Abstract
The adverse consequences of early-life sleep deprivation on mental health are well recognized, yet many aspects remain unknown, therefore, animal studies can offer useful insights. Male Sprague-Dawley rats at postnatal day (PND) 19 were subjected to sleep deprivation (SD) for 14 days (6–8 hours/day). Control (CON) rats were gently handled. Behavior tests were done on PND33, PND60 and PND90. SD rats exhibited anxiety-like behavior at PND33 and PND60, when compared to CON rats. Depression-like behavior was observed at PND90. Evaluation of oxidative stress and inflammatory markers revealed interesting results. Plasma 8-isoprostane and antioxidant defense enzymes; hemeoxygenase-1, superoxide dismutase, glutathione peroxidase in the prefrontal cortex (PFC), were upregulated in SD rats at PND33 but not at PND90. PFC interleukin-6 protein expression was elevated at PND33 and PND90. PFC mitogen activated protein kinase phosphatase-1 (MKP-1) and p-38 protein expression were upregulated at PND90. PFC expression of glutamate receptor subunits, post synaptic density protein (PSD-95), calcium/calmodulin-dependent protein kinase (CaMKII), and extracellular signal-regulated kinase (ERK1/2), were significantly reduced in SD rats at PND33 and PND90. PFC brain derived neurotrophic factor (BDNF) and cAMP response element binding protein (CREB) were reduced in SD rats at PND90. Our postulation is that SD by increasing PFC oxido-inflammation, negatively affects glutamate receptor subunits and PSD95 expression, which disrupts synapse formation and maturation, potentially causing anxiety-like behavior at PND33. Oxido-inflammation further results in MKP-1 and CaMKII-mediated blockade of ERK1/2 activation, which inhibits CREB dependent BDNF expression. This most likely disrupts neuronal circuit development, leading to depression-like behavior at PND90.
Keywords: Sleep deprivation, Oxidative stress, emotional disorders, prefrontal cortex, synaptic density/plasticity
Introduction
According to the National Sleep Foundation, approximately 55% of children and adolescents do not get adequate sleep (National Sleep Foundation, 2014). Relevant to this, sleep deprivation (SD) in youth is believed to be associated with adverse physical, mental, and behavioral outcomes (Astill, Van der Heijden, Van Ijzendoorn, & Van Someren, 2012; Dewald, Meijer, Oort, Kerkhof, & Bogels, 2010; Touchette et al., 2009). Our understanding of the neurobiological basis for negative outcomes of early life sleep deprivation (EL-SD) is limited. Animal studies can offer useful insights, which can be critical for improving mechanistic understanding of EL-SD-induced adverse behavioral outcomes.
In this study, using a customized automated sleep deprivation system (Ward, Wooden, & Kieltyka, 2017), we examined the consequences of EL-SD across different stages of development in rats. The Pinnacle sleep deprivation system has an automated rotating bar which gently pushes the rats to move and constantly disrupts their sleep in a gentle manner, minimizing personnel involvement. Furthermore, two littermate rats were placed together during SD, thus eliminating the issues of social isolation stress. Male Sprague-Dawley rats at postnatal day (PND) 19 were subjected to SD for 6–8 hours per day, for 14 days, lasting until PND32. This developmental stage (PND19-PND32) in rodents mimics childhood to adolescent phase in humans (Semple, Blomgren, Gimlin, Ferriero, & Noble-Haeusslein, 2013). Importantly, synaptic remodeling and neuronal myelination, which are important for development of neuronal circuits and synaptic plasticity, occur during childhood and continue until late adolescence (Semple et al., 2013). Interestingly, sleep architecture shows remarkable changes during development that parallel the time course of postnatal brain maturation, suggesting an important relationship between postnatal brain development and sleep (Kurth, Olini, Huber, & LeBourgeois, 2015; Lopez et al., 2008; Semple et al., 2013). In fact, sleep promotes synaptic pruning (Maret, Faraguna, Nelson, Cirelli, & Tononi, 2011), synaptic plasticity (Dumoulin Bridi et al., 2015), and neuronal myelination (Kurth, Achermann, Rusterholz, & Lebourgeois, 2013; Kurth et al., 2015). Considering that important changes occur during this period, it seemed reasonable to target PND19-PND32 stage, for sleep disruption and follow the behavioral and biochemical impact of EL-SD thereafter. Following the conclusion of EL-SD protocol, behavioral functions including anxiety-like and depression-like behaviors were evaluated at different stages of development including PND33, PND60 and PND90. Biochemical studies included evaluation of oxidative stress and inflammatory processes. Both oxidative stress (Everson, Laatsch, & Hogg, 2005; Melgarejo-Gutierrez et al., 2013; Ramanathan, Hu, Frautschy, & Siegel, 2010; Villafuerte et al., 2015; Vollert et al., 2011) and neuroinflammation (Born, Lange, Hansen, Molle, & Fehm, 1997; Opp, 2005; Redwine, Dang, & Irwin, 2004; Simpson & Dinges, 2007; Vgontzas et al., 1999) are reported to be upregulated during disruption of normal sleep. Specific areas of the brain are considered susceptible to oxidative stress and inflammation including the pre-frontal cortex (PFC) (de Pablos et al., 2006; Zlatkovic et al., 2014). Here, we examined involvement of an interesting cascade initiated within the central nervous system (CNS), by oxido-inflammatory processes, implicating important molecular targets potentially involved in EL-SD-induced behavioral outcomes including glutamate receptor subunits; GluA1 and GluN2b, post synaptic density protein (PSD) 95, mitogen activated protein kinase phosphatase-1 (MKP-1) and p-38, calcium/calmodulin-dependent protein kinase (CaMKII), extracellular signal-regulated kinase (ERK1/2), cAMP response element binding protein (CREB), and brain derived neurotrophic factor (BDNF).
Material and methods
Animals
Consolidated litters of Sprague-Dawley male pups with lactating female rats were purchased from Envigo, USA. The pups arrived in our animal facility at PND11. The rats were acclimatized in the animal facility for 7 days with 12 h light/12 h dark cycle. Lights were on at 7:00 AM central time, considered as Zeitgeber 0 (ZT0). On PND18, the pups were separated from their mother, and housed as two rats per cage and provided with water gel and food. Rat chow (diet pellets) were cut into small pieces and scattered all over the bedding to allow easy access.
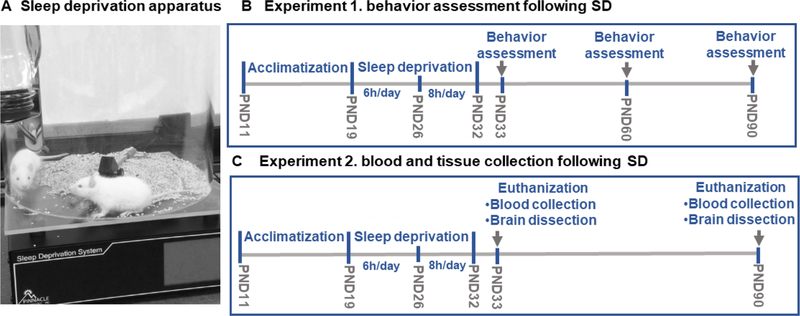
Sleep deprivation apparatus
Pinnacle automated sleep deprivation system was used to induce sleep deprivation in rats (Pinnacle Technology, Lawrence, KS, USA), (Figure 1A). This system effectively produces sleep deprivation in rats as validated by polysomnography in previous studies (Hines, Schmitt, Hines, Moss, & Haydon, 2013; Wooden et al., 2014). The apparatus is a Plexiglas cylindrical cage with a rotating bar at the base. The bar is controlled by a software (Sirenia Acquisition) which enables controlled rotation to maintain desired speed and direction. Random bar rotation was selected to prevent adaptation to the bar rotation. A moderate rotation speed of 10–40 rotations per minute was selected, so that the rotating bars gently touch rat feet and disturb their sleep. The cages were layered with corn cob bedding and equipped with water bottles.
Figure 1.
Early-life Sleep deprivation protocol. The SD apparatus is a Plexiglas cylindrical cage with a rotating bar at the base controlled by a software system (Sirenia Acquisition) (A). In experiment1, the pups arrived at postnatal day (PND) 11 and were acclimatized in the animal facility for 7 days. At PND19, sixteen rats were randomly assigned into sleep deprivation (SD) or control (CON) groups, 8 rats/group. SD group was subjected to SD 6h/day starting at 8:00 AM CT for 7 days. After 7days, at PND26, the SD rats were sleep deprived for 8h/day for 7days. CON rats were placed in a similar apparatus and allowed to sleep in the same room. Behavior tests were performed, at PND33, 60, and 90. Behavior tests: Open field (OF), light/dark (LD), elevated plus maze (EPM), social interaction (SI), and forced swim test (FST) (B). In experiment 2, we followed the same protocol of SD as in experiment1, at PND33, 4 rats were euthanized for blood and brain tissue collection. At PND90, 4 rats were euthanized for blood and brain tissue collection (C).
Early life sleep deprivation protocol
All experiments were conducted in accordance with NIH guidelines using approved protocols from the University of Houston Animal Care Committee. The rats were randomly assigned into two groups; sleep deprivation (SD) and control (CON) groups, 8 rats per group. At PND19, the SD rats were subjected to sleep deprivation 6 hours per day starting at 8:00 AM central time (ZT1) for 7 days, two rats were placed in each sleep deprivation apparatus to exclude social isolation stress. After 7 days, the sleep deprivation duration was increased to 8 hours per day for 7 additional days. This increment was necessary as the percentage of sleep during light phase increases with development in rats (Alfoldi, Tobler, & Borbely, 1990). The CON rats were placed in similar cages (two rats per cage) and left undisturbed in the same room. At the end of the sleep deprivation protocol; at PND33, behavior tests were conducted as described in the following sections. The behavior tests were repeated on the same animals at PND60 and PND90. Separate set of animals were euthanized at PND33 and PND90 for blood collection and brain dissection. A schematic representation of the sleep deprivation protocol is provided in (Figure 1B, C).
