Abstract
Aims
Perfluoroalkyl substances (PFAS) are environmentally and biologically persistent synthetic environmental contaminants linked to adverse health outcomes. Though null to modest inverse relationships between PFAS and coronary heart disease (CHD) have been reported, studies regarding relationships in high risk populations such as those with diabetes are sparse. We investigated the relationship of PFAS with CHD in persons with diabetes.
Methods
Data on 5,270 adults, aged ≥20 years, with diabetes were obtained from the C8 Health Project. Four PFAS were investigated separately: perfluorohexane sulfonate (PFHxS), perfluoroctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), and perfluoronanaoic acid (PFNA).
Results
In logistic regression analyses adjusting for age, sex, diabetes duration, BMI, smoking, lipids, WBC, CRP, eGFR, uric acid, hemoglobin and iron, all PFAS were inversely associated with CHD, ORs (95% CIs) : PFHxS; 0.72 (0.65-0.79), PFOA; 0.90 (0.81-0.96), PFOS; 0.90 (0.81-0.99), PFNA; 0.88 (0.76-1.02). Stratification by chronic kidney disease status revealed similar inverse relationships for those with and without chronic kidney disease.
Conclusions
In this cross-sectional study of over 5,000 adults with diabetes, PFAS showed inverse associations with CHD. These findings may, if confirmed in future studies, provide new physiologic understanding of CHD prevention strategies.
Keywords: diabetes, coronary heart disease, perfluoroalkyl substances, perfluoroctanoic acid, perfluorooctane sulfonate, perfluoronanaoic acid
1. Introduction
Coronary heart disease (CHD) is the major cause of death in diabetes. In addition to traditional risk factors, common environmental exposures, including persistent environment contaminants, may also influence CHD risk. Perfluoroalkyl substances (PFAS) are a class of highly fluorinated chemicals, perfluorocarbons, with a wide variety of functional groups and industrial and consumer uses. PFAS contamination has raised public health concerns because these compounds are mobile and persistent in the environment, are readily absorbed into most vertebrate species, including humans, and have been linked to adverse health effects.(1)
In epidemiological studies, exposure to the common PFAS, perfluoroctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), has been weakly linked to higher cholesterol levels (2, 3) and higher serum uric acid levels,(4) which are risk factors for CHD. However, serum levels of these PFAS have also been inversely associated with obesity and C-reactive protein, a measure of systemic inflammation that has also been linked to CHD. Findings regarding the relationship of PFAS with diabetes have been mixed, with most studies suggesting no (5, 6) or inverse associations,(7-9) while a recent prospective study found elevated risk of diabetes associated with PFOA and PFOS.(10) However, few studies have examined the relationship between PFAS and CHD, with most studies showing no association.(2, 11, 12)
The mixed findings concerning the relationship of PFAS with health outcomes may relate to the high oxygen carrying capacity of perfluorocarbons, (13-16) thus potentially mitigating any adverse effect they may have on vascular health via anti-hypoxia properties. Indeed, we have recently observed an inverse relationship between PFAS and progression of chronic kidney disease,(17) progression that is hypothesized to be due to chronic hypoxia.(18-20) This inverse relationship was stronger in persons with anemia or diabetes, a condition also characterized as a state of low grade chronic hypoxia.(17)
Studies on the relationship of PFAS and CHD among persons with diabetes are lacking. The objective of this cross-sectional study was to investigate the relationship of CHD to concentrations of four common PFAS in adults with diabetes. Our underlying hypothesis behind the analyses presented in this report is that the high oxygen carrying capacity of PFAS is protective against CHD.
2. Materials and Methods
2.1. Source of data
The C8 Health Project was created as part of a settlement after it was found that PFOA had contaminated the drinking water of six water districts in the mid-Ohio Valley in West Virginia and Ohio between 1950 and 2004. A post-hoc agreement between the settling parties of the class action lawsuit created the C8 Health Project, a community-based health survey designed to investigate the effects of exposure to PFOA-contaminated drinking water.(21) From August 2005 to August 2006, baseline data were gathered on 69,030 individuals working or living in the six PFOA-contaminated water districts, including those exposed to contaminated private-well drinking water. Estimated participation rate in the C8 Health Project among adult residents of the affected water districts was 81%.(22)
2.2. Clinical and biochemical analyses
The enrollment and data collection methods for the C8 Health Project have been described in detail previously.(21) The health survey collected a wide range of serum and anthropometric measures, as well as self-reported clinician diagnoses of medical conditions. Coronary heart disease diagnoses included self-report of clinician-based diagnosis of myocardial infarction, arteriosclerosis, and coronary artery disease check off boxes, and an opportunity to write out diagnoses in a drop down from “other heart disease” (specified by participants). Diabetes was also based on self-report of clinician diagnosis, and this field also included a drop down menu for the diabetes type and another drop down for the age at disease onset. We obtained institutional review board approval at West Virginia University for access to the C8 Health Project de-identified data for this study.
