Abstract
Purpose:
To identify the best metrics or combination of metrics that provide the highest predictive power between normal eyes and the clinically unaffected eye of patients with highly asymmetric keratoconus using data from a Dual Scheimpflug/Placido device
Design:
Retrospective case-control study.
Methods:
Combined Dual Scheimpflug/Placido imaging was obtained from the Galilei G 4 device (Ziemer Ophthalmic Systems AG; Port, Switzerland) in 31 clinically unaffected eyes with highly asymmetric keratoconus and 178 eyes from 178 patients with bilaterally normal corneal examinations that underwent uneventful LASIK with at least one year follow-up. Receiver operating characteristic (ROC) curves were generated to determine area under the curve (AUC), sensitivity, and specificity for 87 metrics, and logistic regression modeling was utilized to determine optimal variable combinations.
Results:
No individual metric achieved an AUC greater than 0.79. A combined model consisting of 9 metrics yielded an AUC of 0.96, with 90.3% sensitivity and 92.6% specificity. Among those 9 metrics included, 5 related to corneal pachymetry, Opposite Sector Index (OSI) and Anterior Height BFS Z from the anterior surface, Asphericity and Asymmetry Index (AAI), Posterior Height BFS Z, and Posterior Height BFS X from the posterior surface. The strongest variable in the model was the thinnest point location on the horizontal (x) axis.
Conclusion:
While individual metrics performed poorly, using a combination of metrics from combined Dual Scheimpflug/Placido device provided a useful model for differentiating normal corneas from the clinically normal eyes of patients with highly asymmetric keratoconus. Pachymetry values were the most impactful metrics.
Keywords: Keratoconus, Subclinical, Scheimpflug, Pentacam, Pachymetry, Screening, Epithelial thickness, Ocular coherence tomography, OCT, Dual Scheimpflug, Placido, Galilei
Screening for signs of keratoconus (KC) and related corneal ectatic disorders in the earliest stages remains challenging, and identification using a single device1-8 or a combination of different technologies9, 10 still remains suboptimal. Corneal ectasia after excimer laser refractive surgery is a rare but still a serious complication that can arise with a higher probability in eyes with undiagnosed early signs of keratoconus. 11-13
Initially, most screening for KC detection was performed using Placido reflection-based technology to evaluate anterior surface curvature.13-19 With the evolution of corneal tomography, multiple devices have the ability to measure additional variables such as regional corneal thickness and anterior and posterior surface elevation. Varying technologies utilize scanning-slit beam imaging, Scheimpflug technology, combined Scheimpflug and Placido imaging, and combined Dual Scheimpflug imaging and Placido imaging.
The optimal metric or group of metrics to use for KC detection is still greatly debated in the literature, as some authors suggest that earliest signs of keratoconus can be identified using corneal thickness variables20-22 while other suggest that changes first manifest in posterior corneal surface maps.3,23,24 Various studies have combined multiple variables from the anterior and posterior surfaces to achieve better predicative models, while other groups have utilized wavefront aberration metrics,1,25-29 or optical coherence tomography (OCT) derived corneal thickness changes and epithelial thickness distribution maps.30 There are also devices that aim to measure the corneal biomechanical strength directly31-34 To date none of these individual technologies have been established as optimal or superior to other technologies in identifying keratoconus in its earliest stages.
The purpose of this study was to identify the variables and variable combinations most impactful in differentiating normal control eyes from the minimally affected eye of patients with highly asymmetric keratoconus using a Dual Scheimpflug/Placido-based imaging device.
METHODS
This retrospective, case-controlled study was conducted at the Department of Ophthalmology, Keck School of Medicine, University of South California, USA, and approved separately by the Institutional Review Boards of Emory University (Atlanta, GA, USA), Baylor College of Medicine, and the University of Southern California (Los Angeles, CA, USA). The study was conducted in accordance with the tenets of the Declaration of Helsinki.
