Abstract
Brain-derived neurotrophic factor (BDNF) expression and signaling activity in brain is influenced by chronic ethanol and stress. We previously demonstrated reduced Bdnf mRNA levels in the medial prefrontal cortex (mPFC) following chronic ethanol treatment and forced swim stress (FSS) enhanced escalated drinking associated with chronic ethanol exposure. The present study examined the effects of chronic ethanol and forced swim stress exposure, alone and in combination, on Bdnf mRNA expression in different brain regions, including mPFC, central amygdala (CeA), and hippocampus (HPC). Additionally, since microRNA-206 has been shown to negatively regulate BDNF expression, the effects of chronic ethanol and FSS on its expression in the target brain regions was examined. Mice received 4 weekly cycles of chronic intermittent ethanol (CIE) vapor or air exposure and then starting 72-hr later, the mice received either a single or 5 daily 10-min FSS sessions (or left undisturbed). Brain tissue samples were collected 4-hr following final FSS testing and Bdnf mRNA and miR-206 levels were determined by qPCR assay. Results indicated dynamic brain regional and time-dependent changes in Bdnf mRNA and miR-206 expression. In general, CIE and FSS exposure reduced Bdnf mRNA expression while miR-206 levels were increased in the mPFC, CeA, and HPC. Further, in many instances, these effects were more robust in mice that experienced both CIE and FSS treatments. These results have important implications for the potential link between BDNF signaling in the brain and ethanol consumption related to stress interactions with chronic ethanol experience.
Keywords: Chronic Ethanol, Stress, BDNF, microRNA
INTRODUCTION
Alcohol use disorder (AUD) continues to be a major concern in society, contributing to high health care costs and lost productivity (Rehm et al., 2015). AUD can develop into alcohol (ethanol) dependence, which is a chronic relapsing disorder where individuals compulsively consume ethanol, lose control over ethanol intake, and suffer negative withdrawal-related symptoms. As the addictive process progresses, periods of heavy ethanol use are interrupted by numerous failed attempts at abstinence, triggering a multitude of neurobiological alterations that drive and maintain ethanol dependence (Becker, 2012; Hansson et al., 2008; Koob, 2013; Koob and Le Moal, 2008; Vengeliene et al., 2008).
Several animal models have been developed to examine neuroadaptations associated with chronic ethanol exposure and withdrawal that may underlie enhanced motivation to drink (Becker, 2013; Becker and Ron, 2014; Vendruscolo and Roberts, 2014). We recently employed high-density oligonucleotide arrays to characterize brain regional changes in gene expression in conjunction with a mouse model of dependence-related escalated drinking. The dorsal medial prefrontal cortex (dmPFC) of ethanol dependent mice showed the greatest number of transcriptional changes due to chronic ethanol exposure and withdrawal. One robust and noteworthy gene change found in the dmPFC was a reduction in brain-derived neurotrophic factor (BDNF) transcript levels (Melendez et al., 2012; Smith et al., 2016). This transcriptional change was confirmed in independent studies and reduced Bdnf mRNA levels also extended to BDNF protein levels (Melendez et al., 2012). Subsequently, we demonstrated that replenishing this deficit by direct injection of BDNF into the dmPFC or preventing the deficit via viral-mediated overexpression of the neurotrophic factor in the dmPFC reversed or prevented (respectively) dependence-related escalated drinking (Haun et al., 2018).
Other laboratories have found reduced BDNF expression in the dmPFC after chronic ethanol vapor exposure in rats (Tapocik et al., 2013) and after chronic intermittent/limited access to ethanol in mice (Darcq et al., 2015; Logrip et al., 2009). Similar to results seen in the dmPFC, chronic ethanol exposure also produced reductions in BDNF content in other brain regions. Specifically, in the central nucleus of the amygdala (CeA) chronic ethanol consumption led to decreases in Bdnf mRNA in adult (You et al., 2014) and adolescent rats (Pandey et al., 2015). In addition, reduced BDNF protein within the hippocampus (HPC) of rats was reported following a 10-day ethanol vapor exposure paradigm (Hauser et al., 2011) or chronic binge-drinking procedure (Briones and Woods, 2013). Further, transgenic mice with genetic deficiencies in BDNF expression has been associated with increased ethanol consumption (Hensler et al., 2003; McGough et al., 2004; Pandey et al., 2004). Thus, there is strong evidence that BDNF expression and activity in brain plays a role in regulation of ethanol consumption (Logrip et al., 2015; Ron and Berger, 2018).
Stress is also known to influence BDNF expression in brain, and this neurotrophic factor has been implicated in a number of stress-related neuropsychiatric disorders (Andero et al., 2014; Ninan, 2014). In general, acute and chronic stress experience has been shown to reduce BDNF expression in several brain regions, including prefrontal cortex (Chiba et al., 2012; Fumagalli et al., 2004; Roceri et al., 2004), central amygdala (Gray et al., 2013; Smith et al., 2014), and hippocampus (Duman and Monteggia, 2006). Given that stress is known to significantly influence ethanol consumption (Becker, 2017; Becker et al., 2011; Blaine and Sinha, 2017; Sinha, 2012; Spanagel et al., 2014) and that both stress and ethanol exposure alter BDNF activity in brain, it is surprising that very little is known about their combined effects on BDNF expression/function. As we have recently demonstrated that forced swim stress selectively enhances drinking in dependent mice (Anderson et al., 2016; Anderson et al., 2016; Lopez et al., 2016; Rodberg et al., 2017), the present study was designed to examine the effects of chronic ethanol and forced swim stress exposure, alone and in combination, on Bdnf mRNA expression in different brain regions.
Additionally, several microRNAs, small (~22 nucleotide) non-coding RNAs, have been shown to regulate expression of Bdnf mRNA transcripts, and there is evidence showing a link between microRNAs regulating BDNF and its effects on ethanol drinking behavior (Darcq et al., 2015; Lee et al., 2012; Tapocik et al., 2014; Tapocik et al., 2013) In particular, microRNA-206 (miR-206) has been shown to suppress Bdnf transcriptional activity in brain (Tapocik et al., 2014). Thus, another aim of the present study was to examine whether chronic ethanol and forced swim stress, alone and in combination, alters the inverse relationship between Bdnf mRNA and miR-206 expression in several brain regions.