Behavior tests
All behavior tests were conducted during the day-time starting at ZT2. Behavior tests were carried out in the order of least to most stressful test. Furthermore, there was a gap of 2–3h between tests. We performed open field (OF) test, and light/dark (LD) test, followed by elevated plus maze (EPM) test at PND33, PND60, and PND90. These were followed by social interaction (SI) and forced swim test (FST) at PND34, PND61, and PND91.
Locomotion and exploratory behavior test
Open field (OF) test
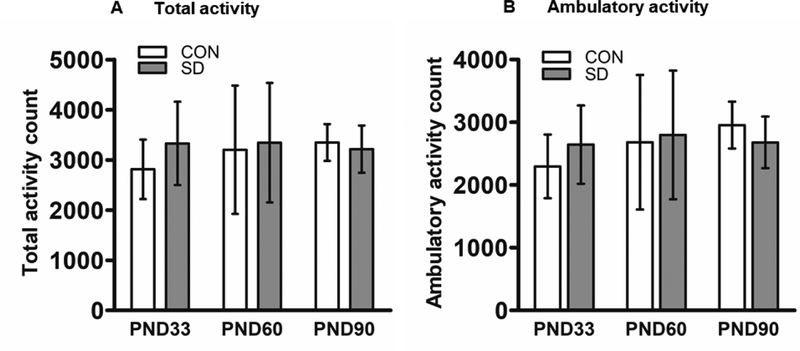
The open field apparatus consists of a rectangular arena (60X40 cm) surrounded by transparent Plexiglas walls. Rats were placed individually in the arena for 15 minutes in a room with dim light. The rat’s movement in the arena was recorded via infra-red sensors and quantified by Opto-Varimex Micro Activity Meter v2.00 system (Optomax, Columbus Instruments, OH). Total activity and ambulatory activity were analyzed. The OF test is routinely used for assessing exploration behavior of rodents (Archer, 1973; Roth & Katz, 1979).
Anxiety-like behavior tests
Light/dark (LD) test
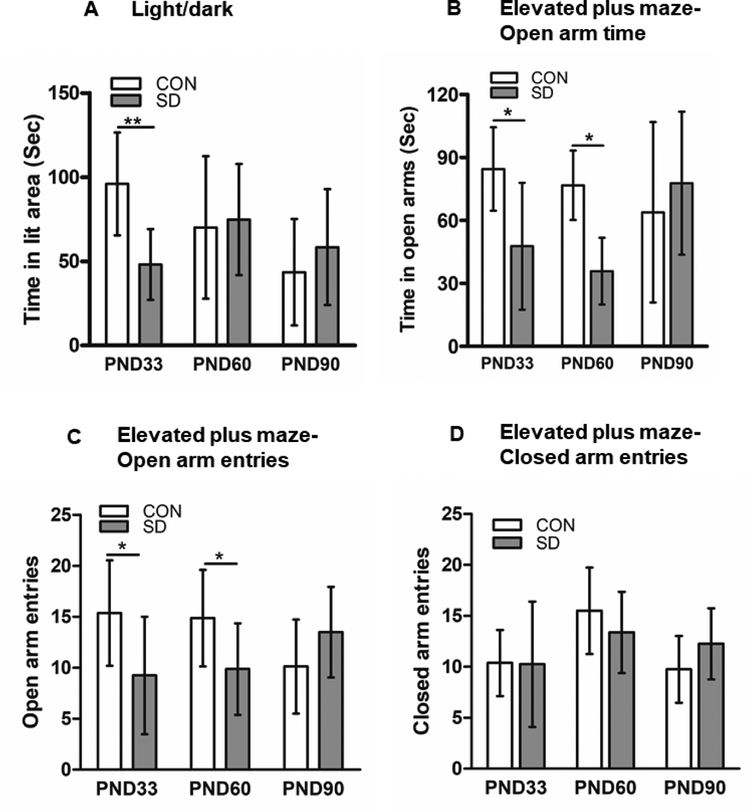
The LD apparatus consists of two compartments; light compartment (27 X 27 X 27 cm) and dark compartment (27 X18 X 27 cm). The rats had free access to both compartments through a single opening (7 X 8 cm) between the two compartments. The rats were placed individually in the light compartment and their movements between the two compartments were recorded for 5 minutes. We calculated the total time spent in the lit area during the total test duration, less time spent in the lit area is an indication of anxiety-like behavior (Vollert et al., 2011).
Elevated plus maze (EPM) test
The EPM apparatus consists of two open and two closed arms (10 X 50 cm) that intersects to create a plus shape. The EPM apparatus is elevated from the floor by 100 cm. The rats were placed individually in the middle between the four arms. Within 5 minutes test duration, the rat’s movement between the arms and the time spent in each arm were recorded. We calculated the total time spent in the open arms, percent time spent in the open arms, the number of open arm entries, the number of closed arm entries, and the percent of number of open arm entries versus total number of all arm entries. Less time spent in open arms and less number of open arm entries are indicators of anxiety-like behavior (Vollert et al., 2011).
Social interaction (SI) test
The social interaction apparatus consists of three-compartments connected via sliding partitions. The middle compartment (25 X 35 X 35 cm) served as habituation compartment, each of the two end compartments (25 X 50 X 35 cm) contained a wire cup. A naïve stranger Sprague Dawley male rat, from a different litter, was placed under one of the wire cups and the second cup left empty. The test consisted of two sessions; habituation session and sociability session. During the habituation session, the test rat was placed in the middle empty compartment for 5 minutes for habituation. In the second “sociability” session, the test rat encountered a stranger rat in one compartment and an empty cup in the other compartment. The sociability session lasted for 10 minutes. The time spent sniffing and interacting with each cup, the time spent in each compartment, and the number of entries into each compartment were recorded. Normal sociability in rats is indicated by their preference to spend more time interacting with another rat (stranger) rather than with the empty cup (Eagle, Fitzpatrick, & Perrine, 2013; Smith, Wilkins, Mogavero, & Veenema, 2015; Toth & Neumann, 2013).
Depression-like behavior test - forced swim test (FST)
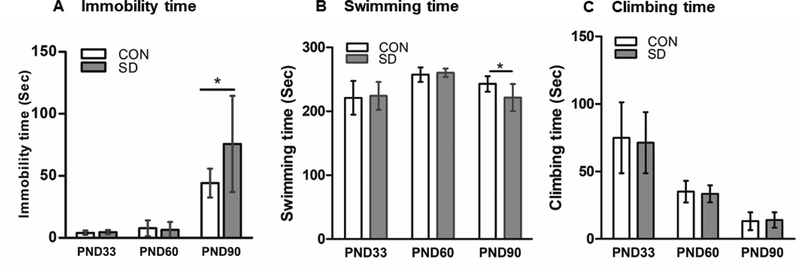
We used FST to assess depression-like behavior in rats. The apparatus consists of a cylindrical Plexiglas tank (24 cm in diameter and 30 cm in height) filled with water (25 °C). The rats were placed individually in the tank and their mobility was recorded for 5 minutes. After struggling in water for a little while, the rats assume an immobile posture. We calculated the total time the rat stayed immobile, as well as the time the rats spent in swimming, or climbing during the total test duration. Longer immobility time and less swimming time are indications of depression-like behavior (Morley-Fletcher et al., 2003; Patki, Solanki, Atrooz, Allam, & Salim, 2013).
Brain dissection and plasma collection
Twenty-four hours after termination of SD protocol, at PND33 and when the rats are adults, at PND90, different set of SD rats were euthanized during the light phase between ZT2–4. Rats were anesthetized using isoflurane (cat. #57319–479–06, Phoenix Pharmaceuticals). Blood was collected from the left ventricle of rat’s heart in ethylene diamine tetra acetic acid (EDTA) tubes. Plasma was separated from the blood by centrifugation at 2000 X g for 20 minutes at 4° C, and stored at −80° C. The rats were quickly decapitated, and brains were removed. The prefrontal cortex (PFC), was dissected out as published (Heffner, Hartman, & Seiden, 1980). The brain tissues were immediately flash frozen in liquid nitrogen and stored at −80° C.
Tissue homogenization and protein estimation
The brain tissues were homogenized using lysis buffer containing 20 mM Tris-HCl, 4 mM ethylene-diamine-tetra-acetic acid (EDTA), protease inhibitors, 100 μg/ml phenyl-methyl-sulfonyl fluoride (PMSF), 1 μg/ml leupeptin, 1 μg/ml aprotinin and 1 μg/ml pepstatin (Salim & Dessauer, 2004). The protein concentration of the lysates was estimated using micro BCA assay kit (Pierce, Rockford, IL).
Measurement of indices of oxidative stress
Plasma 8-isoprostane levels
8-Isoprostane is an eicosanoid generated as a result of phospholipid oxidation by free radicals. Therefore, 8-isoprostane is considered as a marker of oxidative stress. The plasma level of 8-isoprostane was measured using an ELISA kit (cat # 516351, Cayman Chemical, MI) as per manufacturer’s instructions. 8-Isoprostane kit is a competitive ELISA kit which is designed based on the competition between 8-isoprostane in the sample with 8-isoprostane-acetylcholine esterase conjugate for a limited number of 8-isoprostane-specific rabbit antiserum binding sites.