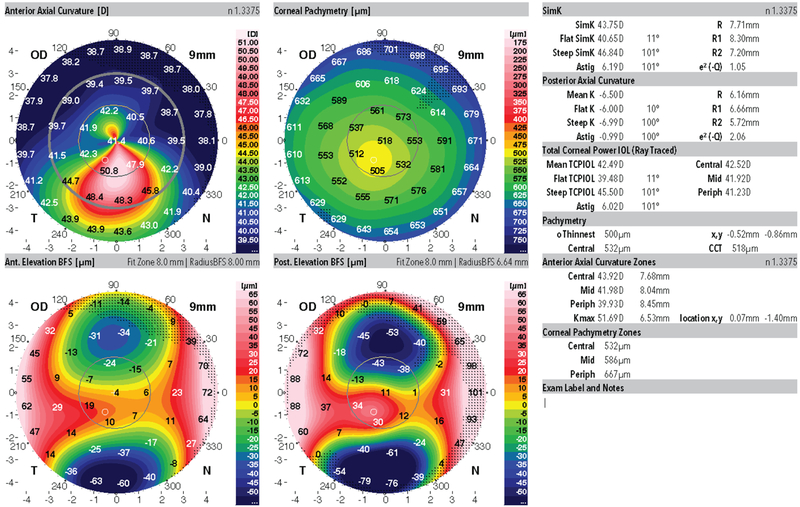
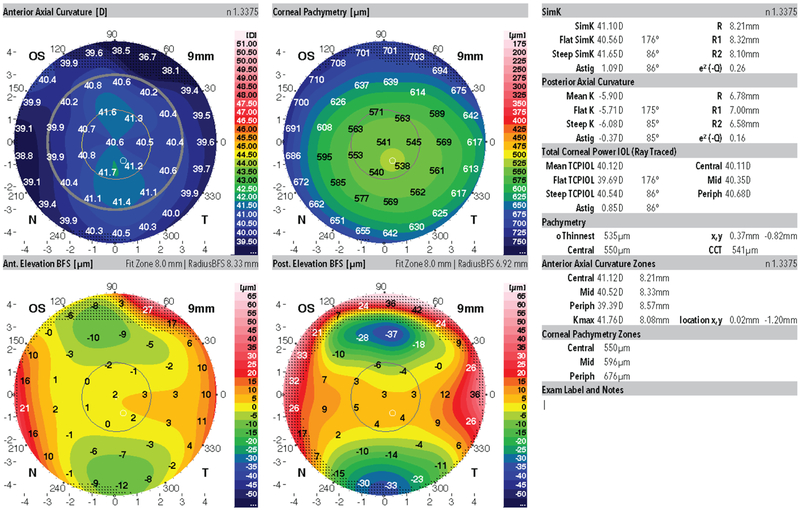
The study and population presented to Emory University, Baylor College of Medicine and the Tel Aviv Sourasky Medical Center, Tel Aviv, Israel from 2014 to 2017. Patients were included in the study group if they had highly asymmetric keratoconus disease presentation. Specifically, all study patients had clear evidence of keratoconus in one eye and no clear evidence of disease in the clinically unaffected eye. For the clinically involved eye, all 31 affected fellow eyes were identified using this screening methodology; patients had abnormal focal anterior corneal steepening, focal corneal thinning, clinical evidence of disease at the slit lamp, scissoring on retinoscopy, subjectively impaired visual acuity, and best corrected distance acuity (CDVA) worse than 20/20. For the unaffected asymmetric keratoconus eye (AKC), there was no clinical evidence of disease, no physical findings on slit lamp examination, no definitive abnormalities on corneal imaging, and CDVA of 20/20 or better. All image screening was performed directly on the computer using a variety of settings and review of different maps; thus no one single output style was relied upon for image screening. Representative study population images are shown for the AKC group in Figures 1 and 2. The complete set of Dual Scheimpflug/Placido imaging is available online as supplemental material.
Figure 1:
Representative Dual Scheimpflug/Placido image from the clinically affected keratoconus eye of a patient from this study
Figure 2:
Representative Dual Scheimpflug/Placido image from the clinically unaffected eye (subclinical asymmetric keratoconus) fellow eye from the same patient shown in Figure 1.
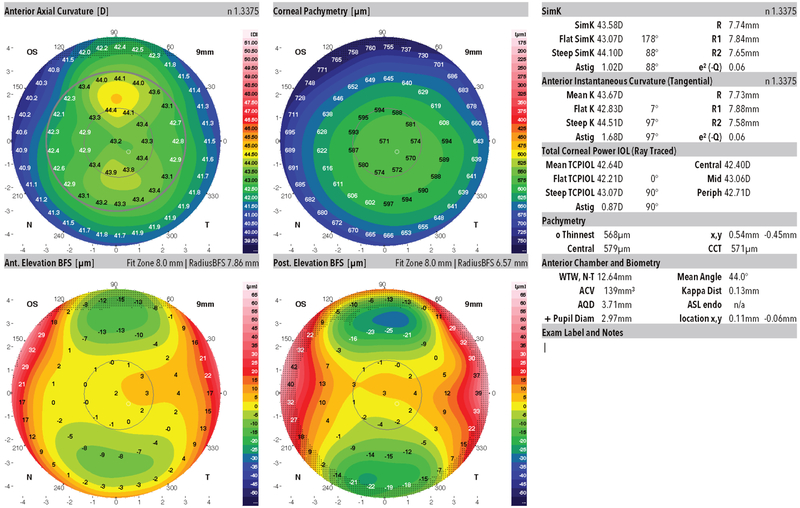
The Control group comprised of patients with pre-operative bilateral normal topography and tomography maps who all underwent LASIK at Emory with at least one-year uneventful follow-up (Figure 3). Eyes with ocular pathology, previous ocular surgery, soft contact lenses worn one week before or rigid contact lens two weeks before and low-quality topography maps that did not fulfill the minimal quality required by the system were excluded from analysis.
Figure 3:
Representative Dual Scheimpflug/Placido image from a bilaterally normal topography and tomography eye of a patient from this study.
