Abstract
INTRODUCTION:
Four less well-studied but promising “emerging” cerebrospinal fluid (CSF) biomarkers are elevated in late onset Alzheimer disease (AD): neurogranin (Ng), synaptosomal-associated protein-25 (SNAP-25), visinin-like protein 1 (VILIP-1) and chitinase-3-like protein 1 (YKL-40).
METHODS:
CSF Ng, SNAP-25, VILIP-1 and YKL-40 were measured in families carrying autosomal dominant AD (ADAD) mutations.
RESULTS:
The four emerging CSF biomarkers were significantly elevated in the mutation carriers (n=235) versus non-carriers (n=145). CSF SNAP-25, VILIP-1 and YKL-40 were altered very early in the AD time course, approximately 15-19 years before estimated symptom onset. All CSF biomarkers predicted important AD-related outcomes including performance on a cognitive composite, brain amyloid burden as measured by amyloid positron emission tomography and the estimated years from symptom onset.
DISCUSSION:
Early abnormalities in CSF tTau, pTau, SNAP-25, VILIP-1 and YKL-40 suggest that synaptic damage, neuronal injury and neuroinflammation begin shortly after the commencement of brain amyloid accumulation.
Keywords: cerebrospinal fluid, biomarkers, dementia, Alzheimer disease, autosomal dominant Alzheimer disease, neuroinflammation
1. Background
Alzheimer disease (AD) is the most common cause of dementia in older adults [1] and is characterized neuropathologically by extracellular amyloid plaques comprised primarily of amyloid β-peptide (Aβ), intraneuronal neurofibrillary tangles comprised primarily of the microtubule-associated protein tau, neuronal dysfunction and eventual cell death. Neuropathological and biomarker findings demonstrate that the pathological changes of AD begin more than 10-20 years before the onset of dementia symptoms in the asymptomatic, preclinical phase of the disease [2-5]. Cerebrospinal fluid (CSF) biomarkers of AD are used to diagnose individuals with AD brain pathology in clinical and research settings and are altered both in preclinical and symptomatic AD [6-8]. The best-established CSF biomarkers for AD are the concentrations of the 42 amino acid form of the amyloid-β peptide (Aβ42), total tau (tTau), and phosphorylated tau181 (pTau) [6].
Less well-studied but promising “emerging” CSF biomarkers of other disease processes have been described that are altered in individuals with symptomatic AD compared to controls, including neurogranin (Ng) [9-16], synaptosomal-associated protein-25 (SNAP-25) [17], visinin-like protein 1 (VILIP-1) [18-20] and chitinase-3-like protein 1, also known as YKL-40 [9, 21-23]. Neurogranin is a post-synaptic calmodulin-binding protein that is enriched in dendritic spines and modulates synaptic plasticity and long-term potentiation [24-26]. SNAP-25 is an essential component of the sensitive factor attachment protein receptor (SNARE) complex, which initiates fusion of synaptic vesicles to the pre-synaptic membrane [27]. Elevated CSF levels of Ng and SNAP-25 are thought to reflect synaptic damage. VILIP-1 is a neuronal calcium sensor protein, and elevated levels may be a marker of neuronal injury [28]. YKL-40 is a secreted glycoprotein that, in the brain, is mainly expressed by astrocytes. It is elevated in a variety of neurological conditions including stroke and multiple sclerosis and is thought to be a general marker of neuroinflammation [29].
In this study, we evaluated these four emerging CSF biomarkers, as well as CSF Aβ42, Aβ42/Aβ40, tTau and pTau, in families with fully-penetrant autosomal dominant Alzheimer Disease (ADAD) mutations. Study of biomarkers in ADAD families is uniquely informative because the age of symptom onset for mutation carriers is predicted by the age at symptom onset in affected family members and the specific ADAD mutation [30, 31]. The estimated years from expected symptom onset (EYO) is calculated as the age of an individual minus the individual’s expected age at symptom onset. Studying biomarkers as a function of EYO allows investigators to determine how biomarkers change over the entire AD time course, including the long preclinical phase [4, 30, 31]. We first examined when the emerging CSF biomarkers became altered in the AD time course. Next, we evaluated how the established and emerging CSF biomarkers, individually and in combination, predicted performance on a cognitive composite and brain amyloid burden as measured by amyloid positron emission tomography (PET). Finally, we assessed whether CSF biomarkers predicted EYO and, therefore, could be useful in staging AD.
2. Methods
2.1. Participants
Individuals with a first-degree family member with a known mutation in Presenilin 1 (PSEN1), Presenilin 2 (PSEN2), or Amyloid Precursor Protein (APP) were enrolled in the Dominantly Inherited Alzheimer Network (DIAN) observational study at one of fourteen sites in the USA, UK, Germany, and Australia. Participants with genetic, clinical, CSF biomarker and neuroimaging data that passed quality control from the 12th semiannual data freeze (last data from June 30, 2017) were included in the analyses. Eighteen individuals carrying the APP Dutch and Flemish mutations were excluded because they manifest a different clinical syndrome (severe cerebral amyloid angiopathy). The institutional review board at Washington University (St. Louis, MO, USA) provided human studies approval. Participants or their caregivers provided written informed consent in accordance with the Declaration of Helsinki and their local institutional review board.
Clinical, biomarker and imaging visits were performed every three years for asymptomatic individuals younger than three years from their familial age of dementia onset, and every year for individuals who either were three or fewer years from their familial age of dementia onset or had dementia symptoms.
2.2. Procedures
Clinical dementia status was assessed using the Clinical Dementia Rating (CDR) scale, which also yields the Clinical Dementia Rating-Sum of Boxes (CDR-SB) [32]. Clinicians assigning the CDR were blinded to the mutation status of participants. The Mini-Mental State Examination (MMSE) [33] was completed as a part of the clinical assessment. The DIAN cognitive composite consists of the delayed recall score from the DIAN Word List Test, the Logical Memory delayed recall score from the Wechsler Memory Scale-Revised (WMS-R), the Digit Symbol Coding test total score from the Wechsler Adult Intelligence Scale-Revised, and the MMSE total score [34]. Scores from each test were converted to z-scores using the baseline mean and standard deviation of non-carriers and averaged to form a composite z-score.
The presence or absence of an ADAD mutation was determined using PCR-based amplification of the appropriate exon followed by Sanger sequencing [4]. The participant's estimated years from expected symptom onset (EYO) was defined as the participant’s age minus their estimated age at symptom onset [4]. The estimated age at symptom onset was based on an individual’s actual age of symptom onset if they were a symptomatic mutation carrier (MC), as well as the age at symptom onset for family members. For the asymptomatic MC and the non-carriers (NC), the estimated age at expected symptom onset was calculated based on the participant's age relative to either the family mutation-specific expected age at dementia onset [30] or parental age at first progressive cognitive decline if the expected age at symptom onset for the mutation was unknown.