2.3. Subjects
There were 13,018 children and adolescents under the age of 20 years who were excluded from analysis. Of the remaining 58,712 study participants, 5,296 reported a physician diagnosis of diabetes. Of the 5,296 with diabetes, 26 had missing data on the four major perfluorocarbons of interest. The remaining 5,270 with diagnoses of diabetes form the primary population for this study.
2.4. Measurements of PFAS (PFHxS, PFOA , PFOS , and PFNA)
As referred to in Conway, Innes et al,(7) perfluoroalkyl substances (PFAS), including PFOA, were analyzed at a single commercial laboratory. Details have been published.(21) Briefly, the protein precipitation extraction method with reverse phase high-performance liquid chromatography/tandem mass spectrometry was utilized for PFAS assays. A triple quadrupole mass spectrometer in pre-selected reaction monitoring mode, monitoring for the M/Z transitions of PFAS species with an internal 13C PFAS standard corresponding to the target compound, was utilized for detection of each PFAS. Four PFAS, PFHxS (perfluorohexane sulfonate), PFOA, PFOS, and PFNA (perfluorononanoic acid) were detected in the serum of over 90% of participants and are the focus of the current study.
2.5. Calculation of eGFR
Serum creatinine was measured using a kinetic rate Jaffe method.(23) Estimated glomerular filtration rate was calculated based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.(24) Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2.
2.6. Statistical analysis
General linear models were used to test for differences in continuous variables and the chi square test was used to test for differences in categorical data. Logistic regression was used to estimate the relationship of each PFAS with coronary heart disease. Interaction terms that included the PFAS were created by the cross products of the potential effect modifying variable and the addition of 1 to the log of the specific PFAS. The criterion for statistical significance was a two-tailed P-value of <0.10 for effect modification and <0.05 otherwise. Statistical analysis was conducted using SAS version 9.4 (Cary, North Carolina).
3. Results
3.1. Characteristics of the population
Characteristics of the adult C8 Health Study participants with diabetes stratified by CHD status are presented in Table 1. Persons with CHD and diabetes tended to be older, male, marginally more likely to be White, and more likely to have had diabetes for a longer period of time. They were also more likely to have a history of smoking, chronic kidney disease, higher uric acid levels, but tended to have a lower BMI, lower kidney function as measured by eGFR, and lower lipid levels. Median PFHxS and PFNA were slightly lower among those with CHD than among those without CHD.
Table 1.
Characteristics of Adult (age ≥20 years) with Diabetes in the C8 Health Population by Coronary Heart Disease Status, mean ± SD, median (IQR) or % (n)
| Characteristics | Coronary heart disease (n=1,489) | No coronary heart disease (n=3,781) | P-value |
|---|---|---|---|
| Age, years | 64.2 (11.0) | 55.3 (13.6) | <0.0001 |
| Sex, male | 60.1 (895) | 45.3 (1,714) | <0.0001 |
| Race, White | 96.9 (1,431) | 96.08 (3,601) | 0.16 |
| Diabetes duration, years a,b | 8.5 (3.9-16.2) | 5.9 (2.9-11.6) | <0.0001 |
| A history of smoking | 63.5 (942) | 53.0 (2,001) | <0.0001 |
| BMI, m/kg2 | 32.6 (7.1) | 33.4 (9.9) | 0.002 |
| eGFR, mL/min/1.73 m 2c | 67.1 (22.4) | 81.3 (21.1) | <0.0001 |
| Chronic kidney disease | 36.4 (524) | 15.9 (582) | <0.0001 |
| HDLc, mg/dL | 43.1 (10.8) | 46.6 (12.5) | <0.0001 |
| LDLc, mg/dL | 88.8 (36.6) | 102.3 (35.8) | <0.0001 |
| White blood cell count, x10e3/uL | 7.6 (4.6) | 7.6 (2.3) | 0.32 |
| C-reactive protein, mg/L | 2.7 (1.2-5.9) | 2.9 (1.2-6.4) | 0.55 |