EXPERIMENTAL PROCEDURES
Subjects
Adult male C57BL/B6J mice purchased from Jackson Laboratories (Bar Harbor, ME) were individually housed under a 12-hr light/dark cycle (lights on at 8:00 AM), with free access to food (Teklad rodent diet) and water in a temperature and humidity controlled AAALAC-accredited facility at the Medical University of South Carolina. All procedures were approved by the Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
General Study Design
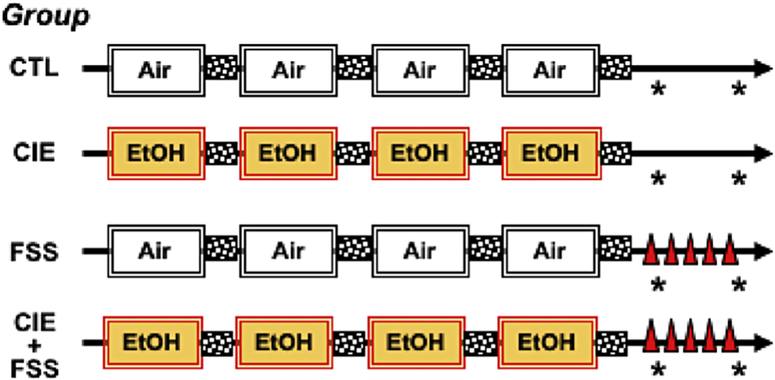
A schematic of the general study design is presented in Figure 1. Briefly, mice received 4 consecutive weekly cycles of chronic intermittent exposure to ethanol vapor or air (described below). One cohort of CIE and Air-exposed mice received a single forced swim stress (FSS) session while corresponding CIE and Air-exposed groups were left undisturbed until sacrifice, which corresponded to 3 days following the final CIE/Air exposure cycle. A separate cohort of mice received the same treatment, except they were sacrificed following 5 daily FSS sessions (or left undisturbed), which corresponded to 7 days post CIE/Air exposure. As shown in Figure 1, this balanced design resulted in 4 distinct groups of mice (CTL, CIE, FSS, and CIE+FSS) that were sacrificed at each of the two time points relative to FSS experience (FSS x 1 or FSS x 5) and CIE/Air final exposure (3 or 7 days post-exposure). Group sizes at the start of the study were 10/group. Problems with RNA isolation (e.g., low yield, poor quality) and qPCR assay resulted in fewer tissue samples included in analyses of Bdnf mRNA and miR-206 expression (N= 6–10/group/time point/brain region).
Figure 1: Schematic of General Study Design.
Four groups of mice were generated based on the factorial combination of Group (CIE vs. Air) x Stress (FSS vs. no-FSS). Mice received 4 weekly cycles of CIE or Air exposure, with each week separated by a 72-hr period (stippled boxes). Following the final CIE/Air exposure cycle, mice received FSS (10-min) (denoted by red triangles) or left undisturbed in the home-cage. Separate groups of CLT, CIE, FSS, and CIE+FSS treated mice were sacrificed 4-hr following a single FSS challenge (corresponding to 3-days post-CIE/Air exposure) or 4-hr following the 5th of 5 daily FSS sessions (corresponding to 7-days post-CIE/Air exposure) – time of sacrifice denoted by asterisk.
Chronic Intermittent Ethanol (CIE) Exposure
Mice were exposed to chronic intermittent ethanol (CIE) vapor or uncontaminated air in inhalation chambers, as previously described (Becker and Lopez, 2004; Griffin et al., 2009; Lopez and Becker, 2005). Ethanol vapor (or air) was administered 16 hr/day for 4 days followed by a 72-hr abstinence period, and this pattern of weekly exposure was repeated over four consecutive weeks. Ethanol concentration in the inhalation chambers were monitored daily and air flow was adjusted to maintain exposure within a range that yielded stable blood ethanol levels within the target range (175–225 mg/dl) throughout each weekly exposure. Ethanol concentration in the inhalation chambers and blood ethanol levels (assessed weekly) were determined as previously described (Becker and Hale, 1993; Lopez and Becker, 2005).
Forced Swim Stress Procedure
After 4 consecutive weeks of CIE or air exposure, some mice were challenged with the forced swim stress (FSS) procedure as previously described (Anderson et al., 2016; Anderson et al., 2016; Lopez et al., 2016) Briefly, mice were placed in glass cylinders filled to a depth of 20 cm with 23–25° C water for 10 minutes. The water was replaced between subjects. One group of mice received a single FSS challenge while another group of mice received 5 daily 10-min FSS sessions. Because our previous work showed that the FSS procedure reliably increases ethanol intake 4 hours later in CIE-exposed mice (Anderson et al., 2016; Anderson et al., 2016; Lopez et al., 2016), mice were sacrificed 4-hr after either the first or the fifth FSS challenge. Separate (unstressed) groups of mice were left undisturbed in their home-cage but were sacrificed at the same times.
Brain Tissue Collection
Mice were sacrificed by rapid decapitation and brains quickly removed. The dorsal medial prefrontal cortex (dmPFC), central nucleus of the amygdala (CeA), and CA1 region of the hippocampus (HPC) were dissected on ice using 1-mm microdissection punches, with the mouse brain atlas serving as a guide (Franklin and Paxinos, 2008). Tissue samples were immediately placed into RNA Later (Thermo Fisher; cat no. AM7021). Total RNA was extracted from the brain tissue samples using mirVana miRNA Extraction Kit (Invitrogen/Life Technologies; cat no. AM1560) according to the manufacturer’s instructions. Total RNA was quantified on a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Inc., Rockland, IL).