CSF was collected in accordance with the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocols. Briefly, ~15 mL CSF was collected in polypropylene tubes at 8:00 am following overnight fasting and was immediately frozen on dry ice. Frozen samples were shipped to the DIAN Biomarker Core, thawed, aliquoted (0.5 ml), flash-frozen and stored at − 84°C until analysis.
2.3. CSF biomarker assays
The assays used for Ng, SNAP-25, and VILIP-1 have been described in detail elsewhere [35]. Briefly, these analytes were measured with microparticle-based immunoassays using Single Molecule Counting [SMC]™ technology originally developed for the Erenna® System by Singulex® and now part of EMD Millipore (Burlington, MA), and employed antibodies developed in the laboratory of Dr. Jack Ladenson at Washington University in St. Louis. YKL-40 was measured with a plate-based enzyme-linked immunoassay (MicroVue ELISA; Quidel, San Diego, CA) [36]. Within-person longitudinal samples were run on the same assay plate.
Aβ42, Aβ40, tTau, and pTau were measured with the corresponding Elecsys immunoassays on the automated Roche cobas e 601 analyzer in the laboratory of Leslie Shaw in the ADNI Biomarker Core at the University of Pennsylvania as previously described [37, 38]. The Elecsys immunoassays are electrochemiluminescence immunoassays employing a quantitative sandwich principle. A single lot of immunoassay reagents for each analyte was used to measure all samples.
2.4. Brain imaging
Structural brain MRI was performed according to the ADNI protocol [39] and is described in detail elsewhere [40]. T1-weighted images were acquired on a 3 Tesla scanner. Volumetric segmentation and cortical surface reconstruction were performed using FreeSurfer 5.3 and subcortical volumes were corrected for intracranial volume using a regression approach. Cortical thickness and volume measures were averaged across hemispheres.
Amyloid PET was performed with 11C-Pittsburgh Compound B (11C-PiB) as previously described [40]. Data was converted to regional standardized uptake value ratios (SUVRs) relative to the cerebellar grey matter and partial volume corrected using a regional spread function technique.
2.5. Statistical analysis
The mean differences between the MC and NC were calculated at each EYO point and standardized [4]. Results were inverted (multiplied by negative 1) for measures where lower levels represent abnormality (Aβ42/Aβ40, Aβ42, MMSE, cognitive composite, hippocampal volume, precuneus thickness and entorhinal cortical thickness) so that the degree of abnormality could be compared more directly. Correlations between the baseline values of CSF biomarkers were estimated using bivariate linear mixed effects (LME) models treating families as random effects and adjusting for covariates including baseline age, sex, years of education and APOE ε4 status [41].
To determine the EYO at which the MC first became significantly different from the NC in the baseline level and rate of intra-individual change for each measure, linear or linear spline mixed effects models with one knot (depending on which model better fit the data) were used to evaluate the intra-individual rate of change [42]. The mutation group (MC or NC), baseline EYO, time, and all possible two-way or three-way interactions among them were included as fixed effects. Sex, years of education, and APOE ε4 status were considered as covariates, but only those effects that were significant were retained in the models. Random slopes and intercepts for each individual with an unstructured covariance matrix were included to account for the within-subject correlation due to repeated measures, and random intercepts were used to account for correlation within the familial cluster. The adjusted difference in the mean level at baseline and difference in the rate of change between the MC and NC was tested using the approximate t-test derived from the models to determine the first EYO point where the difference became significant.
To evaluate the ability of baseline CSF biomarkers individually or in combination to predict outcomes, LME models with random intercepts for familial cluster were utilized. For each outcome measure, a base model was built using significant predictors among potential covariates: baseline age, sex, years of education and APOE ε4 status. Baseline CSF biomarkers were then added to the model as predictors one at a time, then with Aβ42, then with Aβ42 and pTau. The significance of the coefficients for the CSF biomarker(s) were used to determine their prediction performance, in addition to the predictors in the base models and the retained CSF biomarkers.
To prevent inadvertent disclosure of mutation status to research participants, plots do not include the EYO scale, points from EYO outliers are omitted, and points after three data collections are not shown. However, all data was included in the analyses. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC). A p-value <0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of study participants
The baseline characteristics of the study participants are detailed in Table 1. Notably, the 235 mutation carriers (MC) included individuals expected to have widely varying levels of AD brain pathology, while the 145 non-carriers (NC) consisted mainly of cognitively normal individuals expected to have no or very little AD brain pathology due to their relatively young age. At their baseline CSF collection, the MC had a CSF profile consistent with AD brain pathology, with lower Aβ42 and Aβ42/Aβ40 and higher tTau and pTau compared to the NC (p<0.0001). The emerging CSF biomarkers (Ng, SNAP-25, VILIP-1 and YKL-40) were all higher in the MC versus the NC (p<0.0001). Most brain imaging, clinical, and cognitive measures performed within one year of the baseline CSF collection also demonstrated significant abnormalities in the MC compared to NC.
Table 1. Characteristics of study participants at baseline.
Continuous measures are presented as the mean ± standard deviation. For each type of measure, the number of individuals with at least one measurement is provided. The significance of the difference between the MC and NC was calculated by linear mixed effects models with random intercepts for familial clusters.