| Uric acid, mg/dL | 6.4 (1.8) | 5.7 (1.6) | <0.0001 |
| Hemoglobin, g/dL | 13.9 (1.6) | 14.2 (1.5) | <0.0001 |
| Serum iron, μ/dL | 78.0 (28.6) | 78.7 (30.4) | 0.46 |
| Perfluoroalkyl acids | |||
| PFHxS, ng/mLa | 2.6 (1.7-3.9) | 2.8 (1.8-4.4) | <0.0001 |
| PFOA, ng/mLa | 28.4 (12.6-74.9) | 29.0 (12.7-72.8) | 0.52 |
| PFOS, ng/mLa | 22.0 (13.8-32.1) | 21.1 (13.7-31.3) | 0.82 |
| PFNA, ng/mLa | 1.3 (0.9-1.7) | 1.4 (1.0-1.8) | <0.0001 |
PFHxS=perfluorohexane sulfonate PFOA=perfluorooctanoic acid PFOS=perfluorooctane sulfonate PFNA=perfluorononanoic acid
Natural logarithmically transformed before analysis
Addition of 1 to each value before being natural logarithmically transformed
estimated glomerular filtration rate, CKD-EPI formula
3.2. Association between PFAS and CHD
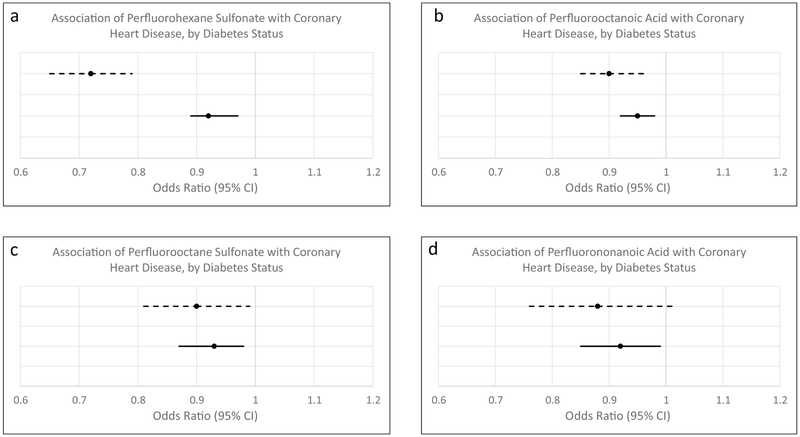
In analyses controlling for age and sex, each PFAS was inversely associated with CHD (ORs = 0.81 (0.78-0.84) for PFHxS; 0.91 (0.89-0.93) for PFOA; 0.85 (0.82-0.89) for PFOS; and 0.83 (0.78-0.88) for PFOS). Upon further controlling for diabetes duration, a history of smoking, BMI, HDLc, LDLc, WBC count, CRP, eGFR, uric acid, hemoglobin and iron, each PFAS remained inversely associated with CHD, with ORs ranging from 0.72 for PFHxS to 0.90 for PFOA and PFOS, and with all but PFNA demonstrating a statistically significant relationship. The multivariable adjusted analyses are presented in Table 2a and Figure 1. Analyses by quintiles showed a stronger inverse relationship with each increasing quintile of PFAS exposure (Table 2b. Results were similar when stratified by sex (Supplementary Table 1).
Table 2.
Multivariable Adjusted Association of Quintiles Perfluoroalkyl Acids with Coronary Heart Disease in those with Diabetes, Odds Ratio (OR) and 95% Confidence Intervals (CI)
| PFHxS | PFOA | PFOS | PFNA | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Table 2a | ||||
| PFASa, b | 0.72 (0.65-0.79) | 0.90 (0.85-0.96) | 0.90 (0.81-0.99) | 0.89 (0.76-1.03) |
| Table 2b | ||||
| PFASa, b | ||||
| Q1 | Ref | Ref | Ref | Ref |
| Q2 | 0.75 (0.58-1.97) | 0.92 (0.71-1.18) | 0.99 (0.76-1.28) | 1.04 (0.80-1.35) |
| Q3 | 0.72 (0.56-0.93) | 0.86 (0.67-1.11) | 0.78 (0.60-1.01) | 0.96 (0.74-1.25) |
| Q4 | 0.66 (0.51-0.85) | 0.74 (0.58-0.96) | 0.85 (0.66-1.10) | 0.93 (0.71-120) |
| Q5 | 0.45 (0.34-0.58) | 0.73 (0.57-0.94) | 0.71 (0.55-0.92) | 0.81 (0.62-1.05) |
| Age | 1.05 (1.04-1.06) | 1.05 (1.04-1.06) | 1.05 (1.04-1.06) | 1.05 (1.04-1.05) |
| Sex, male | 1.65 (1.37-2.00) | 1.60 (1.32-1.93) | 1.62 (1.34-1.96) | 1.61 (1.33-1.94) |
| Diabetes duration | 1.01 (1.01-1.02) | 1.01 (1.01-1.02) | 1.01 (1.01-1.02) | 1.01 (1.01-1.02) |
| A history of smoking | 1.39 (1.18-1.64) | 1.39 (1.18-1.64) | 1.37 (1.16-1.62) | 1.38 (1.17-1.63) |
| BMI | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) | 1.00 (0.99-1.01) | 1.00 (1.00-1.01) |
| HDLc | 0.98 (0.97-0.99) | 0.98 (0.97-0.99) | 0.98 (0.97-0.99) | 0.98 (0.97-0.99) |
| LDLc | 0.99 (0.99-1.00) | 0.99 (0.99-1.00) | 0.99 (0.99-1.00) | 0.99 (0.99-1.00) |
| WBC | 1.03 (0.99-1.07) | 1.03 (0.99-1.07) | 1.03 (0.99-1.06) | 1.03 (0.99-1.06) |
| CRPb | 1.04 (0.96-1.12) | 1.04 (0.97-1.12) | 1.05 (0.97-1.13) | 1.05 (0.98-1.13) |
| eGFRc | 0.99 (0.99-0.99) | 0.99 (0.98-0.99) | 0.99 (0.98-0.99) | 0.99 (0.98-0.99) |
| Uric acid | 1.10 (1.04-1.16) | 1.10 (1.04-1.16) | 1.10 (1.04-1.16) | 1.10 (1.04-1.16) |
| Hemoglobin | 0.93 (0.87-0.99) | 0.91 (0.86-0.98) | 0.91 (0.86-0.98) | 0.91 (0.85-0.97) |
| Iron | 1.00 (1.00-1.01) | 1.00 (0.99-1.01) | 1.00 (1.00-1.01) | 1.00 (1.00-1.01) |
PFAS, perfluoroalkyl substances. This is the model specific PFAS. For example, for the first model it is PFHxS. The first row shows the multivariable adjusted relationship with each PFAS modeled continuously.