Enzymes of antioxidant defense
It is well known that in response to elevation of oxidative stress, the antioxidant defense system is upregulated in order to mitigate accumulation of reactive oxygen species (Kohen & Nyska, 2002; Salim, 2014). Here, we examined the gene expression levels of antioxidant enzymes in the PFC using quantitative PCR (qPCR) technique. Total RNA from 10 mg of PFC tissue was extracted using Allprep DNA/RNA mini kit (#80204, Qiagen, USA) according to supplier’s instructions. RNA samples were treated with Turbo DNAse (AM2238, Invitrogen, USA) for removal of genomic DNA. The quantity of total RNAs was measured using NanoDrop2000 (Thermo Fisher Scientific DE, USA). A total of 2μg RNA was used to generate cDNA using the high-capacity cDNA reverse transcription Kit (#4368814, Thermo Fisher Scientific DE, USA). 100 ng of cDNA template was used with 2X of power SYBER green PCR master mix (# 4367659, Applied Biosystems, USA) and 100 nm of each forward and reverse primers for PCR reaction. A total of 20μl reaction was prepared as triplicates for each sample on the amplification plate. Quantitative PCR was performed on Applied biosystems 7300 real-time PCR system. qPCR cycles consisted of an initialization step at 95°C for 10 min and the amplification was repeated for 40 cycles with denaturation at 95°C for 15 sec, followed by annealing and extension at 60°C for 60 sec. The relative abundance of each RNA target gene transcript was normalized using the endogenous control gene glyceraldehyde-3- phosphate dehydrogenase (Gapdh). Primers were designed from Rattus norvegicus genome using NCBI primer-blast. Primers were provided by (Thermo Fisher Scientific DE, USA). Primers sequence and gene reference are as follow: Glutathione peroxidase (GPx-1), NCBI reference sequence (NM_030826.4), forward primer (5′-CGGTTTCCCGTGCAATCAGTT-3′), reverse primer (5′-ACACCGGGGACCAAATGATG-3′). Hemeoxygenase-1(HO-1), NCBI reference sequence (NM_012580.2), forward primer (5′-TGGAAGAGGAGATAGAGCGA-3′), reverse primer (5′- TGTTGAGCAGGAAGGCGGTC-3′). Catalase (Cat), NCBI reference sequence (NM_012520.2), forward primer (5′-CGACCGAGGGATTCCAGATG-3′), reverse primer (5’-CGACCGAGGGATTCCAGATG −3′). Superoxide dismutase (SOD-1), NCBI reference sequence (NM_017050.1), forward primer (5′-CACTCTAAGAAACATGGCG-3′), reverse primer (5′-CTGAGAGTGAGATCACACG-3′), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh), NCBI reference sequence (NM_017008.4), forward primer (5′-ATGGGAAGCTGGTCATCAAC-3′), reverse primer (5′- CCACAGTCTTCTGAGTGGCA-3′). Delta cycle threshold (dCt) was calculated by comparing the Ct of each gene with the Ct of Gapdh. (ddCt) was determined by comparing the dCt of the SD sample with dCt of the CON sample. The relative fold change of mRNA was calculated as 2^-ddCt.
Markers of inflammation, stress and neurotrophic signaling
Samples for western blotting were prepared by diluting the PFC brain homogenates in 2× Laemmli buffer (50 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS and 0.1 mg/ml bromophenol blue). Samples (approximately 20 μg of total protein per sample) were resolved on standard 15-well, 8–16% SDS-PAGE gels (Biora, CA). The proteins were transferred to PVDF membrane (Biorad, CA) and then detected as immunoreactive bands using specific primary antibodies and horseradish peroxidase-conjugated secondary antibody. β-actin was used as a loading control. Chemiluminescence reagent (#1705060, Biorad, CA) was used for the development of the protein blots. Chemiluminescence was detected using Gene Sys imaging system (Gene Sys V1.4.1.0, GeneSys, USA) and densitometric analysis was performed using Fluorochem FC8800 software.
Proteins detected using western blotting include: IL-6, TNF-α, P38, JNK, MKP-1, ERK1/2, CREB, BDNF, post synaptic density protein (PSD) 95, AMPAR subunit A1 (GluA1), and NMDAR subunit 2b (GluN2b). Primary and secondary antibody dilution used in western blotting are listed in (Table 1, Appendix).
Experimental design and statistical analysis
A schematic of the experimental design is shown in (Figure 1). Two consolidated litters each with one lactating female Sprague Dawely rat and 10 male pups (PND11) were included in experiment 1 (Figure 1 B). Sixteen rats were randomly assigned into 2 groups (CON vs SD), 8 rats per group. After conclusion of SD protocol, at PND32, behavior experiments were conducted on the same animals at PND33, 60, and 90 in the following order; Open field (OF) followed by light/dark (LD) and elevated plus maze (EPM) on day 1, social interaction followed by forced swim test (FST) on day 2. For analysis of OF, LD, EPM, and FST data, we used two-tailed student t-test. For social interaction, we analyzed the data using two-way ANOVA test followed by post hoc Bonferroni test for pair-wise comparison. Experiment 1 was conducted at three different times, for which a total of 6 different consolidated litters were used. For experiment 2 (Figure 1C), two consolidated litters with lactating female rat and 10 male pups (PND11) were included. Eight rats per group were used in this experiment. After conclusion of SD protocol, one set of rats (4 per group) were euthanized for blood and brain tissue collection at PND33, the other set (4 per group) was euthanized at PND90. Experiment 2 was repeated 2 times. Biochemical tests including 8-isoprostane and western blotting included 5–7 samples per group. qPCR experiments included 4 samples per group. 8-isoprostane and western blotting data were analyzed by two-tailed t-test. qPCR data were analyzed by the nonparametric Mann Whitney U test. Graph Pad prism was used for the statistical analysis (Graph Pad 5 Software, Inc., San Diego, CA). All values are reported as mean ± standard deviation. P< 0.05 was used to denote statistically significant groups.
Results
Locomotor and exploratory behavior test
In the open field test, SD rats did not exhibit any significant difference in total activity as compared to CON rats at PND33 (the mean of total activity counts ± standard deviation; CON rats: 2814 ± 594; SD: 3329 ± 831.7, t14= 1.43, p= 0.176). Neither at PND60 (CON: 3202 ± 1280; SD: 3345 ± 1192, t14= 0.23, p= 0.821), nor at PND90 (CON: 3348 ± 336; SD: 3214 ± 470, t14= 0.64, p= 0.535) (Figure 2A). Similarly, SD rats did not exhibit any significant difference in ambulatory activity as compared to CON rats at PND33 (mean of ambulatory activity count ± standard deviation; CON: 2296 ± 507; SD: 2644 ± 625, t14= 1.22, p= 0.121), neither at PND60 (CON: 2682 ± 1074; SD: 2799 ± 1027, t14= 0.22, p= 0.827), nor at PND90 (CON: 2955 ± 374; SD: 2679 ± 412, t14= 1.40, p= 0.183) (Figure 2B). The open field results suggest that SD rats exhibited normal locomotor and exploratory behaviors.
Figure 2.
Examination of locomotor and exploratory behavior using open field test as analyzed by Opto-Varimex software. Total activity counts measured at PND33, 60, and 90 (A). Ambulatory activity counts measured at PND33, 60, and 90 (B). Bars are means ± standard deviation, n=8 rats/group. Data were analyzed using t-test. PND: post-natal day. Group designations: control (CON), sleep deprivation (SD).
Anxiety-like behavior tests
In the light/dark test, SD rats spent significantly less time (48.1 ± 21.0 sec) in the lit area as compared to CON rats (96.0 ± 30.5 sec) at PND33 (t14= 3.66, p= 0.003), suggesting that SD rats exhibited anxiety-like behavior at early-life (95% confidence interval=19.8 to 75.9). However, the SD rats spent (74.8 ± 33.0 sec) in lit area which is comparable to the time CON rats spent in the lit area (70.1 ± 42.3 sec) at PND60 (t14= 0.24, p= 0.811), which suggest that SD rats did not show anxiety-like behavior at this time point. At PND90, SD rats spent an average of (58.4 ± 34.4 sec) in lit area and CON rats spent (43.5 ± 31.6 sec) in the lit area, hence, no significant difference between SD and CON rats was noted (t14= 0.90, p= 0.383) (Figure 3A). The results suggest that SD rats exhibited anxiety like behavior at early life but not at later life.
Figure 3.
Examination of anxiety-like behavior. Light/dark test was used to examine anxiety-like behavior, time spent in lit area was measured at PND33, 60, and 90 (A). Elevated plus maze test was used to examine anxiety-like behavior. Time spent in open arms measured at PND33, 60, and 90 (B). Elevated plus maze test; number of open arm entries, measured at PND33, 60, and 90 (C). Elevated plus maze test; number of closed arm entries, measured at PND33, 60, and 90 (D). Bars are means ± standard deviation, n=8 rats/group. Data were analyzed using t-test. (*) significantly different at p<0.05. PND: post-natal day. Group designations: control (CON), sleep deprivation (SD).
In the elevated plus maze test, at PND33, SD rats spent significantly less time in the open arms (47.8 ± 30.2 sec, ~15.9% of total test time) as compared to CON rats (84.5 ± 19.9 sec, ~28.2% of total test time, t14= 2.87, p= 0.012, 95% confidence interval (9.3 to 64.1) an indication of anxiety-like behavior, the 95% confidence interval is (9.3 to 64.1). Additionally, the number of open arm entries at PND33 was significantly less in SD rats (9.25 ± 5.7) as compared to CON rats (15.38 ± 8.2, t14= 2.24, p= 0.042, with 95% confidence interval of 0.26 to 11.9). However, the number of closed arm entries did not change between SD and CON rats (CON: 10.38 ± 3.25; SD: 10.25 ± 6.16, t14= 0.051, p= 0.960). Interestingly, percent open arm entries versus total arm entries was comparable between CON (57.7 ± 4.8) and SD rats (51.1 ± 11.5, t14= 1.50, p= 0.155), suggesting that SD spent longer time inside the arms.
Similarly, at PND60, SD rats spent significantly less time in open arms (43.3 ± 26.3 sec, ~11.9% of total test time) as compared to the CON rats (69.4 ± 20.0 sec, ~31.4% of total test time, t14= 2.24, p= 0.042, 95% confidence interval: 0.26 to 11.9), an indication of anxiety-like behavior. Moreover, the number of open arm entries at PND60 was significantly less in SD rats (9.9 ± 4.5) as compared to CON rats (14.88 ± 4.7, t14= 2.17, p= 0.047, 95% confidence interval: 0.1 to 8.9). However, the number of closed arm entries did not change between SD and CON rats (CON: 15.50 ± 4.24; SD: 13.38 ± 3.99, t14= 1.03, p= 0.320). Interestingly, the percent open arm entries versus total arm entries was comparable between CON (48.4 ± 9.1) and SD rats (41.1 ± 10.9, t14= 1. 46, p= 0.167).