| Characteristic | All (n=380) |
Mutation carrier (MC) (n=235) |
Mutation Non- carrier (NC) (n=145) |
p-value |
|---|---|---|---|---|
| Estimated years from symptom onset | −8.2 ± 11.6 | −7.6 ± 10.8 | −9.2 ± 12.7 | N.S. |
| Participants with longitudinal CSF, n (%) | 160 (42%) | 105 (44%) | 55 (38%) | N.S. |
| Interval between longitudinal LPs (years) | 2.9 ± 1.4 | 2.8 ± 1.4 | 3.20 ± 1.5 | N.S. |
| Age (years) | 38.6 ± 11.1 | 38.4 ± 10.4 | 38.8 ± 12.1 | N.S. |
| Sex, n (% female) | 216 (57%) | 127 (54%) | 89 (61%) | N.S. |
| Education (years) | 14.6 ± 2.9 | 14.3 ± 3.1 | 14.9 ± 2.5 | N.S. |
| Presence of APOE ε4 allele, n (%) | 121 (32%) | 72 (31%) | 49 (34%) | N.S. |
| Established CSF biomarkers (n = 380) | ||||
| Aβ42 (pg/ml) | 1123 ± 605 | 961 ± 623 | 1385 ± 471 | <0.0001 |
| Aβ42/Aβ40 | 0.07 ± 0.03 | 0.06 ± 0.03 | 0.09 ± 0.01 | <0.0001 |
| tTau (pg/ml) | 243.6 ± 141.9 | 287.9 ± 159.1 | 171.6 ± 58.5 | <0.0001 |
| pTau (pg/ml) | 24.5 ± 19.8 | 30.8 ± 22.7 | 14.2 ± 5.3 | <0.0001 |
| Emerging CSF biomarkers (n = 358) | ||||
| Ng (pg/ml) | 2008 ± 1096 | 2269 ± 1189 | 1572 ± 741 | <0.0001 |
| SNAP-25 (pg/ml) | 4.3 ± 1.8 | 4.6 ± 1.9 | 3.7 ± 1.3 | <0.0001 |
| VILIP-1 (pg/ml) | 158.2 ± 71.5 | 173.4 ± 77.9 | 132.9 ± 50.2 | <0.0001 |
| YKL-40 (ng/ml) | 159.3 ± 83.4 | 173.1 ± 88.7 | 136.4 ± 68.2 | <0.0001 |
| Amyloid PET (n = 319) | ||||
| Amyloid PET SUVR | 1.67 ± 1.00 | 2.06 ± 1.12 | 1.06 ± 0.17 | <0.0001 |
| Structural brain measures (n = 356) | ||||
| Hippocampal volume (mm3) | 8492 ± 1054 | 8310 ± 1201 | 8779 ± 676 | <0.0001 |
| Entorhinal cortical thickness (mm) | 3.44 ± 0.34 | 3.43 ± 0.35 | 3.46 ± 0.32 | N.S. |
| Precuneus thickness (mm) | 2.31 ± 0.2 | 2.27 ± 0.23 | 2.38 ± 0.14 | <0.0001 |
| Clinical measures (n = 380) | ||||
| CDR>0, n (%) | 89 (23%) | 83 (35%) | 6 (4%) | <0.0001 |
| CDR-SB | 0.86 ± 2.33 | 1.37 ± 2.84 | 0.04 ± 0.24 | <0.0001 |
| Cognitive measures (n = 380) | ||||
| DIAN cognitive composite | −0.37 ± 0.92 | −0.60 ± 1.03 | −0.02 ± 0.59 | <0.0001 |
| MMSE | 27.6 ± 4.4 | 26.7 ± 5.3 | 29.1 ± 1.2 | <0.0001 |
Abbreviations: Aβ40, amyloid-β 40; Aβ42, amyloid-β 42; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating, Sum of Boxes; CSF, cerebrospinal fluid; DIAN, Dominantly Inherited Alzheimer Network; LP, lumbar puncture; MMSE, Mini-Mental State Examination; Ng, neurogranin; N.S., not significant; PET, positron emission tomography; pTau, phosphorylated tau181; SNAP-25, synaptosomal-associated protein-25; SUVR, standardized uptake value ratio; tTau, total tau; VILIP-1, visinin-like protein 1; YKL-40, chitinase-3-like protein 1.
3.2. CSF, imaging, clinical and cognitive measures by EYO
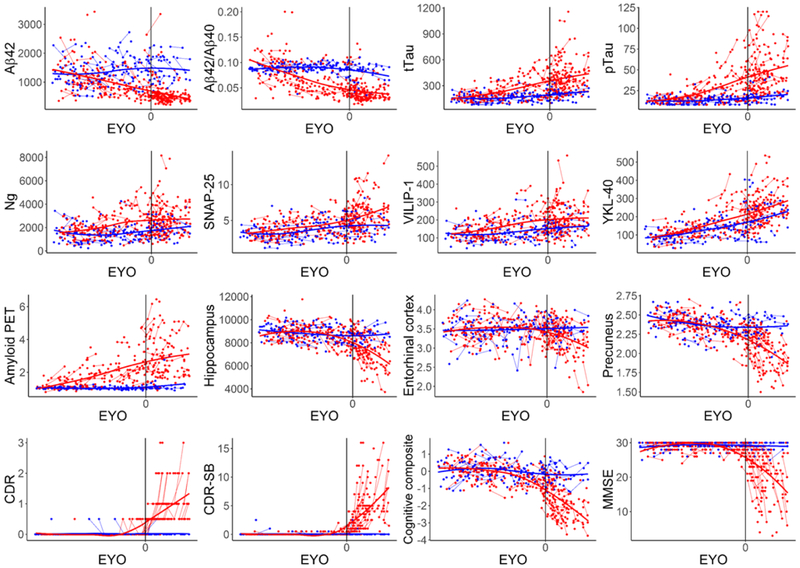
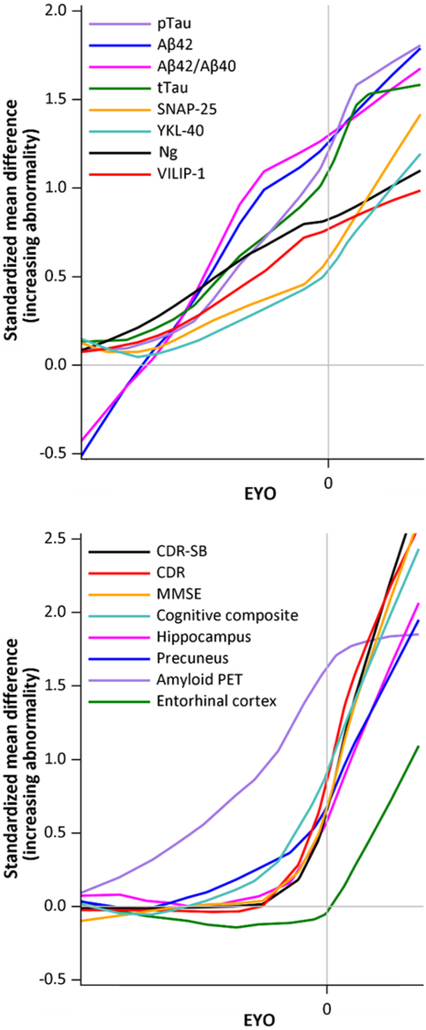
The unadjusted (not adjusted for covariates) levels of all CSF, imaging, clinical and cognitive measures as a function of EYO in the MC and NC were examined (Fig. 1). To visualize the order of biomarker changes, the difference between the MC and NC for the baseline unadjusted standardized levels of the measures was calculated and plotted as a function of EYO, where the y-axis indicates the degree of abnormality (Fig. 2). These plots suggest that amyloid PET as well as the established and emerging CSF biomarkers all change many years prior to the estimated age at expected symptom onset. Because ADAD mutations cause the over-production of Aβ42, CSF Aβ42 levels were higher in the MC versus the NC prior to significant amyloid deposition in the brain, but as brain amyloid burden increased, Aβ42 levels in the MC became equivalent and then lower in the MC versus the NC. The structural brain, clinical, and cognitive measures appear to change shortly before the onset of symptoms (Fig. 2, bottom panel).