Dual Scheimpflug/Placido Device Parameters
All measurements were performed with the Galilei dual Scheimpflug analyzer (G2 and G4, Ziemer Ophthalmic Systems AG; Port, Switzerland) according to the manufacturer’s instructions. The Galilei device is a combined Placido topography and Scheimpflug tomography device with a dual Scheimpflug camera. The system obtains between 15 to 60 Scheimpflug images each scan and two Placido top view images at ninety degrees apart, while the cameras rotate over a central axis. Scheimpflug and Placido data are obtained concurrently. Data extrapolated from the Scheimpflug and Placido images are merged to deliver a surface tailored to the anterior cornea. Data from the posterior corneal surface is measured using the edge detection in images provided by the dual Scheimpflug system. The devices used in this study were set up using the standard settings to acquire 15 images.
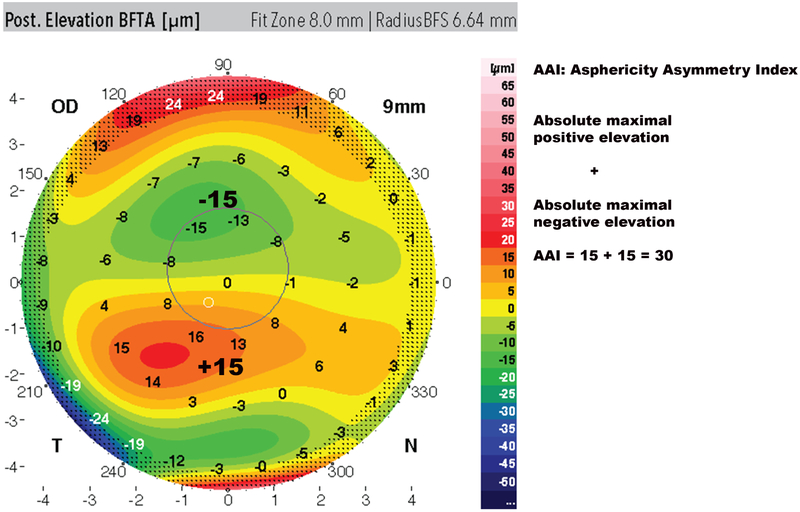
The analysis was performed with the Galilei device’s software version 6.3.1b3 (Ziemer Ophthalmic Systems AG; Port, Switzerland). A total of 87 corneal metrics was collected for the analysis. The majority of the metrics were exported automatically by the Galilei software to csv files except for the high order aberrations, the elevation data from the best fit sphere (BFS) maps, best fit toric and aspheric (BFTA) maps and the Posterior Asymmetry and Asphericity index (AAI) that were collected automatically but exported manually. The Posterior AAI was obtained through manual calculations as previously described.35,36 The Posterior AAI is calculated using the BFTA map as the absolute value of the highest negative elevation and highest positive elevation value within the posterior central 6-mm diameter data zone, 180° opposite 45° zones (Figure 4).
Figure 4:
Asphericity asymmetry Index (AAI) calculation. Adding the absolute value of the maximum positive elevation and the maximum negative depression values within the 6mm zone of the best fit toric aspheric (BFTA) posterior elevation map in 180ø opposite 45° zones, yields the AAI. In this example, AAI = +15 + (15) = 30.
Statistical Analysis
Differences between the data were evaluated using the Levene’s test for equality of variance, and the Student two-sample t-test. Due to the high number of corneal metrics analyzed the level of significance was adjusted with Bonferroni correction and set to p < 0.0005 for individual variables. Data was expressed as mean and standard deviation (SD). Receiver operating characteristics (ROC) curves were used to assess the discriminating ability and to determine the optimal cutoff values for each significant metric and multivariable combination. Cutoff points were calculated using the Youden’s Index. After collecting all significant metrics, for the logistic regression, Wald’s chi-squared test was used to reach the strongest influential combination of variables in a step-wise manner to produce maximal AUROC values using least number of variables. The statistical analysis was performed using the SPSS software, version 24 (IBM Analytics).
RESULTS
This study included 31 highly asymmetric, clinically normal fellow eyes from 31 patients with definitive keratoconus in the contralateral eye (AKC group) and 178 eyes from 178 bilaterally normal control patients (Control group). There were no significant differences between groups for age 31.87 ± 8.57 (17 to 71 years) AKC vs. 28.74 ± 16.55 years (20 to 71 years) Controls (p >0.05). The percentage of males was significantly higher in the AKC group than the control group (71% males AKC vs. 48.3% males Controls (p=0.02). Mean anterior surface astigmatism value was 0.95 ± 0.41D in the AKC group compared to 0.85 ± 0.61 D in the control group (P>0.05) and mean anterior surface inferior-superior ratio was 0.65 ± 0.42 D in the AKC group and 0.65 ± 0.42 D in the control group (p>0.05).
