonadectomy, and pre-pubertal gonadectomy on levels of myelin basic protein (MBP) in the adult orbitofrontal cortex (OFC). Male and female rats were gonadectomized at 28 days of age (Pre-pub GDX), 70 days of age (Adult GDX), or underwent sham surgeries (Intact) and were killed 2.5 months later. (A) Representative western blot images for MBP and the loading control β-actin. MBP is expressed in four isoforms at approximately 21.5kDa, 18.5kDa, 17.0 kDa, and 14.0 kDa. (B) MBP isoforms isolated. Log10 transformation of mean density × area (±SEM) expressed relative to control β-actin. (C) MBP isoforms averaged. Main effects of sex and surgery; * p < .025 vs Female Intact. GDX, gonadectomy; Pre-pub, pre-pubertal.
Discussion
Results of the present experiments indicate that gonadal hormone exposure during puberty mediates a lasting adult sex difference in levels of myelin basic protein (MBP) in the orbitofrontal cortex (OFC) of adult rats. MBP levels were shown to be significantly greater in the OFC of adult intact females as compared to males, replicating results seen previously in our lab (Bayless and Daniel 2015). Gonadectomy in adulthood, and the subsequent loss of adult circulating gonadal hormones, for 3 weeks or 2.5 months did not alter this sex difference and had no impact on levels of MBP. Gonadectomy prior to puberty, however, significantly reduced levels of adult MBP thereby eliminating the adult sex difference. The impact appears to be driven by ovarian hormones acting during puberty, as females that underwent prepubertal gonadectomy displayed significantly reduced level of adult MBP as compared to gonadally intact controls. These results reveal puberty to be a developmental time point that mediates a later increase in MBP leading to a sex difference with adult females exhibiting greater levels of MBP in the OFC than males.
The current finding that pubertal exposure to gonadal hormones in females mediates an adult sex difference in OFC MBP contributes to literature demonstrating puberty and adolescence to be of great importance as an organizational period for brain and behavior (Schulz et al. 2004a., 2006a., 2009; Field et al. 2004; Swithers et al. 2008, O’Donnell 2010; Koss et al. 2015; Drzewiecki et al. 2016, Johnson et al. 2016). The finding also contributes to literature demonstrating this age frame to be of specific importance to myelination within the brain. For example, Xu et al. (2014) observed adolescence specifically to be a critical time point for initiation of myelin generation and production, examining MBP levels in mice. Their findings demonstrate puberty and adolescence to be a period of great increase in MBP concentrations, an increase that continues into adulthood. Additionally, Yates and Juraska (2008) demonstrated that pubertal hormones are responsible for the development of a reported sex difference (Kim and Juraska 1997) in the number of myelinated axons in the rat posterior corpus callosum in which males exhibited greater levels than did females. These results are consistent with the present results indicating puberty as a critical period for myelin generation, although the direction of the effect in males and females may vary across structures. Further, although we did not directly compare levels of MBP across developmental time points, we do show that preventing puberty via pre-pubertal gonadectomy results in a significant reduction of MBP levels of adult females, results consistent with the possibility that actions during puberty result in lasting increases in production of markers of myelination in the OFC.