While at PND90, all CON and SD rats spent comparable time in open arms (CON: 63.9 ± 43.1 sec, ~21.3% of total test time, SD: 77.8 ± 34.10 sec, ~25.9% of total test time, t14= 0.72, p= 0.486), which suggest that the SD rats did not exhibit anxiety-like behavior at the adult life. Furthermore, at PND90, the number and percentage of open arm entries were comparable between CON and SD rats. Open arm entries (CON:10.13 ± 4.6, SD: 13.5 ± 4.4, t14= 1.49, p= 0.158), percent open arm entries (CON: 49.56 ± 5.3, SD: 51.64± 5.4, t14= 0.71, p= 0.488). Moreover, the number of closed arm entries did not change between SD and CON rats (CON: 9.75 ± 3.28; SD: 12.25 ± 3.49, t14= 1.47, p= 0.153). (Figure 3B, C, D). The data suggest that SD rats exhibited anxiety-like behavior at early life but not at adult life.
Depression-like behavior
Forced swim test (FST) was conducted at PND33, 60 and 90. At PND33, SD and CON rats spent comparable time being immobile in FST. CON immobility time: 4.1 ± 1.6 sec, SD immobility time: 4.6 ± 1.6 sec (t14= 0.62, p= 0.55). The swimming time was also comparable in CON and SD rats at PND33. CON swimming time (221.0 ± 26.5 sec), SD swimming time (224.1 ± 21.7 sec) (t14= 0.26, p= 0.80). Additionally, the climbing time did not change between CON and SD rats. CON climbing time (74.88 ± 26.3 sec), SD climbing time (71.25 ± 22.7 sec) (t14= 0.29, p= 0.772) (Figure 4). The data suggest that SD rats did not show depression-like behavior at early life.
Figure 4.
Examination of depression-like behavior. Forced swim test was used to examine depression-like behavior. Immobility time in FST was measured at PND33, 60 and 90 (A). Swimming time was measured at PND33, 60 and 90 (B). The time the rats spent trying to climb the water tank was measured at PND33, 60, and 90 (C). Bars represent the means ± standard deviation, n= 8 rats/group. Data were analyzed using two-tailed unpaired t-test. (*) significantly different at p<0.05. PND: Postnatal day. Group designations: control (CON), sleep deprivation (SD).
Similarly, SD and CON rats exhibited comparable immobility time at PND60. CON immobility time: 7.8 ± 6.4 sec, SD immobility time: 6.4 ± 6.4 sec (t14= 0.43, p= 0.670), which suggest that SD rats did not show depression-like behavior at early life. Moreover, CON and SD rats spent comparable time swimming in FST at PND60. CON swimming time (257.3 ± 11.5 sec), SD swimming time (260.3 ± 6.5 sec) (t14= 0.64, p= 0.531). The climbing time was also comparable between the two groups at PND60. CON climbing time (35.0 ± 8.1 sec), SD climbing time (33.38 ± 6.3 sec) (t14= 0.45, p= 0.660), (Figure 4). Accordingly, SD rats did not show depression-like behavior at PND60.
Interestingly, at PND90, SD rats exhibited significantly longer immobility time (75.8 ± 28.8 sec) as compared to CON rats (44.1 ± 11.7 sec) (t14= 2.20, p= 0.045), an indication of depression-like behavior (95% confidence interval is (−52.3 to −0.87). Moreover, SD rats spent significantly less time swimming (221.5 ± 21.0 sec) when compared to CON rats (242.8 ± 12.2 sec) (t14= 2.47, p= 0.027), with 95% confidence interval (2.8 to 39.7). However, the climbing time did not change between CON and SD rats. CON climbing time (13.13 ± 6.6 sec), SD climbing time (14.00 ± 5.7 sec) (t14= 0.28, p= 0.781) (Figure 4). The results suggest later onset of depression-like behavior in SD rats. Across all developmental stages, the rats in both CON and SD groups showed reduction in the climbing time which might due to habituation of the test.
Social interaction test
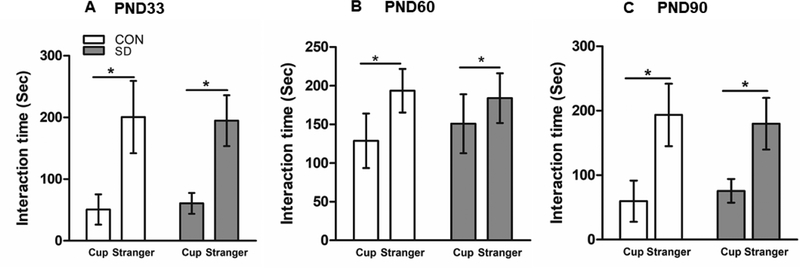
In the social interaction test, rats in SD and CON groups showed significant preference for interaction with the stranger rat rather than the empty cup when assessed at PND33, 60, and 90. At PND33, CON rats spent an average of 50.75 ± 24.5 sec interacting with the cup and 200.6 ± 35.5 sec interacting with the stranger rat, and the SD rats spent 60.75 ± 16.9 sec interacting with the empty cup and an average of 194.8 ± 24.5 sec interacting with the stranger rats. The preference of both groups to interact with the stranger rat rather than the empty cup was significantly higher (F1,28= 107.29, p<0.0001), however, sleep deprivation factor had no effect on the results (F1,28= 0.02, p= 0.881) (Figure 5A),
Figure 5.
Examination of social interaction behavior using the three-compartment paradigm test. Bars represent the time the rats spent interacting with the stranger rat or with the empty cup in seconds (sec) ± standard deviation, measured at PND33 (A), PND60 (B), and PND90 (C). N= 8 rats/group. Data were analyzed using two-way ANOVA followed by post hoc Bonferroni test for pair-wise comparison. (*) significantly different at p<0.05. PND: Postnatal day. Group designations: control (CON), sleep deprivation (SD).
At PND60, both CON and SD groups showed significant preference for interaction with the stranger rat than the empty cup. The CON rats spent 128.8 ± 35.2 sec interacting with the empty cup and 193.5 ± 28.1 sec interacting with the stranger rat. The SD rats spent 150.9 ± 38.1 sec interacting with the empty cup and 183.9 ± 32.1 sec interacting with the stranger rat. The preference of the rats in both groups for interaction with the stranger rat rather than the empty cup was significantly higher (F1,28= 16.92, p=0.0003). Sleep deprivation factor had no effect on the results (F1,28= 0.28, p=0.603) (Figure 5B).
Similarly, at PND90, CON group spent 59.7 ± 31.9 sec interacting with the cup and 193.5 ± 48.4 sec interacting with the stranger rat. The SD rats spent 75.6 ± 18.4 sec interacting with the empty cup and 179.8 ± 40.1 sec interacting with the stranger rat. Both CON and SD rats showed significant preference of interaction with the stranger rat than the empty cup (F1,28= 78.07, p<0.0001), while sleep deprivation factor had no effect on the results (F1,28= 0.07, p= 0.799) (Figure 5C). The results suggest that sleep deprivation did not affect the high sociability behavior in rats.
Oxidative stress markers
Plasma level of 8-isoprostane
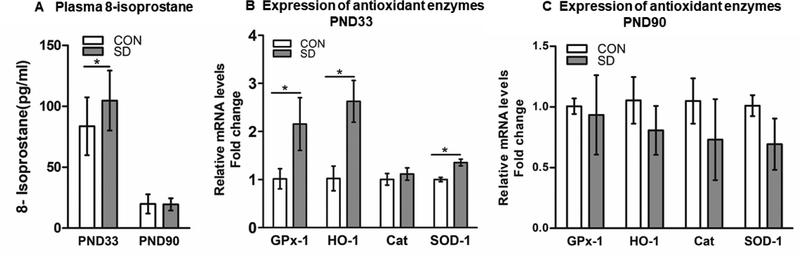
Increased oxidative stress and subsequent buildup of free radicals in the body leads to the oxidation of free fatty acids which results in the production of isoprostanes (Betteridge, 2000). Therefore, the increase in 8-isoprostane levels is considered as an indication of increased oxidative stress. At PND33, plasma 8-Isoprostane levels were significantly elevated in SD rats (104.8 ± 24.6 pg/ml) as compared to CON rats (76.2 ± 14.7 pg/ml), suggesting a systemic elevation of oxidative stress (t10= 2.43, p= 0.035), with 95% confidence interval (−54.6 to −2.4). However, CON and SD rats had comparable plasma 8-isoprostane levels at PND90 (t12= 0.12, p= 0.90), CON: 19.9 ± 7.8 pg/ml; SD: 19.5 ± 4.9 pg/ml) (Figure 6A). The data suggested that systemic oxidative stress normalized with time.
Figure 6.
Examination of plasma 8-isoprostane level and the expression level of antioxidant enzymes in the prefrontal cortex (PFC). 8-Isoprostane concentration was measured in plasma of rats at PND33 and PND90. Bars are means of 8-isoprostane concentration (pg/ml) ± standard deviation, n= 6–7 rats/group. Data were analyzed using two-tailed unpaired t-test. (*) significantly different at p<0.05. Group designations: control (CON), sleep deprivation (SD) (A). Expression level of antioxidant enzymes in the prefrontal cortex (PFC) of rats using relative quantitative PCR technique at PND33 (B), and PND90 (C). Bars are means of fold change in the expression level of the enzymes ± standard deviation as normalized with the reference gene, glyceraldehyde-3-phophate dehydrogenase (GAPDH), and with the control expression level. Glutathione peroxidase-1 (GPx-1), hemeoxygenase-1 (HO-1), catalase (Cat), superoxide dismutase-1 (SOD-1). N= 4 rats/group. Data were analyzed using the nonparametric Mann Whitney U test. (*) significantly different at p<0.05. PND: Postnatal day. Group designations: control (CON), sleep deprivation (SD).