Fig. 1. CSF, imaging, clinical and cognitive measures as a function of EYO in the ADAD mutation carriers and non-carriers.
The unadjusted levels of each measure are shown as a function of EYO. Values for the mutation carriers are shown in red and values for the noncarriers are shown in blue. Each point represents one measurement. Thin lines between points connect measurements within the same individual. Thick lines are the LOESS curves for all values (red for the mutation carriers and blue for the non-carriers) and are depicted to aid with visualization of trends in the data. For Aβ42, tTau, pTau, Ng, SNAP-25, and VILIP-1, units are in pg/ml. For YKL-40, units are in ng/ml. For amyloid PET, units are in mean cortical SUVR. For hippocampus, units are in mm3. For entorhinal cortex and precuneus, units are in mm. The cognitive composite is a Z-score, with a mean of 0 and units in standard deviations from the mean. CDR, CDR-SB and MMSE are the raw test score. To prevent inadvertent disclosure of mutation status to research participants, the plot does not include the EYO scale, points from EYO outliers were omitted, and points after >3 data collections are not shown.
Fig. 2. Comparison of baseline CSF, imaging, clinical and cognitive measures as a function of EYO.
The difference between values in the ADAD mutation carriers and non-carriers for each unadjusted standardized measure at baseline is shown as a function of EYO. The y-axis represents the degree of abnormality consistent with AD pathology so that trajectories are more comparable. Lines are LOESS curves for the values. To prevent inadvertent disclosure of mutation status to research participants, plots do not include the EYO scale.
3.3. Divergence in baseline levels and rates of change for CSF, imaging, clinical and cognitive measures
Linear mixed effects models were used to determine the EYO when CSF, imaging, clinical and cognitive measures first became significantly more abnormal in the MC versus the NC (Table 2). Baseline brain amyloid burden by amyloid PET became significantly higher in the MC versus the NC at EYO −22. Baseline CSF Aβ42/Aβ40 and Aβ42 became significantly lower in the MC versus the NC at EYO −17 and −14, respectively. Baseline levels of CSF tTau, pTau, SNAP-25, VILIP-1 and YKL-40 became significantly higher in the MC versus the NC at EYO −19 to −15. In contrast, baseline structural brain measures did not become significantly smaller and baseline clinical and cognitive measures did not become significantly worse in the MC versus the NC until just prior to symptom onset, at EYO −3 to 0. There was no EYO at which baseline CSF Ng was significantly higher or entorhinal cortical thickness was significantly lower in the MC versus the NC because of high variance in values.
Table 2. Divergence in measures between the ADAD mutation carriers and non-carriers.
Estimated differences between the ADAD mutation carriers (MC) and non-carriers (NC) in the baseline level and intra-individual rate of change for each measure were generated from linear or linear spline mixed effects models. The fixed effects included in the models were baseline EYO (which was highly correlated with baseline age), mutation status (MC or NC), time, and all possible two-way or three-way interactions among the fixed effects. Sex, years of education, and APOE ε4 status were considered as covariates, but only those covariates that were significant were retained in the final models. Random effects included random intercepts for familial clusters as well as individual random intercept and slope for repeated measures. Baseline measures were differences in the mean level ± standard error. Longitudinal measures were differences in the annualized rate of change ± standard error. Only significant differences (p<0.05) are shown below. If the baseline level or intra-individual rate of change did not vary significantly between the MC and NC, the time of divergence is reported as N.A. (not applicable) for that measure.
| Baseline level | Intra-individual annual rate of change |
|||
|---|---|---|---|---|
| Characteristic | Time of divergence (EYO) |
Difference in mean level (MC-NC) |
Time of divergence (EYO) |
Difference in rate of change (MC-NC) |
| Established CSF biomarkers | ||||
| tTau (pg/ml) | −19 | 35.7 ± 15.3 | N.A. | N.A. |
| pTau (pg/ml) | −18 | 4.91 ± 2.03 | N.A. | N.A. |
| Aβ42/Aβ40 | −17 | −0.006 ± 0.003 | −25 | −0.005 ± 0.0008 |
| Aβ42 (pg/ml) | −14 | −210 ± 94 | −19 | −39.3 ± 19.2 |
| Emerging CSF biomarkers | ||||
| VILIP-1 (pg/ml) | −18 | 16.9 ± 8.3 | −25 | 7.47 ± 2.69 |
| SNAP-25 (pg/ml) | −15 | 0.43 ± 0.18 | N.A. | N.A. |
| YKL-40 (ng/ml) | −15 | 16.1 ± 7.7 | N.A. | N.A. |
| Ng (pg/ml) | N.A. | N.A. | N.A. | N.A. |
| Amyloid PET | ||||
| Amyloid PET SUVR | −22 | 0.19 ± 0.09 | −23 | 0.04 ± 0.02 |
| Structural brain measures | ||||
| Precuneus thickness (mm) | −3 | −0.049 ± 0.022 | −4 | −0.021 ± 0.007 |
| Hippocampal volume (mm3) | −1 | −449 ± 140 | −2 | −126 ± 44 |
| Entorhinal cortical thickness (mm) | N.A. | N.A. | −1 | −0.057 ± 0.028 |
| Clinical measures | ||||
| CDR-SB | 0 | 0.70 ± 0.26 | −11 | 0.215 ± 0.100 |
| CDR | −2 | 0.13 ± 0.05 | −2 | 0.060 ± 0.025 |
| Cognitive measures | ||||
| DIAN cognitive composite | −3 | −0.33 ± 0.10 | −3 | −0.102 ± 0.033 |
| MMSE | −2 | −1.77 ± 0.60 | −12 | −0.34 ± 0.16 |
Abbreviations: Aβ40, amyloid-β 40; Aβ42, amyloid-β 42; ADAD, autosomal dominant Alzheimer disease; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating, Sum of Boxes; CSF, cerebrospinal fluid; DIAN, Dominantly Inherited Alzheimer Network; EYO, estimated years from expected symptom onset; LP, lumbar puncture; MC, mutation carriers; MMSE, Mini-Mental State Examination; N.A., not applicable; NC, mutation non-carriers; Ng, neurogranin; PET, positron emission tomography; pTau, phosphorylated tau181; SNAP-25, synaptosomal-associated protein-25; SUVR, standardized uptake value ratio; tTau, total tau; VILIP-1, visinin-like protein 1; YKL-40, chitinase-3-like protein 1.