Increased myelin production is only one mechanism by which female gonadal hormone exposure may act at puberty to mediate adult sex differences in OFC MBP. Myelin basic protein, one of the most abundant proteins in the central nervous system, is responsible for aiding in adhesion to cytosolic surfaces, protein interactions, and is essential for formation of central nervous system myelin (Moscarello, 1997). MBP loss, as observed in transgenic derived MBP deficit models, leads to a decrease in compact CNS myelin and dysmyelination (Carre, 2002; Anaby et al. 2013). It is possible that pubertal gonadal hormone exposure leads to altered levels of MBP survival. Potential mechanisms for hormone influence on myelin protein levels include neuroprotection in females or increased apoptosis in males. Androgens can lead to increased apoptosis and estrogens can be neuroprotective for cell death (Cutolo et al. 2002; 2005). However, in the medial prefrontal cortex, loss of a hormone surge at puberty due to gonadectomy prevents neuron loss and increases neuron and glia counts in female rats (Koss et al. 2015). Willing and Juraska (2015) found this change in neuron count within the female mPFC to coincide specifically with the onset of puberty (days 35 to 45) providing supportive evidence to this time frame’s importance for organizational change. Gonadal hormone exposure influences oligodendrocyte turnover in a sexually dimorphic matter with females exhibiting greater turnover than males in the corpus callosum, fornix, and spinal cord (Cerghet et al. 2006; 2009). Marin-Husstege et al. (2004) also found oligodendrocyte progenitor proliferation and maturation to be differentially regulated by sex steroids in cell cultures. They found delayed exit of female progenitor cells from the cell cycle when exposed to 17β-estradiol than did male derived cells. In the current results, female pubertal gonadal hormone exposure leads to an increase in adult MBP levels supporting the hypothesis that hormone exposure is protective for female oligodendrocyte and neuron count in the OFC.
Previous results in our lab have indicated the importance hormone exposure during development plays in organizing sex differences in behavior. More specifically, we have demonstrated neonatal gonadal hormone exposure is vital in organizing sex differences in impulsive behavior, a behavior mediated by activity of the OFC (Bayless et al. 2013). It is possible that the adult sex difference in OFC MBP, organized at puberty, could also be influenced by neonatal mechanisms that may be activated at puberty. Future studies are needed to determine if this is the case.
Differential connectivity within the OFC and to its downstream targets may contribute to sex differences in inhibition of impulsive behavior. It is known that interconnectivity within the OFC has been shown to be of importance for the encoding and using of associative-information for motivational significance of a particular stimuli (Schoenbaum et al. 2000), an encoding ability especially necessary for performance on a Go/NoGo behavioral task. Abnormal levels of connectivity within the OFC, both hyperconnective (Beucke et al. 2013) and hypoconnective (Frodl et al. 2010), have been linked to the behavioral disorders obsessive compulsive disorder (OCD) and major depressive disorder (MDD) respectively. It remains to be determined if differences in myelin protein and connectivity within the OFC directly impact the ability to inhibit impulsive responses.
The current results collectively demonstrate that a sex difference in MBP levels in the OFC of adult rats results from actions of gonadal hormones during puberty and not during adulthood. This developmental difference in a key structure of the prefrontal cortex important for regulation of behavioral control and decision making could contribute to previously demonstrated sex differences these behaviors. These findings also emphasize the importance of the consideration of puberty as an organizational developmental time point that warrants further investigation in studies of sex differences in the brain and behavior.
Highlights:
A sex difference in adult myelin protein levels in the orbitofrontal cortex exists
Females have greater levels of myelin protein in the orbitofrontal cortex vs males
Adult gonadectomy did not alter myelin protein levels
Prepubertal gonadectomy decreased adult levels of myelin protein
Prepubertal gonadectomy eliminated an adult sex difference in myelin protein
Acknowledgements
We thank Amy Theriot Pierce, Associate Director Uptown Campus of the Tulane Department of Comparative Medicine and the Tulane vivarium staff for their expert animal care and Dr. David Corey of the Tulane Department of Psychology for advice regarding statistical analyses. This work was supported by the National Institute of Health Grant R21DA043072 to JMD, the Carol Lavin Bernick Tulane Faculty Grant to JMD, and the State of Louisiana Board of Regents Graduate Fellowship LEQSF (2013-18)GF-17 to JSD.