Expression levels of antioxidant enzymes in the PFC
The expression level of the antioxidant enzymes in the PFC was examined at PND33 and PND90. At PND33, the relative expression of GPx-1 was significantly increased by 2.12-fold in the PFC of SD rats as compared to CON rats (p= 0.021, data were analyzed using Mann-Whitney U test). However, at PND90, the expression of GPx-1 in the PFC of SD rats and CON rats were comparable (p= 0.69). At PND33, the relative expression of HO-1 was significantly increased by 2.57-fold in the PFC of SD rats as compared to the CON rats (p= 0.029). However, at PND90, the expression of PFC HO-1 in SD rats and CON rats were comparable (p= 0.34). Similarly. at PND33, the relative expression of SOD-1 was significantly increased by 1.36-fold in the PFC of the SD rats as compared to CON rats (p= 0.029). While, at PND90, the expression of SOD-1 in the PFC of SD and CON rats were comparable (p= 0.34). However, the expression level of Catalase in the PFC of SD and CON rats were comparable at PND33 (p= 0.49), and at PND90 (p= 0.34) (Figure 6B, C).
The effect of EL-SD on inflammatory markers in the PFC
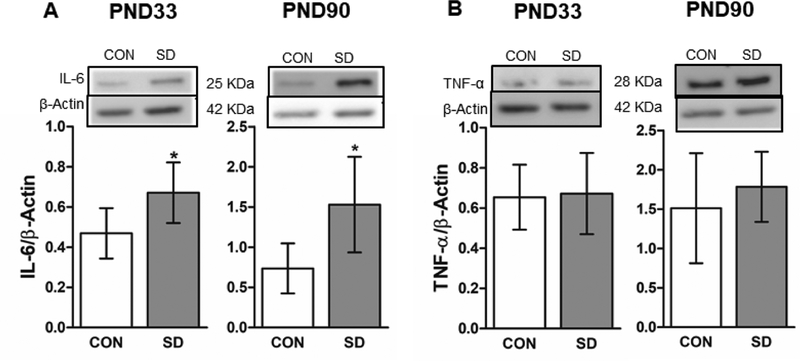
Extended wakefulness enhances cellular metabolism leading to an increase in pro-oxidants and stress pathways which in turn induce an inflammatory response. Therefore, we measured the protein levels of proinflammatory markers including; interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in the PFC of CON and SD rats at PND33 and 90 using western blotting technique. At PND33, the protein levels of IL-6 were significantly increased in the PFC of SD rats (0.671 ± 0.15) as compared to CON rats (0.469 ± 0.12) (t10= 2.45, p= 0.034), with 95% confidence interval (−0.3 to −0.02). Similarly, the protein levels of IL-6 were significantly increased in the PFC of SD rats (1.530 ± 0.59) as compared to CON rats (0.736 ± 0.31) at PND90 (t10= 2.89, p= 0.016), with 95% confidence interval (−1.4 to −0.18) (Figure 7A). The protein levels of TNF-α did not change in the PFC of SD rats (0.672 ± 0.20) as compared to CON rats (0.654 ± 0.16) at PND33 (t10= 0.17, p= 0.868), as well as at PND90 (SD:1.784 ± 0.45; CON: 1.512 ± 0.69, t10= 0.80, p= 0.441) (Figure 7B).
Figure 7.
Examination of the protein levels of the proinflammatory markers in the PFC using the western blotting technique. The protein levels of interleukin-6 (IL-6) in the PFC at PND33 and PND90. The protein levels were normalized with the loading control β-actin (A). The protein levels of the tumor necrosis factor-α (TNF-α) in the PFC at PND33 and PND90. The protein levels were normalized with the loading control β-actin (B). Bars are means ± standard deviation, n= 6 rats/group. Data were analyzed using two-tailed unpaired t-test. (*) significantly different at p<0.05. PND: Postnatal day. Group designations: control (CON), sleep deprivation (SD). Top panels are representative western blotting images.
The effect of EL-SD on PFC MAPKs and stress markers
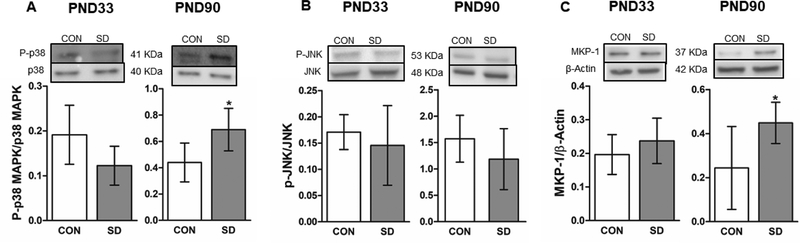
Stress, inflammation or apoptosis lead to activation of p38 and JNK MAPKs, which contributes to alteration of neuronal growth and induction of stress response and inflammation (Collins, Downer, Toulouse, & Nolan, 2015). We measured the phosphorylation levels of p38 and JNK in brain regions at PND33 and 90 using western blotting. At PND33, the phosphorylation levels of p38 MAPK were comparable in the PFC of SD (0.122 ± 0.04) and CON rats (0.191 ± 0.06) (t8=1.96, p= 0.086). While at PND90, the phosphorylation levels of p38 were significantly increased in the PFC of SD rats (0.689 ± 0.16) when compared to the CON rats (0.440 ± 0.15) (t8= 2.58, p= 0.034), with 95% confidence interval (−0.47 to −0.024) (Figure 8A). However, the phosphorylation levels of JNK did not change in the PFC of SD rats (0.146 ± 0.07) when compared to CON rats (0.171 ± 0.03) at PND33 (t12= 0.80, p= 0.434), Similarly, at PND90, the phosphorylation level of JNK did not change in the PFC of SD rats (1.185 ± 0.57) when compared to CON rats (1.572 ± 1.17) (t12= 0.78, p= 0.449) (Figure 8B).
Figure 8.
Examination of the protein levels of stress response markers in the PFC using the western blotting technique. The phosphorylation levels of p38 MAPK were measured in the PFC at PND33 and PND90. The protein phosphorylation levels were normalized with total p38 (A). Phosphorylation levels of JNK MAPK in the brain at PND33 and PND90. The protein phosphorylation levels of JNK were normalized with total JNK (B). The protein levels of the mitogen activated protein kinase phosphatase-1 (MKP-1) were measured in the PFC at PND33 and PND90. The protein levels were normalized with the loading control β-actin (C). Bars are means ± standard deviation, n= 5–7 rats/group. Data were analyzed using two-tailed unpaired t-test. (*) significantly different at p<0.05. PND: Postnatal day. Group designations: control (CON), sleep deprivation (SD). Top panels are representative western blotting images.
Prolonged activation of p38 MAPK is detrimental to the cells due to their role in mediating biosynthesis of proinflammatory cytokines, hence, activation of MAPKs, induces the expression of MAPK phosphatase (MKP-1) which in turn dephosphorylates p38 MAPK and therefore restraints the MAPKs-mediated inflammatory response (Collins et al., 2015). Therefore, we measured the protein levels of MKP-1 in the PFC of the rats at PND33 and PND90 using western blotting technique. The protein levels of MKP-1 did not change in the PFC of the SD rats (0.237 ± 0.07) when compared to CON rats (0.196 ± 0.06) at PND33 (t12= 1.20, p= 0.252), whereas at PND90, MKP-1 protein levels were significantly increased in the PFC of SD rats (0.448 ± 0.09) as compared to the CON rats (0.244 ± 0.18) (t12= 2.38, p= 0.039), with 95% confidence interval (−0.39 to −0.01) (Figure 8C).
The effect of EL-SD on the neurotrophic signaling pathway in the PFC
We measured the effect of EL-SD on the levels of extracellular regulated kinase (ERK1/2), cAMP response element binding protein (CREB), and the brain-derived neurotrophic factor (BDNF) in the PFC of rats at PND33 and 90 using western blotting.
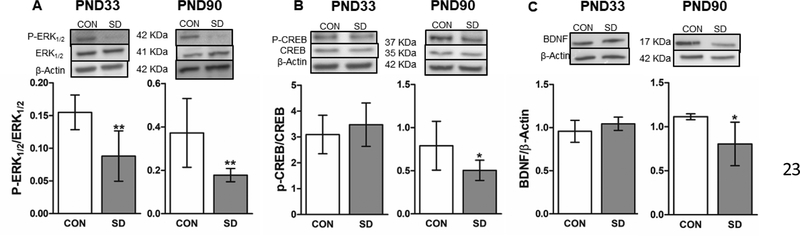
The phosphorylation levels of ERK1/2 were significantly decreased in the PFC of SD rats (0.088 ± 0.03) when compared to CON rats (0.155 ± 0.04) at PND33 (t12= 3.78, p= 0.003), with 95% confidence interval (0.02 to 0.11), as well as at PND90 with comparable values in SD (0.178 ± 0.03) and CON rats (0.372 ± 0.16) (t10= 2.41, p= 0.035), with 95% confidence interval (0.05 to 0.34) (Figure 9A). Moreover, the phosphorylation levels of CREB did not change in the PFC of SD (3.473 ± 0.84) as compared to CON rats (3.093 ± 0.74) at PND33 (t12= 0.80, p= 0.446). While at PND90, the phosphorylation levels of CREB in the PFC of SD rats (0.504 ± 0.11) were significantly reduced when compared to CON rats (0.790 ± 0.28) (t11= 2.30, p= 0.042), with 95% confidence interval (0.01 to 0.56) (Figure 9B).
Figure 9.
Examination of the protein expression levels of ERK1/2, BDNF and CREB. Phosphorylation levels of the extracellular signal-regulated kinase (ERK1/2) in the PFC at PND33 and PND90. The protein phosphorylation levels were normalized with total ERK1/2 (A). Phosphorylation levels of the cAMP response element binding protein (CREB) in PFC were measured at PND33 and PND90. The phosphorylation levels were normalized with total CREB (B). Protein levels of the brain-derived neurotrophic factor (BDNF) in the PFC were measured in PFC at PND33 and PND90. The protein levels were normalized with the loading control β-actin (C). Bars are means ± standard deviation, n= 5–7 rats/group. Data were analyzed using two-tailed unpaired t-test. (*) significantly different at p<0.05. PND: Postnatal day. Group designations: control (CON), sleep deprivation (SD). Top panels are representative western blotting images.