Bdnf mRNA and MicroRNA-206 Measurement
Quantitative real time-polymerase chain reaction (qRT-PCR) assays were performed using similar procedures to those previously published by our group (Melendez et al., 2012). For Bdnf IV mRNA measurements, cDNA was created using QuantiTect Reverse Transcription Kit (Qiagen, Inc.; Cat. No. 205314) according to the manufacturer’s instructions. Bdnf IV mRNA was amplified using custom TaqMan qRT-PCR primers, which were designed to specifically target Bdnf exon IV using Integrated DNA Technologies (IDT) primer designer online software and manufactured by Life Technologies (Forward: GCCTAGATCAAATGGAGCTTCT; Reverse: GCCGATATGTACTCCTGTTCTG; Probe: ACCTCCGCCATGCAATTTCCACTA). Data normalization was performed using the reference gene Cyclophilin (Ppia) (Life Technologies, Inc.). For the reactions, 20 μl of sample cDNA and TaqMan Universal Master Mix II, with UNG (Applied Biosystems, Inc.; Cat. No. 4440042), were loaded in triplicate into a 384-well optical PCR plate and analyzed on a BioRad CFX384 Real Time PCR system. Cycling parameters were 50° C for 2 min, 95° C for 10 min, followed by 40 amplification cycles with melting at 95° C for 15 sec, and annealing/extending at 60° C for 60 sec. Fluorescence readings are obtained after each cycle. The 2^-DDCT method (Livak and Schmittgen, 2001) was used to calculated fold change in expression of the target gene (Bdnf IV) relative to the reference gene (Ppia) using the CTL group as the reference condition.
For microRNA-206 qRT-PCR assays, isolated total RNA was diluted to 2 ng/μl. A 5 μl aliquot was converted to cDNA for both miR-206 and U6 (a short noncoding region serving as the reference gene) using primers purchased from Thermo Fisher Scientific, Inc. and TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Inc.; Cat. No. 4366596) in a final volume of 15 μl. Using TaqMan microRNA qPCR primers (Applied Biosystems) for miR-206 (cat no 4427975, assay ID 001973) and U6 (cat no 4427975, assay ID 000510), microRNA cDNA and TaqMan Universal Master Mix II with UNG were loaded into a 384-well optical PCR plate in 20 μl triplicates. Real-Time PCR system and cycling parameters are identical to that described above for Bdnf IV qPCR, with the exception of the fold change being expressed relative to U6 as the reference gene.
Data Analysis
Data were analyzed by ANOVA, with CIE and Stress as main factors. Separate analyses were performed for Bdnf mRNA and miR-206 data collected following a single FSS session (corresponding to 3-days post CIE/Air exposure) or five consecutive daily FSS sessions (corresponding to 7-days post-CIE/Air exposure). Post-hoc analyses (Newman-Keuls) were performed, when appropriate. Additionally, when indicated, planned pairwise comparisons were performed with Bonferonni corrections. Statistical significance was set at p< 0.05.
RESULTS
Bdnf mRNA and miR-206 Expression in dmPFC
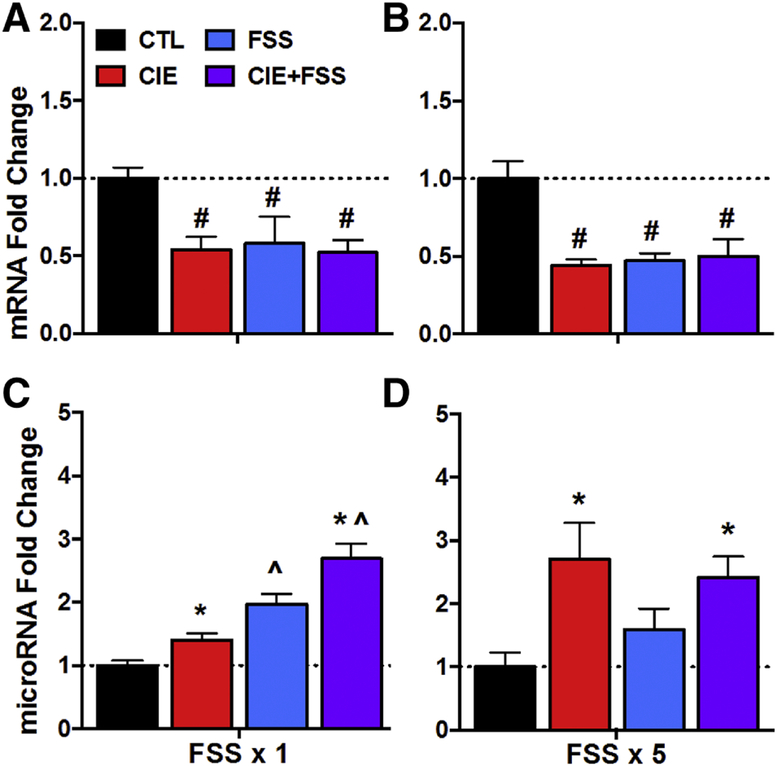
Analysis of Bdnf mRNA levels following a single FSS challenge indicated a main effect of CIE exposure [F(1,32)= 16.72, p< 0.0005], indicating an overall effect of CIE exposure reducing Bdnf mRNA levels (Figure 2A). While FSS alone did not alter Bdnf mRNA expression, this acute stress experience did appear to enhance reduction in Bdnf mRNA in mice with a history of CIE exposure (CIE+FSS group). That is, although the CIE x Stress interaction was not significant [F(1,32)= 3.64, p= 0.07], Bdnf mRNA values were lowest in the CIE+FSS group. Planned pairwise comparisons using the interaction error term revealed that only the CIE+FSS group differed from the CTL condition (p= 0.030). Analysis of Bdnf mRNA data collected at a time corresponding to the fifth daily FSS session indicated main effects of CIE exposure [F(1,37)= 14.33, p< 0.0005] and Stress [F(1,37)= 4.26, p<0.05], but not a significant CIE x Stress interaction [F(1,37)= 0.81, p> 0.10] (Figure 2B). The main effect of CIE was principally due to a Bdnf mRNA reduction in the CIE alone group while the significant effect of Stress was mainly due to an increase in Bdnf mRNA levels in the FSS alone group.
Figure 2: Bdnf mRNA and miR-206 Expression in mPFC.
Bdnf mRNA fold change for CTL, CIE, FSS, and CIE+FSS groups (N= 8–10/group) following (A) a single FSS challenge (or 3-days post-CIE exposure) or following (B) five consecutive daily FSS sessions (or 7-days post-CIE exposure). miR-206 fold change for CTL, CIE, FSS, and CIE+FSS groups (N= 6–8/group) following (C) a single FSS challenge (or 3-days post-CIE exposure) or following (D) five consecutive daily FSS sessions (or 7-days post-CIE exposure). Values are mean ± s.e.m. expression fold change relative to CTL group. Main effect of CIE (*, p< 0.01); Main effect of Stress (^, p< 0.05); CIE x Stress interaction: significantly differs from CTL group (#; p< 0.05).