Out of 87 corneal metrics analyzed, 10 were found highly significant with the student t-test value after Bonferroni correction of p < 0.000574. None of the keratoconus probability metrics (KPI, KPROB, CLMIaa, PPK) were significant (p>0.05). Out of the 10-significant metrics none had an independent strong predictive value; the strongest independent value was the corneal location of the thinnest Point on the X axis (AUC = 0.794) (Table 1). Five of the 10 highly significant metrics were related to pachymetry. Significant anterior surface metrics included the Opposite Sector Index (OSI) and Anterior Height BFS Z, while significant posterior surface metrics included the Asphericity and Asymmetry Index (AAI), Posterior Height BFS Z, and Posterior Height BFS X. The BFS Z anterior and posterior height metrics represent the exact machine coordinate of the distance of the center of the BFS from the Placido disc in the Z direction while the posterior height X describe how far the center of the BFS is away from the measurement Scheimpflug camera head’s rotation axis.
Table 1:
Dual Scheimpflug/Placido Variables That Were Significantly Different Between Asymmetric Keratoconus and Control Study Populations
| Variable | Control | AKC | P-Value | AUC | 95% CI | Cut off | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|
| Anterior Height BFS Z (mm) | 34.36 ± 0.39 (33.32 – 35.2) |
34.02 ± 0.42 (33.03 – 34.88) |
P=0.000006 | 0.723 | 0.625-0.821 | 33.96 | 51.6% | 84.8% |
| OSI [D] | 0.76 ± 0.33 (0.12 – 1.6) |
1.02 ± 0.55 (0.22 – 3.07) |
P=0.00777 | 0.658 | 0.203-0.405 | 0.671 | 83.9% | 45% |
| Posterior Height BFS x (mm) | −0.03 ± 0.02 (−0.11 – 0.04) |
0.0025 ± 0.04 (−0.07 – 0.07) |
P=0.000008 | 0.764 | 0.658-0.879 | −0.01 | 61.3% | 87% |
| Posterior Height BFS z (mm) | 35.05 ± 0.36 (33.9 – 35.9) |
34.78 ± 0.39 (33.86 – 35.58) |
P=0.000008 | 0.696 | 0.595-0.797 | 34.97 | 71% | 61.2% |
| Central Corneal Pachymetry 0 - 4 mm (μm) | 563 ± 26 (505 – 621) |
542 ± 27 (490 – 611) |
P=0.00382 | 0.701 | 0.603-0.800 | 543 | 58% | 75.8% |
| Central Corneal Pachymetry 2 mm (μm) | 555 ± 26 (500 – 613) |
533 ± 27 (482 – 598) |
P=0.000038 | 0.716 | 0.619-0.813 | 534 | 58% | 77.5% |
| Thinnest Corneal Value (μm) | 553 ± 26 (498 – 612) |
529 ± 27 (470 – 595) |
P=0.000001 | 0.733 | 0.640-0.827 | 553 | 87% | 48.8% |
| Thinnest Pachymetry location X axis | −0.50 ± 0.24 (−1.49 – 0.44) |
0.04 ± 0.55 (−1.04 – 1.1) |
P=0.000002 | 0.794 | 0.682-0.906 | −0.15 | 64.5% | 94.3% |
| Thinnest Pachymetry location Y axis | −0.26 ± 0.23 (−0.98 – 0.28) |
−0.52 ± 0.24 (−1.2 – −0.13) |
P=0.0000000036 | 0.784 | 0.703-0.865 | −0.41 | 71% | 75% |
| AAI | 12.46 ± 5.73 (4 – 38) |
18.54 ± 7.8 (6 – 38) |
P=0.00011 | 0.750 | 0.658-0.841 | 13.50 | 77.4% | 67% |
| IAI | 0.42 ± 0.03 (0.35 – 0.54) |
0.411 ± 0.038 (0.32 – 0.50) |
P=0.01 | 0.625 | 0.519-0.730 | 0.4 | 51.6% | 73.6% |
| Corneal Volume (mm3) | 31.61 ± 1.58 (27 – 35) |
30.81 ± 1.57 (27.5 – 34.6) |
P=0.0047 | 0.640 | 0.549-0.740 | 30 | 42% | 82% |
OSI – Anterior Surface Opposite Sector Index
AAI – Posterior Surface Asphericity Asymmetry Index
IAI – Anterior Surface Irregular Astigmatism Index
AUC – Area Under the Curve
AKC – Highly Asymmetric Group
The best combination in logistic regression modeling was achieved with a combination of 9 metrics (Table 2). The model predictive power had a 0.961 AUROC, sensitivity of 90.3% and specificity of 92.6%. The most significant variables in modeling were pachymetry metrics, while the least impactful but still relevant were the anterior and posterior BFS Z variables.