PFHxS=perfluorohexane sulfonate PFOA=perfluorooctanoic acid PFOS=perfluoro octane sulfonate PFNA=perfluorononanoic acid
Natural logarithmically transformed before analysis
estimated glomerular filtration rate, CKD-EPI formula
BMI=body mass index HDLc = high density lipoprotein cholesterol LDLc = low density lipoprotein cholesterol WBC = white blood cell count CRP = C-reactive protein
ORs for non-transformed continuous variables are expressed as per unit increase in the specified variable; ORs for natural logarithmically transformed data are for natural log increase in that variable.
Figure 1.
Multivariable association of perfluoroalkyl substances with coronary heart disease, stratified by diabetes status. Solid lines represent persons without diabetes. Dashed lines represent persons with diabetes. Panel a) association of perfluorohexane sulfonate with coronary heart disease; panel b) association of pefluorooctanoic acid with coronary heart disease; panel c) association of perfluorooctane sulfonate with coronary heart disease; panel d) association of perfluorononanoic acid with coronary heart disease. Analyses are adjusted for age, sex, diabetes duration (in persons with diabetes), a history of smoking, BMI, HDLc, LDLc, WBC count, CRP, eGFR, uric acid, hemoglobin, and iron.
As a posthoc analysis, we also investigated the relationship of PFAS among the 49,161 study subjects without diabetes (Table 3). Inverse relationships between each of the PFAS and CHD were also observed among the non-diabetic population, with ORs (95% Cis) ranging from 0.92 (89-97)-0.95 (0.92-0.98). However, as seen in Figure 1, this relationship appeared to be much stronger for PFHxS among those with diabetes (Figure 1), though significant multiplicative effect modification was not observed (all p-values >0.10).
Table 3.
Multivariable Adjusted Association of Perfluoroalkyl Acids with Coronary Heart Disease in those without Diabetes (n=49,161), Odds Ratio (OR) and 95% Confidence Intervals (CI)
| PFHxS | PFOA | PFOS | PFNA | |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| PFASa,b | 0.92 (0.89-0.97) | 0.95 (0.92-0.98) | 0.93 (0.87-0.98) | 0.92 (0.85-0.99) |
| Age | 1.07 (1.07-1.08) | 1.07 (1.07-1.08) | 1.07 (1.07-1.08) | 1.07 (1.07-1.08) |
| Sex, male | 1.21 (1.09-1.35) | 1.21 (1.09-1.34) | 1.21 (1.09-1.34) | 1.20 (1.08-1.34) |
| A history of smoking | 1.84 (1.69-1.99) | 1.84 (1.69-2.00) | 1.83 (1.68-1.98) | 1.83 (1.69-1.99) |
| BMI | 1.01 (1.00-1.01) | 1.01 (1.00-1.01) | 1.01 (1.00-1.01) | 1.01 (1.00-1.01) |
| HDLc | 0.99 (0.99-0.99) | 0.99 (0.99-0.99) | 0.99 (0.99-0.99) | 0.99 (0.99-0.99) |
| LDLc | 0.99 (0.99-0.99) | 0.99 (0.99-0.99) | 0.99 (0.99-0.99) | 0.99 (0.98-0.99) |
| WBC | 1.02 (1.00-1.04) | 1.02 (1.00-1.04) | 1.02 (1.00-1.04) | 1.02 (1.00-1.04) |
| CRPb | 1.05 (1.01-1.09) | 1.05 (1.01-1.09) | 1.05 (1.01-1.09) | 1.05 (1.01-1.09) |
| eGFRc | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) | 1.00 (0.99-1.00) |
| Uric acid | 1.12 (1.08-1.15) | 1.12 (1.08-1.15) | 1.12 (1.08-1.15) | 1.12 (1.08-1.15) |
| Hemoglobin | 0.96 (0.92-0.99) | 0.95 (0.92-0.98) | 0.96 (0.92-0.99) | 0.95 (0.92-0.99) |
| Iron | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) |
PFAS, perfluoroalkyl substances. This is the model specific PFAS. For example, for the first model it is PFHxS. PFHxS=perfluorohexane sulfonate PFOA=perfluorooctanoic acid PFOS=perfluorooctane sulfonate PFNA=perfluorononanoic acid
Natural logarithmically transformed before analysis
estimated glomerular filtration rate, CKD-EPI formula
BMI=body mass index HDLc = high density lipoprotein cholesterol LDLc = low density lipoprotein cholesterol WBC = white blood cell count CRP = C-reactive protein
3.3. Controlling for kidney function and stratification by chronic kidney disease
Twenty-two percent of the population had chronic kidney disease. To account for potential reverse causation due to reduced kidney function, in addition to controlling for eGFR we also stratified by chronic kidney disease status. Results tended to be similar in both those with and without CKD (Supplementary Table 2). No significant interaction by CKD with any of the PFAS was observed (p interaction all >0.10)
4. Discussion
In this large population-based cross-sectional study of over 5,000 adults with diabetes, we investigated the relationship of coronary heart disease with PFAS, a group of environmental toxicants. To our knowledge, this is the first study to specifically examine the relation of PFAS with CHD in adults with diabetes. Although PFAS have been linked to a number of adverse health outcomes, including dyslipidemia,(2, 3) these compounds have also been inversely associated with other cardiovascular disease risk factors such as CRP(25) and kidney function.(17) In the current study, PFAS showed a significant, inverse relationship with CHD; this association persisted after controlling for kidney function and other potentially confounding factors, and remained after stratification by chronic kidney disease status.