Abbreviations:
- ADHD
attention deficit hyperactivity disorder
- ANOVA
analysis of variance
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- IGG
immunoglobulin
- kDa
kilodalton
- GDX
gonadectomy
- MBP
myelin basic protein
- OFC
orbitofrontal cortex
- PBS
phosphate-buffered saline
- PFC
prefrontal cortex
- TTBS
0.1% Tween/1 X Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Akiyama K, Ichinose S, Omori A, Sakurai Y, Asou H (2002), Study of expression of myelin basic proteins (MBPs) in developing rat brain using a novel antibody reacting with four major isoforms of MBP. J Neurosci Res 68:19–28. [DOI] [PubMed] [Google Scholar]
- Anaby D, Duncan ID, Smith CM, Cohen Y (2013), White matter maturation in the brains of Long Evans shaker myelin mutant rats by ex-vivo QSI and DTI. Magn Reson Imaging 31:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless DW, Daniel JM (2015), Sex differences in myelin-associated protein levels within and density of projections between the orbital frontal cortex and dorsal striatum of adult rats: implications for inhibitory control. Neuroscience 300:286–296. [DOI] [PubMed] [Google Scholar]
- Bayless DW, Darling JS, Daniel JM (2013), Mechanisms by which neonatal testosterone exposure mediates sex differences in impulsivity in prepubertal rats. Horm Behav 64:764–769. [DOI] [PubMed] [Google Scholar]
- Bayless DW, Darling JS, Stout WJ, Daniel JM (2012), Sex differences in attentional processes in adult rats as measured by performance on the 5-choice serial reaction time task. Behav Brain Res 235:48–54. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, et al. (2005), Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–1673. [DOI] [PubMed] [Google Scholar]
- Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, Kaufmann C, Kathmann N, (2013), Abnormally High Degree Connectivity of the Orbitofrontal Cortex in Obsessive-Compulsive Disorder. JAMA Psychiatry . 70(6):619–629. [DOI] [PubMed] [Google Scholar]
- Boggs JM (2006), Myelin basic protein: a multifunctional protein. Cell Mol Life Sci 63:1945–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2002), The role of the frontal cortex in task preparation. Cereb Cortex 12:908–914. [DOI] [PubMed] [Google Scholar]
- Cardinal RN (2006), Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw 19:1277–1301. [DOI] [PubMed] [Google Scholar]
- Carre JL, Goetz BD, O’Connor LT, Bremer Q, Duncan ID (2002), Mutations in the rat myelin basic protein gene are associated with specific alterations in other myelin gene expression. Neurosci Lett 330:17–20. [DOI] [PubMed] [Google Scholar]
- Cerghet M, Skoff RP, Swamydas M, Bessert D (2009), Sexual dimorphism in the white matter of rodents. J Neurol Sci 286:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS (2006), Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci 26:1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW (2003), Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA (2009), Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav 93:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Capellino S, Montagna P, Ghiorzo P, Sulli A, Villaggio B (2005), Sex hormone modulation of cell growth and apoptosis of the human monocytic/macrophage cell line. Arthritis Res Ther 7:R1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Craviotto C, Felli L, Pizzorni C, Seriolo B, Villaggio B (2002), Modulation of cell growth and apoptosis by sex hormones in cultured monocytic THP-1 cells . Ann N Y Acad Sci 966:204–210. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000), Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res 9:103–109. [DOI] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, Juraska JM (2016), Synaptic Number Changes in the Medial Prefrontal Cortex Across Adolescence inM ale and Female Rats: A Role for Pubertal Onset. Synapse 70:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW (2008), Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex 18:178–188. [DOI] [PubMed] [Google Scholar]
- Field EF, Whishaw IQ, Forgie ML, Pellis SM (2004), Neonatal and pubertal, but not adult, ovarian steroids are necessary for the development of female-typical patterns of dodging to protect a food item. Behav Neurosci 118:1293–1304. [DOI] [PubMed] [Google Scholar]
- Frodl T, Bokde AL, Scheuerecker J, Lisiecka D, Schoepf V, Hampel H, Mueller HJ, Breckmann H, et al. (2010), Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry 67:161–167. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ (2006), Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci 26:6885–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN (2004), Structural magnetic resonance imaging of the adolescent brain. Annals of the NY Acad Sci. 1021:77–85. [DOI] [PubMed] [Google Scholar]