The protein levels of BDNF did not change in the PFC of SD (1.045 ± 0.07) when compared to CON (0.958 ± 0.12) rats at PND33 (t9= 1.34, p= 0.215), whereas the protein levels of BDNF were significantly decreased in the PFC of SD rats (0.80 ± 0.24) when compared to the CON rats (1.114 ± 0.03) at PND90 (t9= 3.07, p= 0.013), with 95% confidence interval (0.08 to 0.53) (Figure 9C).
The effect of EL-SD on markers of synaptic density and plasticity in the PFC
A growing body of evidence suggests that alteration of synaptic density and plasticity is associated with mental disorders. Expression of AMPA and NMDA receptors is positively correlated with synaptic density and plasticity (Marsden, 2013). Here, we measured the protein levels of GluA1 (AMPA receptor subunit), GluN2b (NMDA receptor subunit), and postsynaptic density protein (PSD95) in PFC of rats at PND33 and 90. Additionally, we measured the activation of the synaptic plasticity marker calcium/calmodulin kinase II (CaMKII) in the PFC of rats using western blotting.
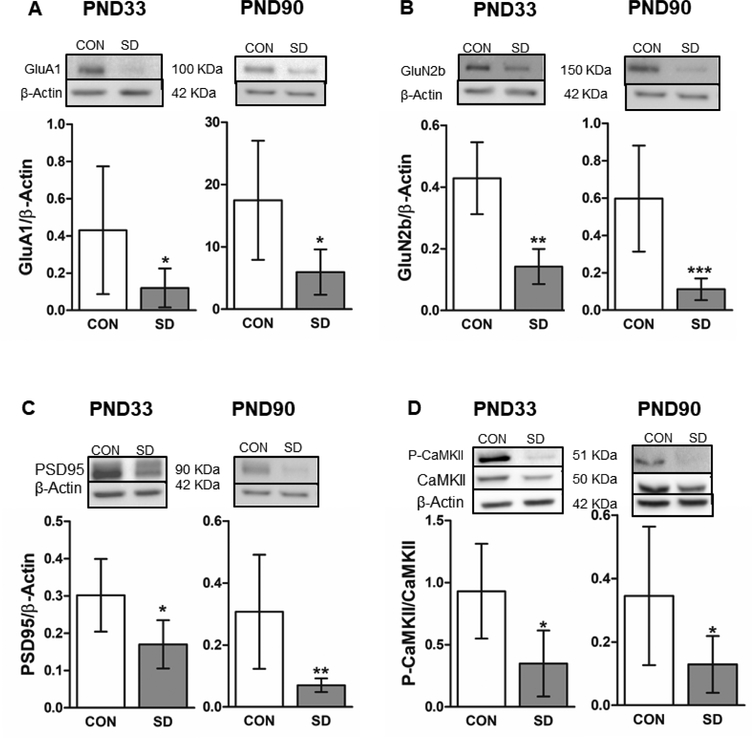
The protein levels of GluA1 were significantly decreased in the PFC of SD rats (0.120 ± 0.10) when compared to CON rats (0.431± 0.14) at PND33 (t12= 2.29, p= 0.041), with 95% confidence interval (0.15 to 0.61), as well as at PND90 (SD: 5.962 ± 3.4; CON: 17.48 ± 9.5) (t9= 2.75, p= 0.023), with 95% confidence interval (2.1 to 21.0) (Figure 10A). Moreover, the protein levels of GluN2b were significantly decreased in the PFC of SD rats (0.142 ± 0.14) when compared to CON rats (0.429 ± 0.12) at PND33 (t12= 4.50, p= 0.0007), with 95% confidence interval (0.13 to 0.44). Similarly, at PND90, the protein levels of GluN2b were significantly decreased in the PFC of SD rats (0.112 ± 0.06) when compared to CON rats (0.597 ± 0.11) (t12= 4.44, p= 0.0008), with 95% confidence interval (0.24 to 0.72) (Figure 10B). Additionally, levels of PSD95 were significantly decreased in the PFC of SD rats (0.170 ± 0.06) when compared to CON rats (0.302 ± 0.09) at PND33 (t11= 2.81, p= 0.017), with 95% confidence interval (0.03 to 0.23). Similarly, at PND90, the protein levels of the PSD95 were significantly decreased in SD (0.070 ± 0.02) as compared to CON rats (0.307 ± 0.18) (t12=3.39, p= 0.005), with 95% confidence interval (0.08 to 0.39) (Figure 10C).
Figure 10.
Examination of the protein expression levels of synaptic density/plasticity markers in the PFC. Protein levels of the AMPA receptor subunit (GluA1) in the PFC were measured at PND33 and PND90. The protein levels were normalized with the loading control β-actin (A). Protein levels of the NMDA receptor subunit (GluN2b) were measured in the PFC at PND33 and PND90. The protein levels were normalized with the loading control β-actin (B). Protein levels of the postsynaptic density protein (PSD95) in PFC at PND33 and PND90. The protein levels were normalized with the loading control β-actin (C). Phosphorylation levels of the calcium/calmodulin-dependent kinase II (CaMKII) were measured in the PFC at PND33 and PND90. The protein phosphorylation levels were normalized with total CaMKII (D). Bars are means ± standard deviation, n= 6–7 rats/group. Data were analyzed using two-tailed unpaired t-test. (*) significantly different at p<0.05. PND: Postnatal day. Group designations: control (CON), sleep deprivation (SD). Top panels are representative western blotting images.
Interestingly, the phosphorylation levels of CaMKII were significantly decreased in the PFC of SD rats (0.349 ± 0.26) as compared to CON rats (0.931 ± 0.38) at PND33 (t10= 3.06, p= 0.012), with 95% confidence interval (0.16 to 1.00). Similarly, the phosphorylation levels of CaMKII at PND90 were significantly reduced in the PFC of SD rats (0.129 ± 0.09) when compared to CON rats (0.345 ± 0.21) (t10= 2.24, p= 0.049), with 95% confidence interval (0.014 to 0.43) (Figure 10D).
Discussion
Sleep by promoting neuronal myelination and synaptic remodeling processes (Bellesi et al., 2013; Maret et al., 2011), not only contributes to brain development and maturation (Kurth et al., 2010; Kurth et al., 2015), but also maintains normal behavior and brain development (Maski & Kothare, 2013). Considering that postnatal brain development occurs early in life, sleep deprivation during early-life can elicit negative consequences in children and adolescents. In fact, sleep deprivation in children and adolescents is commonly associated with emotional problems including anxiety/depression and behavioral difficulties (Astill et al., 2012; Fallone, Acebo, Seifer, & Carskadon, 2005; Gregory & Sadeh, 2012; Maski & Kothare, 2013; Sadeh, Gruber, & Raviv, 2003). One study suggested that sleeping problems in childhood (5 years old) might be associated with increased susceptibility to depression in adult life (35 years old) (Greene, Gregory, Fone, & White, 2015). Another longitudinal study suggested that sleep problems during development (4–19 years old) might be associated with co-occurrence of anxiety/depression and aggressive behavior at adult life (19–32 years old) (Gregory et al., 2005; Gregory, Van der Ende, Willis, & Verhulst, 2008). While in another prospective study, it has been reported that sleep problems in childhood are associated with adulthood anxiety disorders but not depressive disorders (Gregory et al., 2005). Conducting sleep deprivation studies in children is challenging. Therefore, animal studies are useful in studying the impact of EL-SD on behavior across different developmental stages. Relevant to this, the present study offers exciting new insights. Our observations that EL-SD induces an early occurrence of anxiety-like behavior and later onset of depression-like behavior in rats are very interesting and translationally relevant. In this study, the anxiety-like behavior was observed right after induction of SD protocol (PND33) in LD and EPM anxiety tests, but the anxiety-like phenotype seemed to fade by the end of adolescent stage (PND60). At PND60, SD rats showed anxiety-like behavior in EPM test but not in LD test (Figures 3). Test sensitivity seems to play a role in these results. LD test detects anxiety indicated by avoidance of lit areas, but EPM test detects anxiety-like behavior that results from avoidance of open, lit and elevated areas. Thus, while rats at PND60 did not exhibit anxiety-like behavior in LD test, which is a mild test (Salim et al., 2010), anxiety-like phenotype was evident in a more stringent EPM test (Kochi et al., 2017).
Additionally, SD did not impact the high sociability behavior in rats (Figure 5). Rats are very sociable animals and placing the littermates together during SD period might have the advantage of preventing development of social isolation stress after conclusion of SD protocol. Interestingly, at PND60, the rats in both groups showed longer interaction time with the empty cup, without reduction in the time spent interacting with the stranger rat suggesting higher exploration time at this age. In fact, it is well known that social interactions in rats vary with age (Varlinskaya & Spear, 2008), and that periadolescent rats exhibit higher exploration or hyperactivity behavior as compared to younger or older rats (Spear & Brake, 1983). In summary, behavior experiments have suggested that EL-SD was associated with early induction of anxiety-like behavior and late onset of depression-like behavior, which might indicate that anxiety-like behavior appears first, and depression-like behavior occurs later (Liu, Atrooz, Salvi, & Salim, 2017).