Studies investigating the relationship between PFAS and CHD have yielded inconsistent findings, with the majority showing no association. Winquist and Steenland found no meaningful relationship between PFOA and CHD in their study of a worker cohort that included individuals from our broader population, though there was a suggestion of an inverse relationship.(2) In a rural Swedish male population, Mattsson et al found no association between PFAS and CHD overall, with the exception of a significant positive relationship between perfluoroheptanoic acid and likelihood of CHD.(23) While reasons for the disparity in findings are unclear, they may in part reflect differences in study design and population.
Although mechanisms by which PFAS may lower risk for CHD are unknown, the inverse association observed in this study may reflect the anti-inflammatory and/or insulin-sensitizing properties of certain PFAS, as well as their potentially high oxygen transport capacity.(13-16) Inflammation is known to contribute to the development of CHD by promoting atherosclerosis and inducing insulin resistance.(26) PFAS, particularly PFOA and PFOS, have been reported to have potent anti-inflammatory effects. These effects may in part be mediated by activation of peroxisome proliferator-activated receptors (PPARs),(27, 28) which play an important role in lipid and glucose homeostasis and have anti-atherosclerotic properties,(29, 30) including the suppression of vascular inflammation and oxidative stress.(29). CRP is another inflammatory marker generally associated with increased CHD risk; however, CRP has been shown to be inversely related to PFAS.(25) Thus, down regulation of CRP may be another mechanism by which PFAS are associated with a lower likelihood of CHD in our population; reduction of CVD risk factors such as inflammation may partly explain the inverse relationship we observed between PFAS and CHD. Nevertheless, controlling for inflammatory markers such as CRP and WBC count did not eliminate the inverse relationship between PFAS and CHD in our population.
PFAS belong to a broader class of compounds called perfluorocarbons. Perfluorocarbons are hydrogen carbon chains in which the hydrogen atoms have been replaced by fluorine. The fluorine substitution of hydrogen makes perfluorocarbons highly efficient oxygen carriers, as has been shown by some perflurocarbon emulsions such as perfluorooctyl bromide, with peak oxygen solubility reported to be 25 times greater than either blood or water.(13, 14) Another possible mechanism by which PFAS may decrease risk of CHD is by reducing vascular hypoxia. Evidence suggests that hypoxia it is a trigger of inflammation and apoptosis in atherosclerosis.(31, 32) Myocardial hypoxia is also the cause of myocardial infarction.(33) It may be possible that PFAS are protective against atherosclerosis by mitigating hypoxia-induced inflammation and oxidative stress and hypoxia-induced myocardial infarction. Additionally, persons with an already compromised oxygen carrying capacity may suffer from greater harm if they have a myocardial infarction, which further compromises myocardial oxygenation; conversely, someone with superior oxygen carrying capacity may be relatively protected against myocardial infarction.