Table 2:
Variable Rank by Impact on Combined Variable Model to Distinguish Between Asymmetric keratoconus and Control Study Populations
| Variable Rank | Wald Value | 9 Variable Model |
|---|---|---|
| 1 | 15.285 | Thinnest Pachymetry location On the X axis |
| 2 | 9.778 | Thinnest Pachymetry Value |
| 3 | 5.629 | Average Central 2 mm Pachymetry |
| 4 | 5.314 | IAI |
| 5 | 2.927 | AAI |
| 6 | 1.860 | Corneal Volume (mm3) |
| 7 | 1.352 | Average Middle 4-7 mm Pachymetry |
| 8 | 1.215 | Posterior Height BFS Z |
| 9 | 0.948 | Anterior Height BFS Z |
| AUC | 0.961 | |
| Sensitivity | 90.3% | |
| Specificity | 92.6% |
AAI – Posterior Surface Asphericity Asymmetry Index
IAI – Anterior Surface Irregular Astigmatism Index
BFS Z – Best fit sphere in the z direction
AUC – Area Under the Curve
DISCUSSION
In this study we found that, while no individual metric performed well in discriminating between clinically unaffected eyes from patients with highly asymmetric keratoconus and normal corneas, a combination of anterior surface, posterior surface, and corneal thickness metrics yielded a good predictive model.
The majority and most significant metrics of this model were related to corneal pachymetry. In this study 5 out of 9 metrics in the model (55%) were related to corneal pachymetry and volume. The significance of corneal thickness and corneal volume in the detection of subclinical keratoconus has been reported before, both in subclinical keratoconus and in keratoconic eyes, demonstrating a relative thinner cornea with a reduced corneal volume.5,20-22,24,37 Metrics utilized from the posterior surface were the Asphericity and Asymmetry Index (AAI) along with the machine coordinate of the distance between the center of the BFS from the Placido disc in the Z direction (Posterior height BFS Z). Metrics from the anterior surface were the Irregular Astigmatism Index (IAI), which is an objective, machine-derived variable calculated as the difference between average dioptric values in the inferior and superior areas within the 3 mm corneal vertex, and the machine coordinate of the distance of the central BFS from the Placido disc in the Z axis (anterior height BFS Z). The most significant metric in the logistic regression model was the location of the thinnest point temporally on the horizontal (x) axis. The thinnest point on the Y axis was also highly significant; however, its contribution to the logistic regression model was negligible. The Opposite Sector Index (OSI) was highly significant but did not contribute to the final model.
The posterior Asphericity and Asymmetry Index (AAI) is a relatively new, manually derived metric used as a quantitative indicator of the asymmetry and asphericity of the posterior corneal surface. A similar type of index was identified by Nilforoushan and colleagues in studies using Scheimpflug imaging and was found to be predictive for subclinical keratoconus when combined with other variables.38 The variable, ‘high minus low’, was created from the difference between the highest and lowest points on the posterior elevation within the central posterior 7 mm zone.38 Bae and colleagues similarly calculated posterior elevation difference (max-min) for Scheimpflug imaging and found a calculated AUROC of 0.735, although in that study anterior surface curvature variables were best at distinguishing populations.18 In a recent study by Smadja and colleagues, the posterior AAI was selected by an automated decision tree classifier to be the most discriminative variable to differentiate between normal and subclinical keratoconus eyes out of 55 evaluated metrics.35 In that study the cut-off point between subclinical KC and normal eyes was estimated as 21.5, while in this study the optimal value was 13.5, which yielded an AUC of 0.750, with 77% sensitivity and 67% specificity. This discrepancy between studies could be due to the fact that the AAI is still not incorporated into the device’s analyzer and had to be calculated manually and by that is prone to subjective evaluation differences. Ambrosio and colleagues reported a sensitivity of 80% and specificity of 70% for the BAD-D and BAD-DI indexes for distinguishing normal eyes from the uninvolved fellow eye of highly asymmetric keratoconus.34 The sensitivity and specificity improved to 90% and 96%, respectively, using a novel TBI index which combines BAD-D with metrics from a biomechanical evaluation from the Corvis-ST device.
In our study the Irregular astigmatism index (IAI) had an AUROC 0.625 with a 51.6% sensitivity and 73.6% specificity and an optimal cut of value of 0.4. This result approximately agrees with Feizi and colleagues, who found AUROC of 0.664 for a cut-off point of 0.445.39 Shetty and colleagues found better predictive ability with a similar cutoff value of 0.45 (AUROC 0.858, 54% sensitivity, 81.4% specificity).40 The differences in predictive power between studies may be due in part to differing classifications of description asymmetric (subclinical) disease, as the eyes in the study by Shetty included eyes with asymmetric bow tie and skewed radial axis patterns.
In this study none of the higher order aberrations (HOA) were significantly different between groups. Saad and Gatinel used a combined Placido disk and wavefront aberrometry device based on dynamic skiascopy (OPD-Scan Nidek Co. Ltd., Gamagori, Japan) to create a model that combined 4 high order aberration metrics along with 4 Placido disc metrics to identify subclinical keratoconus. A sensitivity and specificity pair of 89% and 92% were calculated, though a sensitivity of 63% was re-calculated in their validation study. 25 Bühren used a scanning slit system (Orbscan IIz, Bausch & Lomb, Rochester, New York, USA) to combine high order aberration metrics from both anterior and posterior corneal surfaces in addition to thickness data to achieve an AUROC of 0.857 and sensitivity/specificity pair of 68.8% and 95.1%, respectively in the validation group.1,41 Reddy and colleagues evaluated HOA extrapolated by the Dual Scheimpflug analyzer (Galilei) and found that 3rd order HOA and Total RMS were the most significant (AUROC of 0. 83 and 0.82, respectively); however, eyes in the subclinical KC group included ones with maps containing skewed anterior radial axes, which have the potential to induce HOA.19 A study using a combination of corneal and total ocular HOA reached a sensitivity and specificity of 91% and 94%, respectively.26 Taken together, these results support our findings that corneal HOA are not particularly predictive in early stages of keratoconus.