Biochemical studies have provided useful insights implicating involvement of oxidative stress and inflammatory processes in EL-SD induced behavioral impairments. A significant increase in oxidative stress was observed in response to EL-SD at PND 33 but not at PND90 (Figure 6). Considering that elevation in oxidative stress is often transitory (Patki et al., 2013; Salim et al., 2010; Solanki, Salvi, Patki, & Salim, 2017) our postulation is that while EL-SD induced oxidative stress observed at PND33 is eventually diminished, hence not observed at PND90, but, it triggered inflammatory processes that led to activation and/or suppression of critical signaling molecules. Proinflammatory markers which often are upregulated together with oxidative stress (Salim et al., 2011) were induced in the PFC of SD rats at PND33 and PND90 (Figure 7A). SD-induced elevation of the proinflammatory marker IL-6 has been reported in humans and in animal models (Chennaoui et al., 2015; Irwin, Olmstead, & Carroll, 2016; Ritter, Kretschmer, Pfennig, & Soltmann, 2013). In fact, SD is reported to directly impact IL-6 levels (Hong, Mills, Loredo, Adler, & Dimsdale, 2005). In addition to IL-6 activation, we observed significant elevation of p38 MAPK phosphorylation (Figure 8A), but not JNK (Figure 8B) at PND90. Activation of MAPKs induces the expression of MAPK phosphatase (MKP-1) (Collins et al., 2015), which is considered as a key factor in regulation of stress responses (Kuwano & Gorospe, 2008). MKP-1 levels were induced in the PFC of SD rats with a parallel enhancement of p38 activation (Figure 8C). This correlated with a significant reduction in the phosphorylation level of ERK1/2 at PND33 and 90 (Figure 9A). These are interesting observations considering the important role MKP-1 plays in neuronal growth, plasticity and survival, which is believed to occur via dephosphorylation of ERK1/2 (Collins et al., 2015; Duman, 2004; Valjent, Caboche, & Vanhoutte, 2001).
Furthermore, ERK1/2 activation indirectly targets CREB phosphorylation which in turn regulates BDNF expression, critical for neuronal growth and plasticity (Impey, Obrietan, & Storm, 1999). Several studies have demonstrated that stress down-regulates BDNF and disrupts CREB function which potentially underlies the molecular mechanism that leads to the pathophysiology of mood disorders (Duman, 2002). We observed reduced activity of ERK1/2 and reduction in the expression of markers of synaptic density/plasticity at PND33 and 90 in PFC of SD rats. Furthermore, CREB phosphorylation (Figure 9B) and BDNF expression (Figure 9C) were significantly reduced in the PFC of SD rats at PND90. Therefore, it is plausible that in PFC of SD rats, the reduced ERK/MAPK activity and disruption of CREB caused an alteration in gene expression and resulted in disruption of the synthesis of proteins responsible for neuronal growth and synaptic plasticity, such as the BDNF and glutamate receptor subunits.
The reduction in synaptic density/plasticity in PFC of SD rats was indicated by the reduction in glutamate receptor markers including GluA1 subunit of AMPARs and a GluN2b subunit of NMDARs, as well as the reduction of the postsynaptic density protein, PSD95 (Figure 10A, B, C). AMPARs expression is positively correlated with the size of the spine head as well as synaptic strength at the excitatory synapse (Marsden, 2013). Moreover, phosphorylation of GluA1 is essential for synaptic potentiation mediated by AMPARs (Marsden, 2013). Furthermore, reduction in GluN2b subunits of NMDA receptors in PFC of SD rats might also contribute to a reduction in synaptic plasticity. Subsequent activation of CaMKII following Ca2+ entry through NMDARs is critical in synaptic plasticity (Marsden, 2013). Interestingly, CaMKII recruitment to NMDARs is facilitated by GluN2b subunit which has a high affinity to CaMKII (Barria & Malinow, 2005). Relevant to this, phosphorylation levels of CaMKII in the PFC of SD rats were significantly reduced in association with the reduction in glutamate receptor markers (Figure 10D). The deleterious effects of sleep deprivation on synaptic plasticity have been reported earlier in rats (Aleisa et al., 2011; Alhaider, Aleisa, Tran, Alzoubi, & Alkadhi, 2010; Alkadhi & Alhaider, 2016; Hagewoud et al., 2010; Kopp, Longordo, Nicholson, & Luthi, 2006).
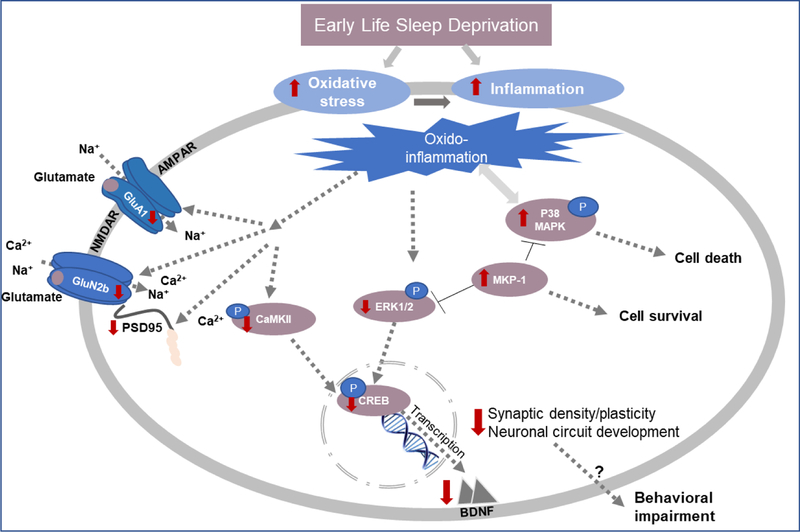
In conclusion, our postulation is that SD by increasing PFC oxido-inflammation, negatively affects GluA1, GluN2b and PSD95 expression, which disrupts synapse formation and maturation, potentially causing anxiety-like behavior at PND33. Oxido-inflammation further results in MKP-1 and CaMKII-mediated blockade of ERK1/2 activation, which inhibits CREB dependent BDNF expression. This most likely disrupts neuronal circuit development, leading to depression-like behavior at PND90 (Figure 11).
Figure 11.
Schematic representation of our hypothesis. Red arrows indicate the changes induced by EL-SD.
Highlights.
This study provides a rat model of early life sleep deprivation (SD).
Early life SD caused age-specific behavioral and cognitive deficits
Early life SD caused anxiety-like behavior at PND33 and 60 but not at PND90.
Early life SD caused depression-like behavior at PND90 but not at PND33 or PND60
Early life SD did not cause learning and memory deficits or social interaction impairment.
Acknowledgements:
This work was supported by a grant from the National Institutes of Health (2R15 MH093918–02) awarded to Dr. Samina Salim.
Appendix
Table 1.
The source and dilution of the antibodies used in western blotting experiments. Dilution for secondary antibodies is 1:2000.
| Protein | Primary antibody Company | Primary antibody Cat # | Primary antibody Dilution | Primary antibody RRID # | Secondary antibody |
|---|---|---|---|---|---|
| TNF-α | Cell Signaling | 3707 | 1:1000 | AB_2240625 | Goat anti-rabbit |
| IL-6 | Thermo Fisher Scientific | ARC0062 | 1:1000 | AB_J501203 | Goat anti-mouse |
| P-P38 MAPK | Abeam | Ab47363 | 1:1000 | AB_881848 | Goat anti-rabbit |
| P38 MAPK | Abeam | Ab31828 | 1:1000 | AB_881839 | Goat anti-mouse |
| P-JNK1,2,3 | Abeam | Ab124956 | 1:1000 | AB_10973183 | Goat anti-rabbit |
| JNK1,2,3 | Abeam | Ab208035 | 1:1000 | Goat anti-rabbit | |
| BDNF | Santa Cruz | SC-546 | 1:500 | AB_630940 | Goat anti-rabbit |
| P-ERK1/2 | Cell Signaling | 9106S | 1:1000 | AB_331768 | Goat anti-mouse |
| ERK1/2 | Cell Signaling | 9107S | 1:1000 | AB_10695739 | Goat anti-rabbit |
| MKP1 | Thermo fisher Scientific | PA5–17973 | 1:1000 | AB_10986429 | Rabbit Anti-goat |
| PSD95 | Thermo fisher Scientific | MA 1–045 | 1:1000 | AB_325399 | Goat anti-mouse |
| GluA1 | Cell Signaling | D4N9V | 1:1000 | AB_2732897 | Goat anti-rabbit |
| GluN2b | Abeam | Ab65783 | 1:1000 | AB_1658870 | Goat anti-rabbit |
| P-CaM KII | Santa Cruz | SC-32289 | 1:1000 | AB_626786 | Goat anti-mouse |
| CaM KII | Santa Cruz | SC-9035 | 1:1000 | AB_634551 | Goat anti-rabbit |
| P-CREB | Cell Signaling | 06–519 | 1:250 | AB_626786 | Goat anti-rabbit |
| CREB | Santa Cruz | SC-58 | 1:1000 | AB_631314 | Goat anti-rabbit |
| β -actin | Cell Signaling | 3700S | 1:1000 | AB_2242334 | Goat anti-mouse |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Aleisa AM, Helal G, Alhaider IA, Alzoubi KH, Srivareerat M, Tran TT, Al -Rejaie SS, & Alkadhi KA 2011. Acute nicotine treatment prevents REM sleep deprivation-induced learning and memory impairment in rat. Hippocampus, 21(8): 899–909. [DOI] [PubMed] [Google Scholar]
- Alfoldi P, Tobler I, & Borbely AA 1990. Sleep regulation in rats during early development. Am J Physiol, 258(3 Pt 2): R634–644. [DOI] [PubMed] [Google Scholar]
- Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, & Alkadhi KA 2010. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep, 33(4): 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkadhi KA, & Alhaider IA 2016. Caffeine and REM sleep deprivation: Effect on basal levels of signaling molecules in area CA1. Mol Cell Neurosci, 71: 125–131. [DOI] [PubMed] [Google Scholar]
- Archer J 1973. A further analysis of responses to a novel environment by testosterone-treated chicks. Behav Biol, 9(3): 389–396. [DOI] [PubMed] [Google Scholar]
- Astill RG, Van der Heijden KB, Van Ijzendoorn MH, & Van Someren EJ 2012. Sleep, cognition, and behavioral problems in school-age children: a century of research meta-analyzed. Psychol Bull, 138(6): 1109–1138. [DOI] [PubMed] [Google Scholar]
- Barria A, & Malinow R 2005. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron, 48(2): 289–301. [DOI] [PubMed] [Google Scholar]
- Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, & Cirelli C 2013. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci, 33(36): 14288–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betteridge DJ 2000. What is oxidative stress? Metabolism, 49(2 Suppl 1): 3–8. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, & Fehm HL 1997. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol, 158(9): 4454–4464. [PubMed] [Google Scholar]
- Chennaoui M, Gomez-Merino D, Drogou C, Geoffroy H, Dispersyn G, Langrume C, Ciret S, Gallopin T, & Sauvet F 2015. Effects of exercise on brain and peripheral inflammatory biomarkers induced by total sleep deprivation in rats. J Inflamm (Lond), 12: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins LM, Downer EJ, Toulouse A, & Nolan YM 2015. Mitogen-Activated Protein Kinase Phosphatase (MKP)-1 in Nervous System Development and Disease. Mol Neurobiol, 51(3): 1158–1167. [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, & Machado A 2006. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci, 26(21): 5709–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, & Bogels SM 2010. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Med Rev, 14(3): 179–189. [DOI] [PubMed] [Google Scholar]
- Duman RS 2002. Synaptic plasticity and mood disorders. Mol Psychiatry, 7 Suppl 1: S29–34. [DOI] [PubMed] [Google Scholar]
- Duman RS 2004. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med, 5(1): 11–25. [DOI] [PubMed] [Google Scholar]