Analysis of miR-206 expression in dmPFC following acute FSS challenge revealed a significant CIE x Stress interaction [F(1,25)= 12.31, p< 0.005]. Post-hoc analysis indicated that the CIE+FSS group evidenced significantly greater miR-206 expression relative to all other groups (p< 0.05 for all comparisons), which did not significantly differ from each other (Figure 2C). ANOVA of data collected after five consecutive daily FSS sessions indicated only a main effect of Stress [F(1,26)= 5.35, p< 0.05], with both FSS and CIE+FSS groups showing elevated miR-206 expression at this time point (Figure 2D).
Bdnf mRNA and miR-206 Expression in CeA
Analysis of Bdnf mRNA expression in CeA measured after a single FSS challenge (3-days post CIE exposure) revealed significant main effects of CIE [F(1,31)= 7.38, p< 0.01] and Stress [F(1,31)= 5.28, p< 0.05], and the CIE x Stress interaction [F(1,31)= 4.41, p< 0.05]. Post-hoc analysis indicated that CIE exposure and acute FSS exposure, both alone and in combination, significantly reduced Bdnf mRNA levels (p< 0.01 for all comparisons) (Figure 3A). These effects persisted, as analysis of Bdnf mRNA levels after 5 daily FSS sessions (7-days post CIE exposure) revealed significant main effect sof CIE [F(1,25)= 8.81, p< 0.01] and Stress [F(1,25)= 6.89, p< 0.01], and the CIE x Stress interaction [F(1,25)= 11.10, p< 0.01]. Post-hoc analysis indicated a similar pattern of results, with significantly reduced Bdnf mRNA levels in CIE, FSS, and CIE+FSS groups relative to the CTL condition (p< 0.01 for all comparisons) (Figure 3B). Thus, CIE exposure alone significantly reduced Bdnf mRNA levels at 3 and 7 days. Additionally, acute and repeated FSS experience alone and in combination with a history of CIE exposure also reduced Bdnf mRNA levels relative to controls. The magnitude of decreased Bdnf mRNA expression in CeA was similar across all experimental conditions.
Figure 3: Bdnf mRNA and miR-206 Expression in CeA.
Bdnf mRNA fold change for CTL, CIE, FSS, and CIE+FSS groups (N= 6–10/group) following (A) a single FSS challenge (or 3-days post-CIE exposure) or following (B) five consecutive daily FSS sessions (or 7-days post-CIE exposure). miR-206 fold change for CTL, CIE, FSS, and CIE+FSS groups (N= 8–10/group) following (C) a single FSS challenge (or 3-days post-CIE exposure) or following (D) five consecutive daily FSS sessions (or 7-days post-CIE exposure). Values are mean ± s.e.m. expression fold change relative to CTL group. Main effect of CIE (*, p< 0.01); Main effect of Stress (^, p< 0.01); CIE x Stress interaction: significantly differs from CTL group (#; p< 0.01).
While CIE and FSS exposure alone and in combination produced robust decreases in Bdnf mRNA levels, miR-206 levels were elevated in all conditions relative to controls. ANOVA indicated a significant main effect of CIE at 3-days [F(1,35)= 12.89, p< 0.001] (Figure 3C) and at 7-days post CIE exposure [F(1,30)= 9.12, p< 0.005] (Figure 3D). The analysis also revealed a main effect of Stress at the 3-day time point [F(1,35)= 51.56, p< 0.0001]. The CIE x Stress interaction was not significant for either 3-day [F(1,35)= 1.06, p> 0.10] or 7-day [F(1,30)= 1.11, p> 0.10] time points.
Bdnf mRNA and miR-206 Expression in HPC
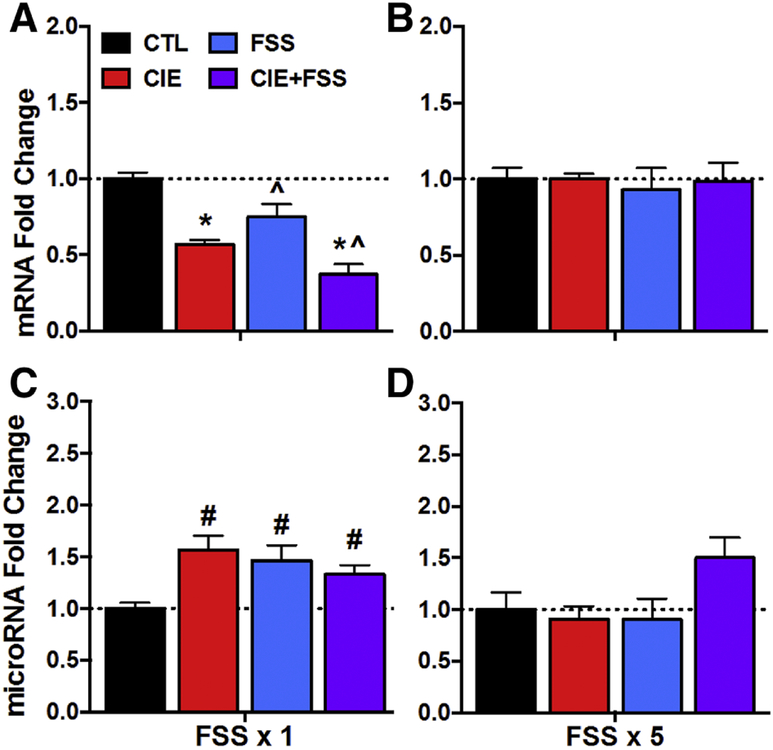
All experimental conditions produced a general decrease in Bdnf mRNA in the HPC relative to controls following a single FSS session (3-day post CIE exposure time point) (Figure 4A). This was supported by ANOVA, which indicated significant main effects of CIE [F(1,34)= 40.68, p< 0.0001] and Stress [F(1,34)= 7.75, p< 0.01]. Although the decrease in Bdnf mRNA was most robust in the CIE+FSS group, the CIE x Stress interaction was not significant [F(1,34= 0.90, p> 0.10]. The reduction in Bdnf mRNA levels normalized for all groups following repeated FSS treatment (7-days post CIE exposure) (Figure 4B).
Figure 4: Bdnf mRNA and miR-206 Expression in HPC.