While the multivariable model in our study achieved a predictive value of 90% sensitivity and 92% specificity, recent work from our group achieved 100% sensitivity and 100% specificity in a similar study population using a combination of metrics from SD-OCT and Scheimpflug imaging.42 That study utilized two separate technologies and incorporated epithelial thickness parameters. Metrics reporting epithelial thickness variability were found to be highly significant and important for optimal modeling to distinguish the study populations. Those epithelial metrics were not available in this cohort and may have further delineated the groups. While the studies were performed in unique cohorts and were therefore not directly comparable, the optimal model achieved using combined Dual Scheimpflug/Placido imaging in this study performed slightly better than optimal models from either SD-OCT only (89% specificity, 89% sensitivity) or Scheimpflug only (83% sensitivity, 83% sensitivity) models form the previous work. We looked at all false positive and false negative cases in detail after analysis but could not discern any specific pattern or deviation that specifically contributed to their misclassification.
Limitations in this study include small sample size and lack of long-term follow-up. Lack of follow-up or intervention in these eyes limits our ability to identify parameters that best indicate a higher risk of keratoconus progression. The cases were obtained from multiple locations and as such do not represent a specific geographic cohort. As patients with highly asymmetric keratoconus represent a heterogenous cohort, we anticipate that a similar analysis performed in a different group may find a slightly different combination of optimal variables; however, we predict that futures optimal models will include pachymetry measures as well as anterior and posterior surface asymmetry metrics. Finally, none of the metrics identified directly measure corneal biomechanics; rather, they all describe corneal morphology.
In conclusion, our findings demonstrate that the clinically unaffected fellow eye in patients with highly asymmetric keratoconus remains challenging to identify using any individual metric from Dual Scheimpflug/Placido imaging; however, a combination of metrics, weighted heavily towards pachymetric variables, yielded good discriminative power.
Ambrosio and colleagues have reported in a recent study, a sensitivity and specificity pair of 0.8/0.7 for the BAD-D and BAD-DI indexes for distinguishing normal from the uninvolved fellow eye of highly asymmetric keratoconus. The sensitivity and specificity pair were improved to 0.9/0.96 using the novel TBI index which combines also metrics from a biomechanical evaluation of the Corvis-ST device.
Supplementary Material
Patient 1: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 2: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 3: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 4: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 5: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 6: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 7: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 8: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 9: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 10: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 11: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 12: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 13: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 14: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 15: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 16: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 17: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 18: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 19: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 20: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 21: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 22: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 23: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 24: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 25: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 26: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 27: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 28: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 29: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 30: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 31: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Highlights.
Dual Scheimpflug/Placido analysis performed well in distinguishing eyes with highly asymmetric keratoconus from normal controls
No individual variable performed adequately
A combination of variables, including 5 pachymetry, 2 anterior surface, and 2 posterior surface variables, distinguished the populations with 90.3% sensitivity and 92.6% specificity
ACKNOWLEDGEMENTS
A. Funding/Support: Supported by NIH grant R01 EY028666 (JBR) and separate unrestricted departmental grants to the USC Roski Eye Institute, and Baylor College of Medicine/Cullen Eye Institute from Research to Prevent Blindness (New York, NY, USA).
B. Financial Disclosures: David Smadja is a paid consultant for Ziemer Ophthalmic System AG. No financial disclosures for authors OG, ALP, ESH, IMG, MK, SSK, JBR.