- Dumoulin Bridi MC, Aton SJ, Seibt J, Renouard L, Coleman T, & Frank MG 2015. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci Adv, 1(6): e1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Fitzpatrick CJ, & Perrine SA 2013. Single prolonged stress impairs social and object novelty recognition in rats. Behav Brain Res, 256: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson CA, Laatsch CD, & Hogg N 2005. Antioxidant defense responses to sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol, 288(2): R374–383. [DOI] [PubMed] [Google Scholar]
- Fallone G, Acebo C, Seifer R, & Carskadon MA 2005. Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep, 28(12): 1561–1567. [DOI] [PubMed] [Google Scholar]
- Greene G, Gregory AM, Fone D, & White J 2015. Childhood sleeping difficulties and depression in adulthood: the 1970 British Cohort Study. J Sleep Res, 24(1): 19–23. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Eley TC, Moffitt TE, Oconnor TG, & Poulton R 2005. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol, 33(2): 157–163. [DOI] [PubMed] [Google Scholar]
- Gregory AM, & Sadeh A 2012. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med Rev, 16(2): 129–136. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Van der Ende J, Willis TA, & Verhulst FC 2008. Parent-reported sleep problems during development and self-reported anxiety/depression, attention problems, and aggressive behavior later in life. Arch Pediatr Adolesc Med, 162(4): 330–335. [DOI] [PubMed] [Google Scholar]
- Hagewoud R, Havekes R, Novati A, Keijser JN, Van der Zee EA, & Meerlo P 2010. Sleep deprivation impairs spatial working memory and reduces hippocampal AMPA receptor phosphorylation. J Sleep Res, 19(2): 280–288. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA, & Seiden LS 1980. A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav, 13(3): 453–456. [DOI] [PubMed] [Google Scholar]
- Hines DJ, Schmitt LI, Hines RM, Moss SJ, & Haydon PG 2013. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl Psychiatry, 3: e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Mills PJ, Loredo JS, Adler KA, & Dimsdale JE 2005. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun, 19(2): 165–172. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, & Storm DR 1999. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron, 23(1): 11–14. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, & Carroll JE 2016. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry, 80(1): 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi C, Liu H, Zaidi S, Atrooz F, Dantoin P, & Salim S 2017. Prior treadmill exercise promotes resilience to vicarious trauma in rats. Prog Neuropsychopharmacol Biol Psychiatry, 77: 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R, & Nyska A 2002. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol, 30(6): 620–650. [DOI] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, & Luthi A 2006. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci, 26(48): 12456–12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Achermann P, Rusterholz T, & Lebourgeois MK 2013. Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role? Brain Sci, 3(4): 1445–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, & Huber R 2010. Characteristics of sleep slow waves in children and adolescents. Sleep, 33(4): 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Olini N, Huber R, & LeBourgeois M 2015. Sleep and Early Cortical Development. Curr Sleep Med Rep, 1(1): 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano Y, & Gorospe M 2008. Protecting the stress response, guarding the MKP-1 mRNA. Cell Cycle, 7(17): 2640–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Atrooz F, Salvi A, & Salim S 2017. Behavioral and cognitive impact of early life stress: Insights from an animal model. Prog Neuropsychopharmacol Biol Psychiatry, 78: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Roffwarg HP, Dreher A, Bissette G, Karolewicz B, & Shaffery JP 2008. Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience, 153(1): 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret S, Faraguna U, Nelson AB, Cirelli C, & Tononi G 2011. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci, 14(11): 1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden WN 2013. Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry, 43: 168–184. [DOI] [PubMed] [Google Scholar]
- Maski KP, & Kothare SV 2013. Sleep deprivation and neurobehavioral functioning in childre n. Int J Psychophysiol, 89(2): 259–264. [DOI] [PubMed] [Google Scholar]
- Melgarejo-Gutierrez M, Acosta-Pena E, Venebra-Munoz A, Escobar C, Santiago-Garcia J, & Garcia-Garcia F 2013. Sleep deprivation reduces neuroglobin immunoreactivity in the rat brain. Neuroreport, 24(3): 120–125. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudery M, Koehl M, Casolini P, Van Reeth O, & Maccari S 2003. Prenatal stress in rats predicts immobility behavior in the forced swim test. Effects of a chronic treatment with tianeptine. Brain Res, 989(2): 246–251. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation, 2014. Sleep in America poll. Available at https://sleepfoundation.org/sites/default/files/2014-NSF-Sleep-in-America-poll-summary-of-findings---FINAL-Updated-3-26-14-.
- Opp MR 2005. Cytokines and sleep. Sleep Med Rev, 9(5): 355–364. [DOI] [PubMed] [Google Scholar]
- Patki G, Solanki N, Atrooz F, Allam F, & Salim S 2013. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res, 1539: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan L, Hu S, Frautschy SA, & Siegel JM 2010. Short-term total sleep deprivation in the rat increases antioxidant responses in multiple brain regions without impairing spontaneous alternation behavior. Behav Brain Res, 207(2): 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine L, Dang J, & Irwin M 2004. Cellular adhesion molecule expression, nocturnal sleep, and partial night sleep deprivation. Brain Behav Immun, 18(4): 333–340. [DOI] [PubMed] [Google Scholar]
- Ritter PS, Kretschmer K, Pfennig A, & Soltmann B 2013. Disturbed sleep in bipolar disorder is related to an elevation of IL-6 in peripheral monocytes. Med Hypotheses, 81(6): 1031–1033. [DOI] [PubMed] [Google Scholar]
- Roth KA, & Katz RJ 1979. Stress, behavioral arousal, and open field activity--a reexamination of emotionality in the rat. Neurosci Biobehav Rev, 3(4): 247–263. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, & Raviv A 2003. The effects of sleep restriction and extension on school -age children: what a difference an hour makes. Child Dev, 74(2): 444–455. [DOI] [PubMed] [Google Scholar]
- Salim S 2014. Oxidative stress and psychological disorders. Curr Neuropharmacol, 12(2): 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Asghar M, Taneja M, Hovatta I, Chugh G, Vollert C, & Vu A 2011. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res, 1404: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, & Dessauer CW 2004. Analysis of the interaction between RGS2 and adenylyl cyclase. Methods Enzymol, 390: 83–99. [DOI] [PubMed] [Google Scholar]
- Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, & Chugh G 2010. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res, 208(2): 545–552. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, & Noble-Haeusslein LJ 2013. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol, 106–107: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson N, & Dinges DF 2007. Sleep and inflammation. Nutr Rev, 65(12 Pt 2): S244–252. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Wilkins KB, Mogavero JN, & Veenema AH 2015. Social Novelty Investigation in the Juvenile Rat: Modulation by the mu-Opioid System. J Neuroendocrinol, 27(10): 752–764. [DOI] [PubMed] [Google Scholar]
- Solanki N, Salvi A, Patki G, & Salim S 2017. Modulating Oxidative Stress Relieves Stress-Induced Behavioral and Cognitive Impairments in Rats. Int J Neuropsychopharmacol, 20(7): 550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, & Brake SC 1983. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol, 16(2): 83–109. [DOI] [PubMed] [Google Scholar]
- Toth I, & Neumann ID 2013. Animal models of social avoidance and social fear. Cell Tissue Res, 354(1): 107–118. [DOI] [PubMed] [Google Scholar]
- Touchette E, Cote SM, Petit D, Liu X, Boivin M, Falissard B, Tremblay RE, & Montplaisir JY 2009. Short nighttime sleep-duration and hyperactivity trajectories in early childhood. Pediatrics, 124(5): e985–993. [DOI] [PubMed] [Google Scholar]
- Trivedi AN, Nsa W, Hausmann LR, Lee JS, Ma A, Bratzler DW, Mor MK, Baus K, Larbi F, & Fine MJ 2014. Quality and equity of care in U.S. hospitals. N Engl J Med, 371(24): 2298–2308. [DOI] [PubMed] [Google Scholar]
- Valjent E, Caboche J, & Vanhoutte P 2001. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol Neurobiol, 23(2–3): 83–99. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP 2008. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res, 188(2): 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, & Chrousos GP 1999. Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf), 51(2): 205–215. [DOI] [PubMed] [Google Scholar]
- Villafuerte G, Miguel-Puga A, Rodriguez EM, Machado S, Manjarrez E, & Arias-Carrion O 2015. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev, 2015: 234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, & Salim S 2011. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res, 224(2): 233–240. [DOI] [PubMed] [Google Scholar]
- Ward CP, Wooden JI, & Kieltyka R 2017. Effects of Sleep Deprivation on Spatial Learning and Memory in Juvenile and Young Adult Rats. Psychol Neurosci, 10(1): 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooden JI, Pido J, Mathews H, Kieltyka R, Montemayor BA, & Ward CP 2014. Sleep deprivation impairs recall of social transmission of food preference in rats. Nat Sci Sleep, 6: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkovic J, Todorovic N, Boskovic M, Pajovic SB, Demajo M, & Filipovic D 2014. Different susceptibility of prefrontal cortex and hippocampus to oxidative stress following chronic social isolation stress. Mol Cell Biochem, 393(1–2): 43–57. [DOI] [PubMed] [Google Scholar]