The intra-individual rates of change for the CSF, imaging, clinical and cognitive measures as a function of EYO became significantly different in the MC versus the NC at some point for most measures (Table 2, see Supplementary Table 1 for detailed analyses on rates of change as a function of EYO). CSF Aβ42/Aβ40 and Aβ42 began declining, and VILIP-1 levels began increasing more rapidly, in the MC versus the NC very early in the AD time course, at EYO −25, −19, and −25, respectively. Given the complexity of Aβ42 levels in the MC at early EYOs due to mutation related over-production, the rate of change of Aβ42 or Aβ42/Aβ40, rather than the absolute level, may be a more reliable indicator of when Aβ42 levels start to change in the MC. Around the same time that the rates of change of CSF biomarkers became different in the two groups, the MC began accumulating brain amyloid more quickly than the NC as measured by amyloid PET (EYO −23). The first changes in clinical and cognitive measures were observed around EYO −12 or −11, but this may be an artifact of the ceiling effect and floor effect of MMSE and CDR-SB, respectively, since at this EYO nearly all individuals scored at or near the maximum score for MMSE and minimum score for CDR-SB. The MC did not develop significantly faster rates of brain atrophy or more rapid decline on the DIAN cognitive composite or global CDR versus the NC until shortly before symptom onset (EYO −4 to −1).
3.4. Baseline CSF biomarkers as predictors of imaging, clinical and cognitive measures
The ability of baseline levels of CSF biomarkers to predict baseline levels and intra-individual rates of change of structural brain, clinical and cognitive measures was evaluated. In the NC, baseline CSF biomarker levels did not significantly predict either the baseline levels or rates of change of any of the imaging, clinical or cognitive measures (data not shown). However, in the MC, most CSF biomarkers were highly predictive of baseline values for most measures, including the DIAN cognitive composite, amyloid PET SUVR, and hippocampal volume (Table 3, see Supplementary Table 2 for other imaging, clinical and cognitive measures). Additionally, most CSF biomarkers were highly predictive of the rate of change of structural brain, clinical, and cognitive measures; the rate of change of amyloid PET was an exception and was significantly predicted by only tTau, pTau and Ng (Table 3).
Table 3. Baseline CSF biomarkers as predictors of the difference between the ADAD mutation carriers and non-carriers in the baseline level and intra-individual rate of change of imaging, clinical and cognitive measures.
Linear mixed effects models were used to estimate the amount of change in imaging, clinical and cognitive measures per unit change in CSF biomarkers. Fixed effects included in the models were biomarker baseline level, mutation status (MC or NC), time, and all possible two-way or three way-interaction among the fixed effects. Baseline age, sex, years of education, APOE ε4 status and their interaction with mutation status were considered as covariates, but only those covariates that were significant were retained in the models. Random effects included random intercepts for familial clusters, as well as individual random intercept and slope for repeated measures. The results for entorhinal cortical thickness, precuneus thickness, CDR, CDR-SB and MMSE are shown in Supplementary Table 2.
| Baseline level | Aβ42 (pg/ml) |
Aβ42/Aβ40 | tTau (pg/ml) |
pTau (pg/ml) |
Ng (pg/ml) |
SNAP-25 (pg/ml) |
VILIP-1 (pg/ml) |
YKL-40 (ng/ml) |
|
|---|---|---|---|---|---|---|---|---|---|
| DIAN cognitive composite | Estimate | 0.0003 | 6.9 | −0.002 | −0.01 | −0.0001 | −0.1 | −0.002 | −0.003 |
| S.E. | 0.00008 | 1.6 | 0.0003 | 0.002 | 0.00004 | 0.03 | 0.0006 | 0.0007 | |
| p-value | 0.0002 | <0.0001 | <0.0001 | <0.0001 | 0.003 | <0.0001 | 0.0009 | 0.0004 | |
| Amyloid PET SUVR | Estimate | −0.0007 | −11.9 | 0.001 | 0.01 | 0.0001 | 0.05 | 0.002 | 0.003 |
| S.E. | 0.00009 | 1.7 | 0.0004 | 0.003 | 0.00005 | 0.03 | 0.0008 | 0.0008 | |
| p-value | <0.0001 | <0.0001 | 0.0007 | 0.0001 | 0.04 | N.S. | 0.004 | 0.0003 | |
| Hippocampal volume (mm3) | Estimate | 0.2 | 4097.2 | −2.2 | −16.4 | −0.2 | −97.1 | −2.3 | −4.6 |
| S.E. | 0.1 | 2235.5 | 0.4 | 3.1 | 0.06 | 39.2 | 0.9 | 0.9 | |
| p-value | 0.08 | 0.07 | <0.0001 | <0.0001 | 0.008 | 0.02 | 0.01 | <0.0001 | |
| Rate of change | Aβ42 (pg/ml) |
Aβ42/Aβ40 | tTau (pg/ml) |
pTau (pg/ml) |
Ng (pg/ml) |
SNAP-25 (pg/ml) |
VILIP-1 (pg/ml) |
YKL-40 (ng/ml) |
|
| DIAN cognitive composite | Estimate | 0.00009 | 1.7 | −0.0007 | −0.005 | −0.00007 | −0.04 | −0.0009 | −0.0009 |
| S.E. | 0.00003 | 0.6 | 0.0001 | 0.0009 | 0.00002 | 0.01 | 0.0003 | 0.0002 | |
| p-value | 0.008 | 0.004 | <0.0001 | <0.0001 | 0.007 | 0.0003 | 0.007 | 0.0003 | |
| Amyloid PET SUVR | Estimate | −0.00002 | −0.2 | 0.0003 | 0.002 | 0.00004 | −0.005 | 0.0003 | 0.00003 |
| S.E. | 0.00002 | 0.4 | 0.0001 | 0.0006 | 0.00001 | 0.009 | 0.0002 | 0.0002 | |
| p-value | N.S. | N.S. | 0.002 | 0.009 | 0.002 | N.S. | N.S. | N.S. | |
| Hippocampal volume (mm3) | Estimate | 0.2 | 2662.4 | −0.9 | −6.1 | −0.09 | −66.1 | −1.3 | −1.6 |
| S.E. | 0.03 | 588.5 | 0.1 | 0.9 | 0.02 | 13.8 | 0.3 | 0.2 | |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
Abbreviations: Aβ40, amyloid-β 40; Aβ42, amyloid-β 42; ADAD, autosomal dominant Alzheimer disease; CSF, cerebrospinal fluid; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating, Sum of Boxes; DIAN, Dominantly Inherited Alzheimer Network; MMSE, Mini-Mental State Examination; Ng, neurogranin; N.S., not significant; PET, positron emission tomography; pTau, phosphorylated tau181; S.E., standard error; SNAP-25, synaptosomal-associated protein-25; SUVR, standardized uptake value ratio; tTau, total tau; VILIP-1, visinin-like protein 1; YKL-40, chitinase-3-like protein 1.