C. No other acknowledgements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presented in part at the Annual Association for Research in Vision and Ophthalmology May 2018
References
- 1.Buhren J, Kook D, Yoon G, Kohnen T. Detection of subclinical keratoconus by using corneal anterior and posterior surface aberrations and thickness spatial profiles. Invest Ophthalmol Vis Sci. 2010;51(7):3424–3432. [DOI] [PubMed] [Google Scholar]
- 2.Cui J, Zhang X, Hu Q, Zhou WY, Yang F. Evaluation of Corneal Thickness and Volume Parameters of Subclinical Keratoconus Using a Pentacam Scheimflug System. Curr Eye Res 2016;41(7):923–926. [DOI] [PubMed] [Google Scholar]
- 3.de Sanctis U, Loiacono C, Richiardi L, Turco D, Mutani B, Grignolo FM. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology. 2008; 115(9):1534–1539. [DOI] [PubMed] [Google Scholar]
- 4.Jia Y, Zhu H, Zhou J. Pentacam scheimpflug tomography findings in topographically normal patients and subclinical keratoconus cases. Am J Ophthalmol 2015;159(1):209. [DOI] [PubMed] [Google Scholar]
- 5.Ruisenor Vazquez PR, Galletti JD, Minguez N, et al. Pentacam Scheimpflug tomography findings in topographically normal patients and subclinical keratoconus cases. Am J Ophthalmol. 2014;158(1):32–40 e32. [DOI] [PubMed] [Google Scholar]
- 6.Smadja D, Santhiago MR, Mello GR, Krueger RR, Colin J, Touboul D. Influence of the reference surface shape for discriminating between normal corneas, subclinical keratoconus, and keratoconus. J Refract Surg 2013;29(4):274–281. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg J, Aubke-Schultz S, Frings A, et al. Correlation of the KISA% index and Scheimpflug tomography in 'normal', 'subclinical', 'keratoconus-suspect' and 'clinically manifest' keratoconus eyes. Acta Ophthalmol 2015;93(3):e199–207. [DOI] [PubMed] [Google Scholar]
- 8.Ucakhan OO, Cetinkor V, Ozkan M, Kanpolat A. Evaluation of Scheimpflug imaging parameters in subclinical keratoconus, keratoconus, and normal eyes. J Cataract Refract Surg 2011. ;37(6): 1116–1124. [DOI] [PubMed] [Google Scholar]
- 9.Luz A, Lopes B, Hallahan KM, et al. Enhanced Combined Tomography and Biomechanics Data for Distinguishing Forme Fruste Keratoconus. J Refract Surg 2016;32(7):479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinowitz YS, Li X, Canedo AL, Ambrosio R Jr., Bykhovskaya Y Optical coherence tomography combined with videokeratography to differentiate mild keratoconus subtypes. J Refract Surg 2014;30(2):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003; 110(2):267–275. [DOI] [PubMed] [Google Scholar]
- 12.Randleman JB, Trattler WB, Stulting RD. Validation of the Ectasia Risk Score System for preoperative laser in situ keratomileusis screening. Am J Ophthalmol 2008;145(5):813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115(1):37–50. [DOI] [PubMed] [Google Scholar]
- 14.Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg 1999;25(10): 1327–1335. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Yang H, Rabinowitz YS. Longitudinal study of keratoconus progression. Exp Eye Res 2007;85(4):502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Rabinowitz YS, Rasheed K, Yang H. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology. 2004;111(3):440–446. [DOI] [PubMed] [Google Scholar]
- 17.Klyce SD, Karon MD, Smolek MK. Screening patients with the corneal navigator. J Refract Surg 2005;21(5 Suppl):S617–622. [DOI] [PubMed] [Google Scholar]
- 18.Bae GH, Kim JR, Kim CH, Lim DH, Chung ES, Chung TY. Corneal topographic and tomographic analysis of fellow eyes in unilateral keratoconus patients using Pentacam. Am J Ophthalmol 2014;157(1):103–109 e101. [DOI] [PubMed] [Google Scholar]
- 19.Reddy JC, Rapuano CJ, Cater JR, Suri K, Nagra PK, Hammersmith KM. Comparative evaluation of dual Scheimpflug imaging parameters in keratoconus, early keratoconus, and normal eyes. J Cataract Refract Surg 2014;40(4):582–592. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosio R Jr., Caiado AL, Guerra FP, et al. Novel pachymetric parameters based on corneal tomography for diagnosing keratoconus. J Refract Surg 2011. ;27(10):753–758. [DOI] [PubMed] [Google Scholar]
- 21.Ambrosio R Jr., Alonso RS, Luz A, Coca Velarde LG. Corneal-thickness spatial profile and corneal-volume distribution: tomographic indices to detect keratoconus. J Cataract Refract Surg 2006;32(11): 1851–1859. [DOI] [PubMed] [Google Scholar]
- 22.Saad A, Gatinel D. Topographic and tomographic properties of forme fruste keratoconus corneas. Invest Ophthalmol Vis Sci 2010;51(11):5546–5555. [DOI] [PubMed] [Google Scholar]
- 23.Schlegel Z, Hoang-Xuan T, Gatinel D. Comparison of and correlation between anterior and posterior corneal elevation maps in normal eyes and keratoconus-suspect eyes. J Cataract Refract Surg 2008;34(5):789–795. [DOI] [PubMed] [Google Scholar]
- 24.Pinero DP, Alio JL, Aleson A, Escaf Vergara M, Miranda M. Corneal volume, pachymetry, and correlation of anterior and posterior corneal shape in subclinical and different stages of clinical keratoconus. J Cataract Refract Surg 2010;36(5):814–825. [DOI] [PubMed] [Google Scholar]
- 25.Saad A, Gatinel D. Combining Placido and Corneal Wavefront Data for the Detection of Forme Fruste Keratoconus. J Refract Surg 2016;32(8):510–516. [DOI] [PubMed] [Google Scholar]
- 26.Saad A, Gatinel D. Evaluation of total and corneal wavefront high order aberrations for the detection of forme fruste keratoconus. Invest Ophthalmol Vis Sci 2012;53(6):2978–2992. [DOI] [PubMed] [Google Scholar]