We cannot explain the much stronger inverse relationship of PFHxS with CHD than for the other PFAS, nor why this was only observed in the diabetic population. This strong inverse relationship was observed when PFHxS was modeled linearly as a continuous variable and when grouped into quitiles. In an animal toxicity study of PFHxS by the Minnesota Department of Health,(34) PFHxS exposure among rats resulted in a decrease in body weight, cholesterol levels and an increase in prothrombin time, all factors likely to lower the risk of CHD.(34) BMI and lipids were included in our models and did not explain the relationship between PFHxS and CHD. While we cannot fully explain why the strong inverse relationship of PFHxS exposure with CHD was only observed among those with diabetes in our population, this may in part reflect the more generalized chronic hypoxia among persons with diabetes, coupled with the longer half lives of the sulfur containing PFAS.(35, 36) Consistent with this explanation, of the four PFAS investigated, we have previously observed the strongest inverse relationship with PFHxS, followed by PFOS, for the likelihood of having diabetes in our prior investigation of PFAS exposure and diabetes risk.(7) Though this inverse relationship was particularly pronounced for Type 1 diabetes, a condition that has been characterized as a state of chronic hypoxia, a similar finding for Type 2 diabetes was also reported by Donat-Vargas et al.(37)
Strengths of this study include the population-based design, the large sample size of adults who reported a diagnosis of diabetes, and the high study participation rate. As noted above, this is the first study to specifically investigate the relation of PFAS levels with CHD in adults with diabetes. Additional strengths include our ability to evaluate persistent biomarkers of PFAS exposure obtained concurrently with survey information regarding diagnosis of diabetes, CHD, and other conditions, the measurement of a broad array of biomarkers and other clinical data, and the extensive information available on potential confounders. We were also able to assess the association of PFAS which CHD by chronic kidney disease status, helping to clarify the possible modifying effects of this condition.
Our study also has several limitations. First, because of the cross-sectional nature of the data, we cannot draw conclusions regarding causal associations and cannot rule out the possibility of survival bias. For example, it is possible that sicker persons with CHD and higher prior PFAS exposure had already died. In a cross-sectional study, we were unable to assess this; however, prospective data on PFAS and CHD has failed to show an association with the contaminants.(11) It remains possible that the observed associations are due to chance; however, this is unlikely given that the relationships of PFAS with CHD remained consistently robust after adjustment for multiple covariates. Unmeasured confounding might also contribute to our findings, although our ability to control for a large number of both known and potential risk factors for CHD renders this possibility less probable. Finally, for the primary analyses, ascertainment of diabetes was based on participant-reported physician diagnosis, which may have introduced misclassification bias. However, agreement between self-report and medical record-verified data for diabetes in this study population was good (over 80%).(9)
In conclusion, in this cross-sectional analysis of over 5000 adults with diabetes, PFAS demonstrated significant inverse relationships with CHD that were not modified by the presence of chronic kidney disease. While our results should not be interpreted as suggesting that exposure to these environmental contaminants is beneficial, these findings may, if confirmed in future studies, may provide new physiologic understanding of CHD prevention strategies.
Supplementary Material
Acknowledgments
KK wrote the manuscript. AMD provided database expertise and critically reviewed the manuscript for scientific content and contributed to the discussion. KEI critically reviewed the manuscript for scientific content and contributed to the discussion. BNC designed the study, analyzed the data, contributed to the discussion and critically reviewed the manuscript for scientific content. Dr. Baqiyyah N. Conway is the guarantor of this work and, as such, had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. This work was presented in part at the 2015 American Heart Association’s Epidemiology and Prevention ∣ Lifestyle and Cardiometabolic Health, New Orleans, LA.
Funding Statement
This work was supported in part by the National Institutes of Health grant U54GM1049 to the West Virginia University CTSI.
Footnotes
Data Availability
The C8 Health Project data used to support the findings of this study have not been made available because of court mandated order related to the legal settlement, which resulted in the C8 Health Project, that only West Virginia University Investigators may access the data.
Conflicts of Interests