3.5. Combinations of CSF biomarkers as predictors of cognitive performance, brain amyloid burden and EYO
Pair-wise correlations between the baseline CSF biomarkers in the MC were determined (Table 4). The correlation between CSF Aβ42 and the other CSF biomarkers was the lowest, suggesting that Aβ42 may be the least redundant with the other biomarkers. The ability of combinations of baseline CSF biomarkers to predict the baseline DIAN cognitive composite and amyloid PET SUVR was evaluated in the MC. These two measures were chosen for further analyses because they were strongly predicted by CSF biomarkers and were well modeled by linear relationships. Baseline levels of all CSF biomarkers individually predicted the baseline DIAN cognitive composite in the MC (Supplemental Table 3). In combination with Aβ42, all other CSF biomarkers (tTau, pTau, Ng, SNAP-25, VILIP-1 or YKL-40) improved prediction of the baseline DIAN cognitive composite. When combined with both Aβ42 and pTau, Ng or SNAP-25 further improved prediction significantly, although it was a very small improvement (adjusted R2 of 0.566 and 0.572, respectively, versus 0.555).
Table 4. Correlations between baseline CSF biomarkers in ADAD mutation carriers.
The upper number in the cell is the correlation coefficient (ρ) estimated using a bivariate linear mixed model that accounts for familial clusters. The lower number in the cell is the significance of the correlation. Calculations of significance were adjusted for baseline age, years of education, sex and APOE ε4 status.
| tTau | pTau | Ng | VILIP-1 | SNAP-25 | YKL-40 | Aβ42/Aβ40 | Aβ42 | |
|---|---|---|---|---|---|---|---|---|
| tTau | 1 | 0.955 | 0.868 | 0.832 | 0.731 | 0.616 | −0.690 | −0.426 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| pTau | 1 | 0.774 | 0.744 | 0.681 | 0.587 | −0.698 | −0.498 | |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||
| Ng | 1 | 0.844 | 0.655 | 0.568 | −0.605 | −0.222 | ||
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.009 | ||||
| VILIP-1 | 1 | 0.744 | 0.567 | −0.515 | −0.157 | |||
| <0.0001 | <0.0001 | <0.0001 | 0.08 | |||||
| SNAP-25 | 1 | 0.479 | −0.579 | −0.355 | ||||
| <0.0001 | <0.0001 | <0.0001 | ||||||
| YKL-40 | 1 | −0.686 | −0.516 | |||||
| <0.0001 | <0.0001 | |||||||
| Aβ42/Aβ40 | 1 | 0.817 | ||||||
| <0.0001 | ||||||||
| Aβ42 | 1 |
Abbreviations: Aβ40, amyloid-β 40; Aβ42, amyloid-β 42; ADAD, autosomal dominant Alzheimer disease; CSF, cerebrospinal fluid; EYO, estimated years from expected symptom onset; Ng, neurogranin; pTau, phosphorylated tau181; SNAP-25, synaptosomal-associated protein-25; tTau, total tau; VILIP-1, visinin-like protein 1; YKL-40, chitinase-3-like protein 1.
In the MC, the baseline amyloid PET SUVR was predicted by all baseline CSF biomarkers except Ng or SNAP-25 (Supplementary Table 4). Prediction of baseline amyloid PET SUVR was improved by the combination of CSF Aβ42 with either tTau, pTau, VILIP-1 or YKL-40 (adjusted R2 of 0.314, 0.316, 0.332, and 0.328, respectively, versus 0.296). The combination of CSF Aβ42, pTau and a third CSF biomarker was no better at predicting amyloid PET SUVR than the combination of Aβ42 and pTau.
The ability of baseline CSF biomarkers to predict EYO was also examined (Supplementary Table 5). EYO was significantly predicted by Aβ42, Aβ42/Aβ40, tTau, pTau, Ng, SNAP-25, or VILIP-1, but not YKL-40. In combination with Aβ42, addition of tTau, Ng, SNAP-25 or VILIP-1 significantly improved prediction of EYO. When combined with Aβ42 and pTau, no additional biomarker improved prediction of EYO.
4. Discussion
Despite some data suggesting that amyloid PET positivity lags behind abnormalities in CSF biomarkers [43], we found that amyloid PET SUVR changes a small but measurable amount very early in the AD time course (EYO −22), shortly before absolute levels of the established CSF biomarkers (Aβ42, tTau and pTau) show significant alterations. In this study, the EYO at which the established CSF biomarkers became significantly more abnormal in the MC versus the NC (EYO −19 to −14) was approximately four years earlier than in previous reports [4, 42, 44-46], likely because of increased precision with the automated Elecsys immunoassays and increased statistical power due to the larger number of samples analyzed. Interestingly, CSF SNAP-25, VILIP-1 and YKL-40 also changed very early in the AD time course (EYO −18 to −15). These findings suggest that the processes reflected by these emerging CSF biomarkers, as well as by CSF tTau and pTau, and which include synaptic damage, neuronal injury and neuroinflammation, begin at or shortly after the commencement of amyloid accumulation as defined by amyloid PET. In contrast to hypothetical models that propose a significant lag period between amyloid accumulation and neuronal injury [47], amyloid deposition appears to be associated with other pathological changes almost immediately.
One major limitation of our study is that findings in ADAD may not be generalizable to typical sporadic late onset AD (LOAD). This is particularly true for findings involving CSF Aβ42 because ADAD mutations cause over-production of Aβ42 [48]. In a LOAD cohort the differences between CSF biomarkers in individuals with and without AD brain pathology may be different because of the increased prevalence of other neurological diseases or conditions in older individuals that may alter CSF biomarker levels. Another limitation is that we did not evaluate all promising CSF biomarkers such as neurofilament light chain (NfL) or soluble triggering receptor expressed on myeloid cells 2 (sTREM2) [49, 50], which have been measured [49] or are in the process of being measured in baseline samples from a smaller cohort of families with ADAD. Finally, some of the results, especially the EYO at which measures became significantly different in the MC versus the NC, are sensitive to a variety of factors including measurement precision, power (number of measurements), and statistical modeling approach, and therefore different studies may reach different conclusions about the exact timing of biomarker changes.
Despite these limitations, our work has important implications for the field. We have found that CSF Ng, SNAP-25, VILIP-1 and YKL-40 are all significantly altered in ADAD mutation carriers and predict important AD-related outcomes measures. Relative to a single emerging CSF biomarker, the combination of one emerging CSF biomarker with CSF Aβ42 often improved prediction of performance on a cognitive composite or brain amyloid burden as defined by amyloid PET. In this side-by-side comparison, one emerging biomarker was not consistently superior in the prediction of a variety of outcome measures. Instead, all four of the emerging CSF biomarkers could potentially substitute for tTau and pTau as markers of AD brain pathology in studies where tau-related biomarkers may be directly affected (e.g. drug trials of anti-tau antibodies where CSF biomarkers are outcome measures). Finally, a unique strength of studying biomarkers in ADAD is that it provides reliable estimates of where individuals are located in the AD time course (estimated years from symptom onset, EYO). Importantly, we found that CSF biomarkers predict EYO. Clinical trials for AD, particularly prevention trials, may benefit from including AD stage as estimated by CSF or imaging biomarkers as a factor in their analyses because different drugs may be efficacious at different stages of the disease.