- Harauz G, Boggs JM, (2013), Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic isoforms. Journal of Neurochemistry. 125:334–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CM, Loucks A, Peckler H, Thomas AW, Janak P, Wilbrecht L (2016), Long-range orbitofrontal and amygdala axons show divergent patterns of maturation in the frontal cortex across adolescence. Dev Cogn Neurosci. 18:113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Juraska JM (1997), Sex differences in the development of axon number in the splenium of the rat corpus callosum from postnatal day 15 through 60. Brain Res Dev Brain Res. 102:77–85. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL, Gilmore JH. (2010), Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cerebral Cortex. 20:1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Lloyd MM, Sadowski RN, Wise LM, Juraska JM (2015), Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev Psychobiol 3:305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstage M, Muggironi M, Raban D, Skoff RP, Casscia-Bonnfeil P (2006), Oligodendrocyte Progenitor Proliferation and Maturation Is Differentially Regulated by Male and Female Sex Steroid Hormones. Dev Neurosci 26:245–254 [DOI] [PubMed] [Google Scholar]
- Moscarello MA (1997), Myelin basic protein, the ‘executive’ molecule of the myelin membrane In: Cell Biology and Pathology of Myelin: Evolving Biological Concepts and Therapeutic Approaches (Juurlink BHJ, Devon RM, Doucette JR, Nazarali AJ, Schreyer DJ and Verge VMK Eds.), pp. 13–25, Plenum, New York [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM (2002), Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol 52:312–321. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, (2010), Adolescent Maturation of Cortical Dopamine. Neurotoxicity Research. 3:306–312 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998), The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press. [Google Scholar]
- Peper JS, Mandl RC, Braams BR, de Water E, Heijboer AC, Koolschijn PC, Crone EA (2013), Delay discounting and frontostriatal fiber tracts: a combined DTI and MTR study on impulsive choices in healthy young adults. Cereb Cortex 23:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, et al. (2011), Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev 65:124–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ (2008), The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett 432:40–45. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M, (2000), Changes in Functional Connectivity in Orbitofrontal Cortex and Basolateral Amygdala during Learning and Reversal Training. Journal of Neuroscience . 20(13):5179–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL (2009), Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav 55:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL (2006a), Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav.50:477–83. PMID: [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL (2004a), Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster.Horm Behav. 45:242–249. [DOI] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ (2005), Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci 6:683–690. [DOI] [PubMed] [Google Scholar]
- Steel Z, Blaszczynski A (1998), Impulsivity, personality disorders and pathological gambling severity. Addiction 93:895–905. [DOI] [PubMed] [Google Scholar]
- Swithers SE, McCurley M, Hamilton E, Doerflinger A (2008), Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav 54:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent S, Davies W (2012). The influence of sex-linked genetic mechanisms on attention and impulsivity. Biol Psychol 89:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Francis R, Cabassa J, Kuhn CM (2001), Effect of ovarian hormones and estrous cycle on stimulation of the hypothalamo-pituitary-adrenal axis by cocaine. J Pharmacol Exp Ther 297:291–298. [PubMed] [Google Scholar]
- Wallen Kim and J. Baum Michael, (2002), Masculinization and Defeminization in Altricial and Precocial MammalsComparative Aspects of Steroid Hormone Action. Hormones, Brain and Behavior. 4 385–423. [Google Scholar]
- Watanabe G, Terasawa E (1989), In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 125:92–99. [DOI] [PubMed] [Google Scholar]
- Weafer J, de WH (2014), Sex differences in impulsive action and impulsive choice. Addict Behav. 39:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Juraska JM (2015), The Timing of Neuronal Loss Across Adolescence in the Medial Prefrontal Cortex of Male and Female Rats. Neuroscience 301:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield JC, Farner DS (1993), Endocrinology of reproduction in wild species. In: Avian Biology. Vol. 9:164–327. [Google Scholar]
- Xu W, Xin C, Lin Q, Ding F, Gong W, Zhou Y, Yu J, Cui P, et al. (2014), Adolescent mouse takes on an active transcriptomic expression during postnatal cerebral development. Genomics Proteomics Bioinformatics 12:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MA, Juraska JM (2008), Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp Neurol 209:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]