AMD has provided expert testimony that communities benefit from medical monitoring of PFAS. The remaining authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Post GB, Gleason JA, Cooper KR. Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: Contaminants of emerging concern. PLoS biology. 2017;15(12):e2002855. Epub 2017/12/21. doi: 10.1371/journal.pbio.2002855. PubMed PMID: 29261653; PMCID: PMC5737881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winquist A, Steenland K. Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environmental health perspectives. 2014;122(12):1299–305. Epub 2014/09/27. doi: 10.1289/ehp.1307943 PubMed PMID: 25260175; PMCID: PMC4256699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starling AP, Engel SM, Whitworth KW, Richardson DB, Stuebe AM, Daniels JL, Haug LS, Eggesbo M, Becher G, Sabaredzovic A, Thomsen C, Wilson RE, Travlos GS, Hoppin JA, Baird DD, Longnecker MP. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environment international. 2014;62:104–12. Epub 2013/11/06. doi: 10.1016/j.envint.2013.10.004. PubMed PMID: 24189199; PMCID: PMC3870471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin XD, Qian Z, Vaughn MG, Huang J, Ward P, Zeng XW, Zhou Y, Zhu Y, Yuan P, Li M, Bai Z, Paul G, Hao YT, Chen W, Chen PC, Dong GH, Lee YL. Positive associations of serum perfluoroalkyl substances with uric acid and hyperuricemia in children from Taiwan. Environmental pollution (Barking, Essex : 1987). 2016;212:519–24. Epub 2016/03/14. doi: 10.1016/j.envpol.2016.02.050. PubMed PMID: 26970855. [DOI] [PubMed] [Google Scholar]
- 5.Karnes C, Winquist A, Steenland K. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environmental research. 2014;128:78–83. Epub 2013/12/05. doi: 10.1016/j.envres.2013.11.003. PubMed PMID: 24299613. [DOI] [PubMed] [Google Scholar]
- 6.Lind L, Zethelius B, Salihovic S, van Bavel B, Lind PM. Circulating levels of perfluoroalkyl substances and prevalent diabetes in the elderly. Diabetologia. 2014;57(3):473–9. Epub 2013/12/18. doi: 10.1007/s00125-013-3126-3. PubMed PMID: 24337155. [DOI] [PubMed] [Google Scholar]
- 7.Conway B, Innes KE, Long D. Perfluoroalkyl substances and beta cell deficient diabetes. J Diabetes Complications. 2016;30(6):993–8. Epub 2016/06/18. doi: 10.1016/j.jdiacomp.2016.05.001. PubMed PMID: 27311784; PMCID: PMC5556924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environmental health perspectives. 2010;118(5):686–92. Epub 2010/01/22. doi: 10.1289/ehp.0901584. PubMed PMID: 20089479; PMCID: PMC2866686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacNeil J, Steenland NK, Shankar A, Ducatman A. A cross-sectional analysis of type II diabetes in a community with exposure to perfluorooctanoic acid (PFOA). Environmental research. 2009;109(8):997–1003. Epub 2009/09/11. doi: 10.1016/j.envres.2009.08.002. PubMed PMID: 19740462. [DOI] [PubMed] [Google Scholar]
- 10.Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environmental health perspectives. 2018;126(3):037001. Epub 2018/03/03. doi: 10.1289/ehp2619. PubMed PMID: 29498927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattsson K, Rignell-Hydbom A, Holmberg S, Thelin A, Jonsson BA, Lindh CH, Sehlstedt A, Rylander L. Levels of perfluoroalkyl substances and risk of coronary heart disease: Findings from a population-based longitudinal study. Environmental research. 2015;142:148–54. Epub 2015/07/06. doi: 10.1016/j.envres.2015.06.033. PubMed PMID: 26142720. [DOI] [PubMed] [Google Scholar]
- 12.Christensen KY, Raymond M, Thompson BA, Anderson HA. Perfluoroalkyl substances in older male anglers in Wisconsin. Environment international. 2016;91:312–8. Epub 2016/03/24. doi: 10.1016/j.envint.2016.03.012. PubMed PMID: 27003842. [DOI] [PubMed] [Google Scholar]
- 13.Hancock JB, Davidson S, Guinn C, Zachary R. Using liquid ventilation to improve lung function in patients with respiratory distress syndrome: a comprehensive review of the literature. AANA J. 2004;72(3):218–24. Epub 2004/06/24. PubMed PMID: 15208970. [PubMed] [Google Scholar]
- 14.Hosgood SA, Nicholson ML. The role of perfluorocarbon in organ preservation. Transplantation. 2010;89(10):1169–75. Epub 2010/04/16. doi: 10.1097/TP.0b013e3181da6064. PubMed PMID: 20393403. [DOI] [PubMed] [Google Scholar]
- 15.Atias S, Mizrahi SS, Shaco-Levy R, Yussim A. Preservation of pancreatic tissue morphology, viability and energy metabolism during extended cold storage in two-layer oxygenated University of Wisconsin/perfluorocarbon solution. The Israel Medical Association journal : IMAJ. 2008;10(4):273–6. Epub 2008/06/14. PubMed PMID: 18548980. [PubMed] [Google Scholar]
- 16.Reznik ON, Bagnenko SF, Loginov IV, Iljina VA, Ananyev AN, Moysyuk YG. The use of oxygenated perfluorocarbonic emulsion for initial in situ kidney perfusion. Transplantation proceedings. 2008;40(4):1027–8. Epub 2008/06/17. doi: 10.1016/j.transproceed.2008.03.053. PubMed PMID: 18555106. [DOI] [PubMed] [Google Scholar]
- 17.Conway Baqiyyah, Badders Ashley, Costacou Tina, Arthur John, Innes Kim. Perfluoroalkyl substances and kidney function in chronic kidney disease, anemia, and diabetes. Diabetes, metabolic syndrome and obesity : targets and therapy. 2018;11:707–16. doi: 10.2147/DMSO.S173809; PMCID: PMC6244585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Q, Colgan SP, Shelley CS. Hypoxia: The Force that Drives Chronic Kidney Disease. Clinical medicine & research. 2016;14(1):15–39. Epub 2016/02/06. doi: 10.3121/cmr.2015.1282. PubMed PMID: 26847481; PMCID: PMC4851450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine LG, Orphanides C, Norman TJ. Progressive renal disease: the chronic hypoxia theory. Kidney Int Suppl. 1998;65:S74–S8. [PubMed] [Google Scholar]