- 27.Jafri B, Li X, Yang H, Rabinowitz YS. Higher order wavefront aberrations and topography in early and suspected keratoconus. J Refract Surg 2007;23(8):774–781. [DOI] [PubMed] [Google Scholar]
- 28.Colak HN, Kantarci FA, Yildirim A, et al. Comparison of corneal topographic measurements and high order aberrations in keratoconus and normal eyes. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2016;39(5):380–384. [DOI] [PubMed] [Google Scholar]
- 29.Buhren J, Kuhne C, Kohnen T. Defining subclinical keratoconus using corneal first-surface higher-order aberrations. Am J Ophthalmol 2007;143(3):381–389. [DOI] [PubMed] [Google Scholar]
- 30.Temstet C, Sandali O, Bouheraoua N, et al. Corneal epithelial thickness mapping using Fourier-domain optical coherence tomography for detection of form fruste keratoconus. J Cataract Refract Surg 2015;41(4):812–820. [DOI] [PubMed] [Google Scholar]
- 31.Vinciguerra R, Ambrosio R Jr., Roberts CJ, Azzolini C, Vinciguerra P Biomechanical Characterization of Subclinical Keratoconus Without Topographic or Tomographic Abnormalities. J Refract Surg 2017;33(6):399–407. [DOI] [PubMed] [Google Scholar]
- 32.Scarcelli G, Besner S, Pineda R, Yun SH. Biomechanical characterization of keratoconus corneas ex vivo with Brillouin microscopy. Invest Ophthalmol Vis Sci 2014;55(7):4490–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarcelli G, Besner S, Pineda R, Kalout P, Yun SH. In vivo biomechanical mapping of normal and keratoconus corneas. JAMA Ophthalmol 2015; 133(4):480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrosio R Jr., Lopes BT, Faria-Correia F, et al. Integration of Scheimpflug-Based Corneal Tomography and Biomechanical Assessments for Enhancing Ectasia Detection. J Refract Surg 2017;33(7):434–443. [DOI] [PubMed] [Google Scholar]
- 35.Smadja D, Touboul D, Cohen A, et al. Detection of subclinical keratoconus using an automated decision tree classification. Am J Ophthalmol 2013; 156(2):237–246 e231. [DOI] [PubMed] [Google Scholar]
- 36.Golan O, Hwang ES, Lang P, Santhiago MR, Abulafia A, Touboul D, Krauthammer M, Smadja D Differences in Posterior Corneal Features Between Normal Corneas and Subclinical Keratoconus. J Refract Surg 2018;34(10):664–670. [DOI] [PubMed] [Google Scholar]
- 37.Tellouck J, Touboul D, Santhiago MR, Tellouck L, Paya C, Smadja D. Evolution Profiles of Different Corneal Parameters in Progressive Keratoconus. Cornea. 2016;35(6):807–813. [DOI] [PubMed] [Google Scholar]
- 38.Nilforoushan MR, Speaker M, Marmor M, et al. Comparative evaluation of refractive surgery candidates with Placido topography, Orbscan II, Pentacam, and wavefront analysis. J Cataract Refract Surg 2008;34(4):623–631. [DOI] [PubMed] [Google Scholar]
- 39.Feizi S, Yaseri M, Kheiri B. Predictive Ability of Galilei to Distinguish Subclinical Keratoconus and Keratoconus from Normal Corneas. Journal of ophthalmic & vision research. 2016; 11 (1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shetty R, Rao H, Khamar P, et al. Keratoconus Screening Indices and Their Diagnostic Ability to Distinguish Normal From Ectatic Corneas. Am J Ophthalmol. 2017;181:140–148. 41. [DOI] [PubMed] [Google Scholar]
- 41.Buhren J, Schaffeler T, Kohnen T. Validation of metrics for the detection of subclinical keratoconus in a new patient collective. J Cataract Refract Surg 2014;40(2):259–268. [DOI] [PubMed] [Google Scholar]
- 42.Hwang E, Perez-Straziota C, Kim S, Santhiago M, Randleman J. Distinguishing Highly Asymmetric Keratoconus Eyes Using Combined Scheimpflug And Spectral Domain OCT Analysis. Ophthalmology. 2018. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient 1: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 2: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 3: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 4: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 5: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 6: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 7: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 8: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 9: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 10: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 11: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 12: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 13: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 14: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 15: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 16: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 17: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 18: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 19: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 20: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 21: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 22: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 23: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 24: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 25: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 26: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 27: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 28: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 29: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 30: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).
Patient 31: Dual Scheimpflug/Placido image from both eyes of a patient from this study with highly asymmetric keratoconus. The left image shows the clinically unaffected eye (subclinical eye analyzed in this study), while the right image shows the clinically affected keratoconus eye (used to identify the patient population).