Bdnf mRNA fold change for CTL, CIE, FSS, and CIE+FSS groups (N= 8–10/group) following (A) a single FSS challenge (or 3-days post-CIE exposure) or following (B) five consecutive daily FSS sessions (or 7-days post-CIE exposure). miR-206 fold change for CTL, CIE, FSS, and CIE+FSS groups (N= 7–10/group) following (C) a single FSS challenge (or 3-days post-CIE exposure) or following (D) five consecutive daily FSS sessions (or 7-days post-CIE exposure). Values are mean ± s.e.m. expression fold change relative to CTL group. Main effect of CIE (*, p< 0.01); Main effect of Stress (^, p< 0.01); CIE x Stress interaction: significantly differs from CTL group (#; p< 0.05).
Analysis of miR-206 expression in HPC following acute FSS challenge revealed a significant CIE x Stress interaction [F(1,29)= 8.47, p< 0.01]. Post-hoc analysis indicated that CIE, FSS, and CIE+FSS groups exhibited elevated miR-206 expression relative to the CTL group (p< 0.05 for all comparisons), with no differences between groups (Figure 4C). These changes appeared to normalize in the CIE group (7-days post exposure) and FSS group (after 5 sessions), and although there was increased expression of miR-206 expression in the CIE+FSS group, this effect did not achieve statistical significance at this time point (Figure 4D).
DISCUSSION
Results from this study show unique brain regional and time-dependent changes in Bdnf IV mRNA expression produced by ethanol dependence (CIE exposure), acute and repeated forced swim stress, and the combination of both CIE and FSS exposure. Similarly, expression levels for miR-206, a microRNA known to regulate Bdnf mRNA transcripts and implicated in ethanol dependence-related drinking, were altered in a brain region-specific and time-dependent manner. In general, these results parallel previous findings involving broad genomic analyses and demonstrating dynamic changes in transcriptional activity across different brain regions associated with ethanol dependence (CIE exposure) and withdrawal (Melendez et al., 2012; Smith et al., 2016). The present findings extend these observations to acute and repeated stress challenge and the combination of stress and chronic ethanol exposure.
Prolonged ethanol exposure has been shown to reduce Bdnf mRNA levels in a number of brain regions including cortex (Darcq et al., 2015; Fernandez et al., 2017; Logrip et al., 2009; Tapocik et al., 2014), central amygdala (Pandey et al., 2006; Pandey et al., 2008), hippocampus (Briones and Woods, 2013; Hauser et al., 2011), and striatum (Jeanblanc et al., 2013; Logrip et al., 2008). Consistent with these reports and previous work in our laboratory (Haun et al., 2018; Melendez et al., 2012; Smith et al., 2016), the present findings indicated reduced Bdnf mRNA levels in dmPFC, CeA, and HPC 3 days following repeated cycles of CIE exposure. Since separate groups of mice were used as controls for the two time points evaluated in this study, it is not appropriate to analyze data collected at 3- and 7-days post-CIE exposure. Nevertheless, it is interesting that the decrease in Bdnf mRNA levels persisted and were more robust 7 days following CIE exposure in dmPFC and CeA but normalized to control levels at this time point in HPC. Thus, while numerous chronic ethanol exposure paradigms have been shown to produce a general reduction in Bdnf mRNA expression in brain, the persistence of this effect appears to vary depending on the brain region.
As noted earlier, stress exposure also has been shown to influence BDNF expression in several brain regions. For example, repeated forced swim stress, chronic restraint stress, and early developmental stress reduced BDNF levels in PFC (Borsoi et al., 2015; Chiba et al., 2012; Fumagalli et al., 2004; Roceri et al., 2004). Similarly, social defeat stress was shown to produce reductions in Bdnf mRNA levels in the CeA (Smith et al., 2014), although other stress procedures produced opposite effects (Gray et al., 2013; Machida et al., 2018). Likewise, a relatively large number of studies have shown various stress procedures (including forced swim stress) to reduce BDNF mRNA and protein content in the HPC of mice and rats, although there are some exceptions (Duman and Monteggia, 2006). Consistent with this literature, the present results indicate that single and repeated forced swim stress experience alone altered Bdnf mRNA expression in the target brain regions in a dynamic manner. In the dmPFC, acute FSS challenge had no effect but repeated FSS treatment resulted in increased Bdnf mRNA levels. In contrast, Bdnf mRNA expression was reduced following a single FSS session in CeA and HPC, and this effect grew more robust in CeA while normalizing to control levels in HPC following repeated FSS treatment. There is no clear explanation for this pattern of results or the varied outcomes reported in the aforementioned published studies. However, it is clear that stress produces a complex pattern of changes in Bdnf expression that are brain region-specific. Differences in results are also likely related to the nature and chronicity of the stressor, the time interval following stress exposure when measurements are performed, and procedures for assessing transcriptional activity of the neurotrophic factor.
A major aim of the present study was to examine effects of acute and repeated FSS experience on Bdnf mRNA expression in mice with a history of CIE exposure. Here again, results showed a distinct pattern of changes that differed across the target brain regions. The combination of a single FSS session with a history of CIE exposure enhanced reductions in Bdnf mRNA levels in mPFC and HPC while this effect was of a similar magnitude compared to CIE treatment alone in the CeA. Repeated FSS exposure following CIE treatment attenuated CIE-induced reductions in Bdnf mRNA levels in mPFC and HPC, but decreased Bdnf mRNA levels persisted in CeA. Taken together, these results suggest that acute stress enhances CIE-induced reduction in Bdnf mRNA expression across several brain regions, and this effect is differentially modified following repeated FSS treatment in a brain region-specific manner. The significance of these changes as they may relate to our previous demonstration that stress enhances dependence-related ethanol consumption remains to be determined.
The regulation of BDNF transcription and translation is complex, mediated through multiple epigenetic processes such as DNA methylation (Hing et al., 2018) and histone acetylation (Bredy et al., 2007), as well as microRNA regulation of expression of the various target Bdnf mRNA transcripts (Caputo et al., 2011). Studies have shown that several microRNAs that target BDNF expression modulate ethanol consumption (Bahi and Dreyer, 2013; Darcq et al., 2015; Miranda et al., 2010; Pietrzykowski et al., 2008; Tapocik et al., 2014). Additionally, microRNAs are known to be involved in stress-responsive events (Dwivedi, 2014; Rinaldi et al., 2010). Among several microRNAs that regulate BDNF expression, miR-206 is known to influence ethanol drinking (Tapocik et al., 2014; Tapocik et al., 2013), and stress-related behaviors (Miao et al., 2018). In the present study, CIE exposure, FSS exposure, and the combination of CIE and FSS experience all produced altered miR-206 expression in the target brain regions.