Supplementary Material
Highlights.
Ng, SNAP-25, VILIP-1 and YKL-40 are emerging CSF biomarkers of Alzheimer disease
The emerging CSF biomarkers are elevated in autosomal dominant Alzheimer disease
CSF SNAP-25, VILIP-1 and YKL-40 increase 15-19 years before estimated symptom onset
The emerging CSF biomarkers predict Alzheimer disease-related outcomes
CSF biomarkers predict estimated years from symptom onset in ADAD mutation carriers
Research in Context.
Systematic review:
Literature was reviewed on the association of late onset Alzheimer disease (AD) and CSF levels of neurogranin (Ng), synaptosomal-associated protein-25 (SNAP-25), visinin-like protein 1 (VILIP-1) and chitinase-3-like protein 1 (YKL-40).
Interpretation:
CSF SNAP-25, VILIP-1 and YKL-40 were significantly elevated in autosomal dominant AD mutation carriers approximately 15-19 years before estimated symptom onset, suggesting that the pathological processes reflected by these biomarkers begin very early in the AD time course. Additionally, all four CSF biomarkers predicted important AD-related outcomes including performance on a cognitive composite, brain amyloid burden as measured by amyloid positron emission tomography and the estimated years from symptom onset.
Future directions:
CSF Ng, SNAP-25, VILIP-1 and YKL-40 may be useful biomarkers of AD brain pathology in drug trials. CSF biomarkers may help stage preclinical AD, which could enable investigators to examine whether different therapies are more efficacious at different points in the AD time course.
Acknowledgments
We acknowledge Leona Fields, who performed the Roche Elecsys assays on the CSF samples. Funding for this study of the emerging CSF biomarkers (Ng, SNAP-25, YKL-40, and VILIP-1) in autosomal dominant Alzheimer disease was provided by Biogen, Cambridge MA. S.E.S. is supported by K23AG053426. Data collection and sharing for this project was supported by The Dominantly Inherited Alzheimer Network (DIAN, U19AG032438) funded by the National Institute on Aging (NIA), the German Center for Neurodegenerative Diseases (DZNE), Raul Carrea Institute for Neurological Research (FLENI), partial support by the Research and Development Grants for Dementia from Japan Agency for Medical Research and Development, AMED, and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI). This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications. We acknowledge the altruism of the participants and their families and contributions of the DIAN research and support staff at each of the participating sites for their contributions to this study.
Abbreviations
- Aβ40
amyloid-β 40
- Aβ42
amyloid-β 42
- AD
Alzheimer disease
- ADAD
autosomal dominant Alzheimer disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- CDR
Clinical Dementia Rating
- CDR-SB
Clinical Dementia Rating, Sum of Boxes
- CSF
cerebrospinal fluid
- DIAN
Dominantly Inherited Alzheimer Network
- EYO
estimated years from expected symptom onset
- LOAD
late onset Alzheimer disease
- LP
lumbar puncture
- MC
mutation carrier
- MMSE
Mini-Mental State Examination
- NC
mutation non-carrier
- Ng
neurogranin
- N.A.
not applicable
- N.S.
not significant
- PET
positron emission tomography
- pTau
phosphorylated tau181
- SNAP-25
synaptosomal-associated protein-25
- SUVR
standardized uptake value ratio
- tTau
total tau
- VILIP-l
visinin-like protein 1
- YKL-40
chitinase-3-like protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest
S.E.S. has a family member with stock in Eli Lilly, which is developing drugs for Alzheimer disease. D.L.G. is a full time employee at Biogen, which funded this study and is developing drugs for Alzheimer disease. L.M.S. receives research support from Eli Lilly, Hoffman LaRoche, MJFox Foundation for Parkinson’s Research for Biofind study and has served as consultant and/or advisory boards for Roche Diagnostics and Eli Lilly. He provides quality control oversight for Roche Elecsys immunoassays in the ADNI study. J.J.H. is on the advisory board and consults for both Biogen and Lundbeck A/S. T.L.S.B. consults for Eli Lilly and receives research funding from Avid Radiopharmaceuticals. J.L. reports personal fees from Aesku, Bayer Vital, the Willi Gross Foundation, Axon Neuroscience, and Ionis Pharmaceuticals. He has received non-financial support from AbbVie that is outside the submitted work. D.M.H. co-founded and is on the scientific advisory board of C2N Diagnostics. D.M.H. consults for Genentech, AbbVie, Eli Lilly, Proclara, and Denali. Washington University receives research grants to the lab of D.M.H. from C2N Diagnostics, Eli Lilly, AbbVie, and Denali. J.H.L. reports being named on patents related to the use of VILIP-1. These are being managed by Washington University in accordance with University policy. J.H.L. is a co-inventor on patent 11/630582 (2005) (Markers for brain damage) and patent 60957132 (2008) (Alzheimer’s diagnosis). J.C.M. has or is currently participating in clinical trials of anti-dementia drugs sponsored by Janssen Immunotherapy, Eli Lilly and Company, and Pfizer. He has served as a consultant for or has received speaking honoraria from Eisai, Esteve, Janssen Alzheimer Immunotherapy Program/Elan, GlaxoSmithKline, Novartis, and Pfizer. He receives research support from Eli Lilly/Avid Radiopharmaceuticals. R.J.B. co-founded and is on the scientific advisory board of C2N Diagnostics. He consults for Roche, Genentech, AbbVie, Pfizer, Boehringer-Ingelheim, and Merck. A.M.F. has received research funding from Biogen, Fujirebio and Roche Diagnostics. She is a member of the scientific advisory boards for Roche, Genentech and AbbVie and also consults for Araclon/Griffols and DiamiR. Y.L., K.W.T., E.M.H., R.L.H., J.D.G., G.W., J.Q.T., C.C., M.J., J.P.C., J.M.N., J.M.R., N.R.G-R., and C.X. have nothing to report.