- 20.Ow CPC, Ngo JP, Ullah MM, Hilliard LM, Evans RG. Renal hypoxia in kidney disease: Cause or consequence? Acta physiologica (Oxford, England). 2018;222(4):e12999. Epub 2017/11/22. doi: 10.1111/apha.12999. PubMed PMID: 29159875. [DOI] [PubMed] [Google Scholar]
- 21.Frisbee SJ, Brooks AP Jr., Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, Halverson JA, Vieira VM, Jin C, Leyden KM, Ducatman AM. The C8 health project: design, methods, and participants. Environmental health perspectives. 2009;117(12):1873–82. Epub 2010/01/06. doi: 10.1289/ehp.0800379. PubMed PMID: 20049206; PMCID: PMC2799461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steenland K, Tinker S, Shankar A, Ducatman A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environmental health perspectives. 2010;118(2):229–33. Epub 2010/02/04. doi: 10.1289/ehp.0900940. PubMed PMID: 20123605; PMCID: PMC2831922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cain L, Shankar A, Ducatman AM, Steenland K. The relationship between serum uric acid and chronic kidney disease among Appalachian adults. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(11):3593–9. Epub 2010/05/27. doi: 10.1093/ndt/gfq262. PubMed PMID: 20501458; PMCID: PMC2980994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. Epub 2009/05/06. PubMed PMID: 19414839; PMCID: PMC2763564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genser B, Teles CA, Barreto ML, Fischer JE. Within- and between-group regression for improving the robustness of causal claims in cross-sectional analysis. Environmental health : a global access science source. 2015;14:60. Epub 2015/07/15. doi: 10.1186/s12940-015-0047-2. PubMed PMID: 26159541; PMCID: PMC4702298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montero-Vega MT. The inflammatory process underlying atherosclerosis. Critical reviews in immunology. 2012;32(5):373–462. Epub 2013/01/24. PubMed PMID: 23339672. [DOI] [PubMed] [Google Scholar]
- 27.Mollenhauer MA, Bradshaw SG, Fair PA, McGuinn WD, Peden-Adams MM. Effects of perfluorooctane sulfonate (PFOS) exposure on markers of inflammation in female B6C3F1 mice. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2011;46(2):97–108. Epub 2010/12/21. doi: 10.1080/10934529.2011.532418. PubMed PMID: 21170772. [DOI] [PubMed] [Google Scholar]
- 28.DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden-Adams MM, Luebke RW, Luster MI. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Critical reviews in toxicology. 2009;39(1):76–94. Epub 2008/09/20. doi: 10.1080/10408440802209804. PubMed PMID: 18802816. [DOI] [PubMed] [Google Scholar]
- 29.Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochimica et biophysica acta. 2007;1771(8):972–82. Epub 2007/07/17. doi: 10.1016/j.bbalip.2007.04.021. PubMed PMID: 17631413; PMCID: PMC2083576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi ZG, Zhao X, Zhong W, Xie ML. Osthole improves glucose and lipid metabolism via modulation of PPARalpha/gamma-mediated target gene expression in liver, adipose tissue, and skeletal muscle in fatty liver rats. Pharmaceutical biology. 2016;54(5):882–8. Epub 2015/10/13. doi: 10.3109/13880209.2015.1089295. PubMed PMID: 26455539. [DOI] [PubMed] [Google Scholar]
- 31.Bitto A, De Caridi G, Polito F, Calo M, Irrera N, Altavilla D, Spinelli F, Squadrito F. Evidence for markers of hypoxia and apoptosis in explanted human carotid atherosclerotic plaques. Journal of vascular surgery. 2010;52(4):1015–21. Epub 2010/08/20. doi: 10.1016/j.jvs.2010.05.116. PubMed PMID: 20719466. [DOI] [PubMed] [Google Scholar]
- 32.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. Journal of the American College of Cardiology. 2008;51(13):1258–65. Epub 2008/03/29. doi: 10.1016/j.jacc.2007.12.025. PubMed PMID: 18371555. [DOI] [PubMed] [Google Scholar]
- 33.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine Gn, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–53. Epub 2007/10/24. doi: 10.1161/circulationaha.107.187397. PubMed PMID: 17951284. [DOI] [PubMed] [Google Scholar]
- 34.Perfluorohexane sulfonate In: Environment Health Division HRAU, editor. Minnesota: Minnesota Department of Health; 2009. [Google Scholar]
- 35.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environmental health perspectives. 2007;115(9):1298–305. Epub 2007/09/07. doi: 10.1289/ehp.10009. PubMed PMID: 17805419; PMCID: PMC1964923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, Jakobsson K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational and environmental medicine. 2018;75(1):46–51. Epub 2017/11/15. doi: 10.1136/oemed-2017-104651. PubMed PMID: 29133598; PMCID: PMC5749314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donat-Vargas C, Bergdahl IA, Tornevi A, Wennberg M, Sommar J, Kiviranta H, Koponen J, Rolandsson O, Akesson A. Perfluoroalkyl substances and risk of type II diabetes: A prospective nested case-control study. Environment international. 2019;123:390–8. Epub 2019/01/10. doi: 10.1016/j.envint.2018.12.026. PubMed PMID: 30622063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.