In general, reduced Bdnf mRNA levels were associated with elevated miR-206 expression across the experimental groups. This inverse relationship supports the known suppressive effects miR-206 exerts on Bdnf transcriptional activity (Lee et al., 2012; Tapocik et al., 2014). However, this effect varied across brain regions as well as time when brain tissue samples were harvested for analyses. The negative relationship between Bdnf mRNA and miR-206 expression was most clearly and consistently observed in the CeA. This effect was also evident in mPFC and HPC following a single FSS challenge (and 3-days post CIE exposure). The relationship between Bdnf mRNA and miR-206 expression in mPFC and HPC following repeated FSS treatment (and 7-days post-CIE exposure) was more varied. Taken together, these data indicate associated changes in Bdnf mRNA and miR-206 expression in response to CIE exposure and FSS exposure alone, as well as combined CIE and FSS experience, but the changes are brain region-specific and time-dependent. An explanation for these differences will require further investigation.
One possibility is that brain regional and temporal differences in the relationship between miR-206 and Bdnf transcript levels may be due, in part, to alterations in Dicer and Drosha, two RNA-specific endoribonuclease enzymes involved in microRNA biosynthesis (Ameres and Zamore, 2013). Studies in rats and BXD mouse strains have shown ethanol significantly reduces Dicer and Drosha mRNA in select brain regions, including the PFC and HPC (Mulligan et al., 2013; Prins et al., 2014). For example, Dicer and Drosha expression in PFC of BXD mice was elevated 4-hr following acute injection of ethanol (1.8 g/kg) (Mulligan et al., 2013). In rats, Prin et al. (2014) reported an interaction between binge drinking and age on Drosha expression in different sub-regions of the HPC. In the dorsal HPC, adolescent binge drinking increased Drosha, an effect that persisted for a month. In contrast, Drosha levels in the ventral HPC were significantly elevated during peri-puberty but were significantly reduced during late puberty. In both the dorsal and ventral regions of the HPC, adolescent binge ethanol drinking increased Dicer in expression during peri-puberty but levels were reduced during late puberty (Prin et al., 2014). Thus, the timing and duration of ethanol exposure may alter expression of key elements of microRNA biogenesis machinery, which in turn, may affect the time course of microRNA expression in various brain regions.
Further complicating the relationship between Bdnf mRNA and miR-206 expression is the existence of two possible isoforms of the Bdnf 3’ untranslated region (3’UTR), the long form and the short form. The long form 3’UTR is thought to be more susceptible to translation inhibition or mRNA degradation by miR-206 because there are 3 binding sites for miR-206 to attach and induce silencing or degradation of the transcript, while the short 3’UTR isoform only has 1 binding site (Miura et al., 2012). Whether CIE or FSS exposure, alone and/or in combination, alter microRNA biosynthesis remains to be determined.
There are a few limitations in this study that should be noted in considering these results. First, changes in expression of a single Bdnf mRNA exon, Bdnf IV, were examined. The mouse Bdnf gene is comprised of nine different exons, each acting as a unique promoter. Each exon is individually spliced to the same BDNF coding sequence resulting in the identical BDNF protein being translated, but under the control of different transcription factors (Aid et al., 2007; Pruunsild et al., 2007; West et al., 2014). The multiple exon variants are known to be expressed to different degrees in different brain regions and trafficked to distinctive parts of the neuron for localized translation of BDNF protein (Baj et al., 2013; Tongiorgi et al., 2004; Tongiorgi and Baj, 2008). Evidence indicates the various BDNF transcripts respond to specific stimuli (Vaghi et al., 2014). We focused on the Bdnf IV exon because our previous work showed reduced Bdnf IV mRNA expression following chronic ethanol treatment (Haun et al., 2018; Melendez et al., 2012) and others, likewise, have shown this exon to be responsive to ethanol exposure (Logrip et al., 2009) and stress (Fuchikami et al., 2010). However, chronic ethanol and stress also influence expression of other Bdnf exons (Fuchikami et al., 2010; Ieraci et al., 2015; Sakharkar et al., 2016; Shojaei et al., 2015). Thus, given the complicated nature of BDNF transcription, translation, and cellular trafficking, focusing on a single exon variant may overlook small but significant change caused by CIE, FSS, or the combination of CIE and FSS exposure.
Another limitation is focusing on a single Bdnf mRNA targeting microRNA (miR-206). It is known through microarray analysis that CIE exposure (Tapocik et al., 2013) and stress (Ma et al., 2016) alters the expression of many microRNAs which can target Bdnf mRNA. Therefore, regulation of Bdnf transcriptional activity may be controlled, to varying degrees, by a combination of microRNAs. Also, these effects are likely to be brain region-specific as well as time-dependent (Osterndorff-Kahanek et al., 2018). A more complete understanding of the role of microRNA (and other epigenetic mechanisms) regulating BDNF expression in response to chronic ethanol and stress as it relates to enhanced motivation to drink will require further investigation.
An additional limitation to consider is that only a single time point following FSS exposure (4-hr) was analyzed in the present study. It has been demonstrated that immobilization stress produced dynamic changes in Bdnf mRNA expression, with early increases followed by a decrease in Bdnf mRNA levels (Marmigere et al., 2003). Likewise, temporal changes in Bdnf mRNA (and related microRNAs) expression in relation to chronic ethanol exposure and withdrawal have been reported across a number of brain regions (Darq et al., 2015; Jeanblanc et al., 2013; Logrip et al., 2009; Melendez et al., 2012; Tapocik et al., 2014). Thus, changes in Bdnf mRNA and miR-206 expression across different brain regions will likely vary as a function of time in relation to stress experience in mice with or without a history of chronic ethanol exposure.