References
- [1].Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80:1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Price J, Ko A, Wade M, Tsou S, McKeel D, Morris J. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer's disease. Arch Neurol. 2001;58:1395–402. [DOI] [PubMed] [Google Scholar]
- [3].Morris J, Price J. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–18. [DOI] [PubMed] [Google Scholar]
- [4].Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. The New England journal of medicine. 2012;367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7:280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Blennow K, Dubois B, Fagan AM, Lewczuk P, de Leon MJ, Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. The Lancet Neurology. 2003;2:605–13. [DOI] [PubMed] [Google Scholar]
- [8].Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature reviews Neurology. 2010;6:131–44. [DOI] [PubMed] [Google Scholar]
- [9].Kester MI, Teunissen CE, Crimmins DL, Herries EM, Ladenson JH, Scheltens P, et al. Neurogranin as a Cerebrospinal Fluid Biomarker for Synaptic Loss in Symptomatic Alzheimer Disease. JAMA neurology. 2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thorsell A, Bjerke M, Gobom J, Brunhage E, Vanmechelen E, Andreasen N, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer's disease. Brain research. 2010;1362:13–22. [DOI] [PubMed] [Google Scholar]
- [11].Kvartsberg H, Portelius E, Andreasson U, Brinkmalm G, Hellwig K, Lelental N, et al. Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer's disease patients and healthy controls. Alzheimer's research & therapy. 2015;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kvartsberg H, Duits FH, Ingelsson M, Andreasen N, Ohrfelt A, Andersson K, et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014. [DOI] [PubMed] [Google Scholar]
- [13].De Vos A, Jacobs D, Struyfs H, Fransen E, Andersson K, Portelius E, et al. C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015. [DOI] [PubMed] [Google Scholar]
- [14].Portelius E, Zetterberg H, Skillback T, Tornqvist U, Andreasson U, Trojanowski JQ, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer's disease. Brain : a journal of neurology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tarawneh R, D'Angelo G, Crimmins D, Herries E, Griest T, Fagan AM, et al. Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease. JAMA neurology. 2016;73:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer's disease. EMBO molecular medicine. 2016;8:1184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brinkmalm A, Brinkmalm G, Honer WG, Frolich L, Hausner L, Minthon L, et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer's disease. Molecular neurodegeneration. 2014;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tarawneh R, D'Angelo G, Macy E, Xiong C, Carter D, Cairns NJ, et al. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011;70:274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Babic Leko M, Borovecki F, Dejanovic N, Hof PR, Simic G. Predictive Value of Cerebrospinal Fluid Visinin-Like Protein-1 Levels for Alzheimer's Disease Early Detection and Differential Diagnosis in Patients with Mild Cognitive Impairment. J Alzheimers Dis. 2015;50:765–78. [DOI] [PubMed] [Google Scholar]
- [20].Lee JM, Blennow K, Andreasen N, Laterza O, Modur V, Olander J, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008;54:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Janelidze S, Hertze J, Zetterberg H, Landqvist Waldo M, Santillo A, Blennow K, et al. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer's disease. Ann Clin Transl Neurol. 2016;3:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wennstrom M, Surova Y, Hall S, Nilsson C, Minthon L, Hansson O, et al. The Inflammatory Marker YKL-40 Is Elevated in Cerebrospinal Fluid from Patients with Alzheimer's but Not Parkinson's Disease or Dementia with Lewy Bodies. PloS one. 2015;10:e0135458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, et al. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biological psychiatry. 2010;68:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhong L, Cherry T, Bies CE, Florence MA, Gerges NZ. Neurogranin enhances synaptic strength through its interaction with calmodulin. The EMBO journal. 2009;28:3027–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang KP, Huang FL, Jager T, Li J, Reymann KG, Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gerendasy DD, Sutcliffe JG. RC3/neurogranin, a postsynaptic calpacitin for setting the response threshold to calcium influxes. Molecular neurobiology. 1997;15:131–63. [DOI] [PubMed] [Google Scholar]
- [27].Shin OH. Exocytosis and synaptic vesicle function. Compr Physiol. 2014;4:149–75. [DOI] [PubMed] [Google Scholar]
- [28].Laterza OF, Modur VR, Crimmins DL, Olander JV, Landt Y, Lee JM, et al. Identification of novel brain biomarkers. Clin Chem. 2006;52:1713–21. [DOI] [PubMed] [Google Scholar]
- [29].Prakash M, Bodas M, Prakash D, Nawani N, Khetmalas M, Mandal A, et al. Diverse pathological implications of YKL-40: answers may lie in 'outside-in' signaling. Cell Signal. 2013;25:1567–73. [DOI] [PubMed] [Google Scholar]
- [30].Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014;83:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schindler SE, Fagan AM. Autosomal Dominant Alzheimer Disease: A Unique Resource to Study CSF Biomarker Changes in Preclinical AD. Frontiers in neurology. 2015;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- [33].Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [34].Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, et al. The DIAN-TU Next Generation Alzheimer's prevention trial: Adaptive design and disease progression model. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2017;13:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sutphen CL, McCue L, Herries EM, Xiong C, Ladenson JH, Holtzman DM, et al. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sutphen CL, Jasielec MS, Shah AR, Macy EM, Xiong C, Vlassenko AG, et al. Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age. JAMA neurology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bittner T, Zetterberg H, Teunissen CE, Ostlund RE Jr., Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta-amyloid (1-42) in human cerebrospinal fluid. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2016;12:517–26. [DOI] [PubMed] [Google Scholar]
- [38].Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-beta PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jack CR Jr., Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, et al. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2010;6:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer's disease: a longitudinal study. The Lancet Neurology. 2018;17:241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Luo J, D'Angela G, Gao F, Ding J, Xiong C. Bivariate correlation coefficients in family-type clustered studies. Biom J. 2015;57:1084–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McDade E, Wang G, Gordon BA, Hassenstab J, Benzinger TLS, Buckles V, et al. Longitudinal Cognitive and Biomarker Changes in Dominantly Inherited Alzheimer's Disease. Neurology, in press. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Palmqvist S, Mattsson N, Hansson O, Alzheimer's Disease Neuroimaging I. Cerebrospinal fluid analysis detects cerebral amyloid-beta accumulation earlier than positron emission tomography. Brain : a journal of neurology. 2016;139:1226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TL, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease. Science translational medicine. 2014;6:226ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gutierrez Gomez M, Langois CM, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA neurology. 2015;72:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ringman JM, Younkin SG, Pratico D, Seltzer W, Cole GM, Geschwind DH, et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology. 2008;71:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. The Lancet Neurology. 2010;9:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. The Lancet Neurology. 2012;11:1048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Suarez-Calvet M, Araque Caballero MA, Kleinberger G, Bateman RJ, Fagan AM, Morris JC, et al. Early changes in CSF sTREM2 in dominantly inherited Alzheimer's disease occur after amyloid deposition and neuronal injury. Science translational medicine. 2016;8:369ra178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hampel H, Toschi N, Baldacci F, Zetterberg H, Blennow K, Kilimann I, et al. Alzheimer's disease biomarker-guided diagnostic workflow using the added value of six combined cerebrospinal fluid candidates: Abeta1-42, total-tau, phosphorylated-tau, NFL, neurogranin, and YKL-40. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018;14:492–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.