In conclusion, results from the present study demonstrate dynamic brain regional and time-dependent changes in Bdnf mRNA and miR-206 expression following chronic ethanol (CIE) exposure, single and repeated stress (FSS) experience, and the combination of both CIE and FSS experience. In general, CIE and FSS exposure reduced Bdnf mRNA expression and this was associated with an increase in miR-206 expression in the target brain regions (mPFC, CeA, HPC). This profile of results was most consistently observed in the CeA. Further, in many instances, these effects were more robust in mice that experienced both CIE and FSS treatments. As BDNF has been shown to play a role in escalated drinking associated with ethanol dependence (Haun et al., 2018), and stress (FSS) selectively enhances ethanol consumption in dependent mice (Anderson et al., 2016; Anderson et al., 2016), these results have important implications for the potential link between BDNF signaling in the brain and ethanol consumption related to stress interactions with chronic ethanol experience.
Highlights.
Chronic ethanol, stress, and their combination alter Bdnf mRNA levels in brain.
Chronic ethanol, stress, and their combination alter microRNA-206 levels in brain.
Chronic ethanol +/− stress changes in Bdnf mRNA levels are brain region and time-dependent.
ACKNOWLEDGEMENTS
MGS performed the experiments. MGS, WCG, MFL, and HCB analyzed study results and participated in conceptual discussions. MGS and WCG wrote the initial draft and all authors contributed to the final manuscript.
Funding: This work was supported by the Department of Veterans Affairs Medical Research grant (BLR&D BX000813) to HCB, and the National Institutes of Health grants (P50 AA010761, U01 AA014095, U24 AA020929, T32 AA007474) to HCB.
ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- miR-206
microRNA-206
- CIE
chronic intermittent ethanol
- FSS
forced swim stress
- mPFC
medial prefrontal cortex
- CeA
central nucleus of the amygdala
- HPC
hippocampus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T (2007), Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res 85:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameres SL, Zamore PD (2013), Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 14:475–488. [DOI] [PubMed] [Google Scholar]
- Andero R, Choi DC, Ressler KJ (2014), BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci 122:169–192. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC (2016), Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology (Berl) 233:2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC (2016), Stress-Induced Enhancement of Ethanol Intake in C57BL/6J Mice with a History of Chronic Ethanol Exposure: Involvement of Kappa Opioid Receptors. Front Cell Neurosci 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL (2013), Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci 38:2328–2337. [DOI] [PubMed] [Google Scholar]
- Baj G, Del Turco D, Schlaudraff J, Torelli L, Deller T, Tongiorgi E (2013), Regulation of the spatial code for BDNF mRNA isoforms in the rat hippocampus following pilocarpine-treatment: a systematic analysis using laser microdissection and quantitative real-time PCR. Hippocampus 23:413–423. [DOI] [PubMed] [Google Scholar]
- Becker HC (2012), Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res 34:448–458. [PMC free article] [PubMed] [Google Scholar]
- Becker HC (2013), Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci 13:355–377. [DOI] [PubMed] [Google Scholar]
- Becker HC (2017), Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology 122:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL (1993), Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res 17:94–98. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF (2004), Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28:1829–1838. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL (2011), Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 218:131–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Ron D (2014), Animal models of excessive alcohol consumption: recent advances and future challenges. Alcohol 48:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Sinha R (2017), Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsoi M, Antonio CB, Viana AF, Nardin P, Goncalves CA, Rates SM (2015), Immobility behavior during the forced swim test correlates with BNDF levels in the frontal cortex, but not with cognitive impairments. Physiol Behav 140:79–88. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M (2007), Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem 14:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Woods J (2013), Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience 254:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo V, Sinibaldi L, Fiorentino A, Parisi C, Catalanotto C, Pasini A, Cogoni C, Pizzuti A (2011), Brain derived neurotrophic factor (BDNF) expression is regulated by microRNAs miR-26a and miR-26b allele-specific binding. PLoS One 6:e28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H (2012), Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 39:112–119. [DOI] [PubMed] [Google Scholar]
- Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F, Ron D (2015), MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Mol Psychiatry 20:1219–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM (2006), A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y (2014), Emerging role of microRNAs in major depressive disorder: diagnosis and therapeutic implications. Dialogues Clin Neurosci 16:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez GM, Lew BJ, Vedder LC, Savage LM (2017), Chronic intermittent ethanol exposure leads to alterations in brain-derived neurotrophic factor within the frontal cortex and impaired behavioral flexibility in both adolescent and adult rats. Neuroscience 348:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (2008) The Mouse Brain in Stereotaxic Coordinates, Third Edition San Diego: Academic Press. [Google Scholar]
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S (2010), Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig 7:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Perez J, Racagni G, Riva MA (2004), Corticostriatal brain-derived neurotrophic factor dysregulation in adult rats following prenatal stress. Eur J Neurosci 20:1348–1354. [DOI] [PubMed] [Google Scholar]
- Gray JD, Milner TA, McEwen BS (2013), Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 239:214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC(2009), Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 201:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M (2008), Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci 27:1912–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun HL, Griffin WC, Lopez MF, Solomon MG, Mulholland PJ, Woodward JJ, McGinty JF, Ron D, et al. (2018), Increasing Brain-Derived Neurotrophic Factor (BDNF) in medial prefrontal cortex selectively reduces excessive drinking in ethanol dependent mice. Neuropharmacology 140:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Taylor RE, Tizabi Y (2011), Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler JG, Ladenheim EE, Lyons WE (2003), Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. J Neurochem 85:1139–1147. [DOI] [PubMed] [Google Scholar]
- Hing B, Sathyaputri L, Potash JB (2018), A comprehensive review of genetic and epigenetic mechanisms that regulate BDNF expression and function with relevance to major depressive disorder. Am J Med Genet B Neuropsychiatr Genet 177:143–167. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Mallei A, Musazzi L, Popoli M (2015), Physical exercise and acute restraint stress differentially modulate hippocampal brain-derived neurotrophic factor transcripts and epigenetic mechanisms in mice. Hippocampus 25:1380–1392. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Logrip ML, Janak PH, Ron D (2013), BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci 37:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2013), Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2008), Addiction and the brain antireward system. Annu Rev Psychol 59:29–53. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim JH, Huh JY, Yoon H, Park DK, Lim JY, et al. (2012), miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann Neurol 72:269–277. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001), Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Barak S, Warnault V, Ron D (2015), Corticostriatal BDNF and alcohol addiction. Brain Res 1628:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D (2008), Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J 22:2393–2404. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D (2009), Escalating ethanol intake is associated with altered corticostriatal BDNF expression. J Neurochem 109:1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC (2016), Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol 51:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Becker HC (2005), Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181:688–696. [DOI] [PubMed] [Google Scholar]
- Ma K, Guo L, Xu A, Cui S, Wang JH (2016), Molecular Mechanism for Stress-Induced Depression Assessed by Sequencing miRNA and mRNA in Medial Prefrontal Cortex. PLoS One 11:e0159093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida M, Lonart G, Sanford LD (2018), Effects of stressor controllability on transcriptional levels of c-fos, Arc, and brain-derived neurotrophic factor in mouse amygdala and medial prefrontal cortex. Neuroreport 29:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigere F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L (2003), Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus 13:646–655. [DOI] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, et al. (2004), RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci 24:10542–10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, McGinty JF, Kalivas PW, Becker HC (2012), Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addict Biol 17:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z, Mao F, Liang J, Szyf M, Wang Y, Sun ZS (2018), Anxiety-Related Behaviours Associated with microRNA-206–3p and BDNF Expression in Pregnant Female Mice Following Psychological Social Stress. Mol Neurobiol 55:1097–1111. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Pietrzykowski AZ, Tang Y, Sathyan P, Mayfield D, Keshavarzian A, Sampson W, Hereld D (2010), MicroRNAs: master regulators of ethanol abuse and toxicity? Alcohol Clin Exp Res 34:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura P, Amirouche A, Clow C, Belanger G, Jasmin BJ (2012), Brain-derived neurotrophic factor expression is repressed during myogenic differentiation by miR-206. J Neurochem 120:230–238. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Dubose C, Yue J, Miles MF, Lu L, Hamre KM (2013), Expression, covariation, and genetic regulation of miRNA Biogenesis genes in brain supports their role in addiction, psychiatric disorders, and disease. Front Genet 4:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan I (2014), Synaptic regulation of affective behaviors; role of BDNF. Neuropharmacology 76 Pt C:684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterndorff-Kahanek EA, Tiwari GR, Lopez MF, Becker HC, Harris RA, Mayfield RD (2018), Long-term ethanol exposure: Temporal pattern of microRNA expression and associated mRNA gene networks in mouse brain. PLoS One 13:e0190841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T (2004), Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. J Neurosci 24:5022–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H (2015), Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis 82:607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K (2006), Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci 26:8320–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K (2008), Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci 28:2589–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN (2008), Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron 59:274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins SA, Przybycien-Szymanska MM, Rao YS, Pak TR (2014), Long-term effects of peripubertal binge EtOH exposure on hippocampal microRNA expression in the rat. PLoS One 9:e83166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruunsild P, Kazantseva A, Aid T, Palm K, Timmusk T (2007), Dissecting the human BDNF locus: bidirectional transcription, complex splicing, and multiple promoters. Genomics 90:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Manthey J, Struzzo P, Gual A, Wojnar M (2015), Who receives treatment for alcohol use disorders in the European Union? A cross-sectional representative study in primary and specialized health care. Eur Psychiatry 30:885–893. [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Vincenti S, De Vito F, Bozzoni I, Oliverio A, Presutti C, Fragapane P, Mele A (2010), Stress induces region specific alterations in microRNAs expression in mice. Behav Brain Res 208:265–269. [DOI] [PubMed] [Google Scholar]
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA (2004), Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry 55:708–714. [DOI] [PubMed] [Google Scholar]
- Rodberg EM, den Hartog CR, Anderson RI, Becker HC, Moorman DE, Vazey EM (2017), Stress Facilitates the Development of Cognitive Dysfunction After Chronic Ethanol Exposure. Alcohol Clin Exp Res 41:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Berger A (2018), Targeting the intracellular signaling “STOP” and “GO” pathways for the treatment of alcohol use disorders. Psychopharmacology (Berl) 235:1727–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC (2016), A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct 221:4691–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei S, Ghavami S, Panjehshahin MR, Owji AA (2015), Effects of Ethanol on the Expression Level of Various BDNF mRNA Isoforms and Their Encoded Protein in the Hippocampus of Adult and Embryonic Rats. Int J Mol Sci 16:30422–30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2012), How does stress lead to risk of alcohol relapse? Alcohol Res 34:432–440. [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Achua JK, Summers TR, Ronan PJ, Summers CH (2014), Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Front Behav Neurosci 8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Lopez MF, Archer KJ, Wolen AR, Becker HC, Miles MF (2016), Time-Course Analysis of Brain Regional Expression Network Responses to Chronic Intermittent Ethanol and Withdrawal: Implications for Mechanisms Underlying Excessive Ethanol Consumption. PLoS One 11:e0146257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Vengeliene V, Jandeleit B, Fischer WN, Grindstaff K, Zhang X, Gallop MA, Krstew EV, et al. (2014), Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology 39:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, Sun H, Schank JR, et al. (2014), microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci 34:4581–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Solomon M, Flanigan M, Meinhardt M, Barbier E, Schank JR, Schwandt M, Sommer WH, et al. (2013), Coordinated dysregulation of mRNAs and microRNAs in the rat medial prefrontal cortex following a history of alcohol dependence. Pharmacogenomics J 13:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongiorgi E, Armellin M, Giulianini PG, Bregola G, Zucchini S, Paradiso B, Steward O, Cattaneo A, et al. (2004), Brain-derived neurotrophic factor mRNA and protein are targeted to discrete dendritic laminas by events that trigger epileptogenesis. J Neurosci 24:6842–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongiorgi E, Baj G (2008), Functions and mechanisms of BDNF mRNA trafficking. Novartis Found Symp 289:136–147; discussion 147–151, 193–135. [DOI] [PubMed] [Google Scholar]
- Vaghi V, Polacchini A, Baj G, Pinheiro VL, Vicario A, Tongiorgi E (2014), Pharmacological profile of brain-derived neurotrophic factor (BDNF) splice variant translation using a novel drug screening assay: a “quantitative code”. J Biol Chem 289:27702–27713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ (2014), Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol 48:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R (2008), Neuropharmacology of alcohol addiction. Br J Pharmacol 154:299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Pruunsild P, Timmusk T (2014), Neurotrophins: transcription and translation. Handb Exp Pharmacol 220:67–100. [DOI] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC (2014), Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol 17:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]