Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a disabling condition accompanying several cancer drugs, including the front-line chemotherapeutic agent paclitaxel. Although CIPN can force dose reduction or even discontinuation of chemotherapy affecting survival in cancer patients, there is no FDA-approved treatment for CIPN. CIPN in mice is characterized by neuropathic pain (e.g., mechanical allodynia) in association with oxidative stress and neuroinflammation in dorsal root ganglia (DRGs), as well as retraction of intraepidermal nerve fibers. Here, we report that paclitaxel-induced mechanical allodynia is associated with transcriptional increased in matrix metalloproteinase (MMP) 2 and 9, and decreased of metallopeptidase inhibitor 1 (TIMP1), a strong endogenous MMP9 inhibitor. Consistently, MMP9 protein levels are increased in DRG neurons in vivo and in vitro after paclitaxel treatment and demonstrated, for the first time, that intrathecal injections of exogenous TIMP1 or a monoclonal antibody targeting MMP9 (MMP9 mAb) significantly prevented and reversed paclitaxel-induced mechanical allodynia in male and female mice. Analyses of DRG tissues showed that MMP9 mAb significantly decreased oxidative stress and neuroinflammatory mediators IL-6 and TNFα, as well as prevented paclitaxel-induced loss of intraepidermal nerve fibers. These findings suggest that MMP signaling plays a key role in paclitaxel-induced peripheral neuropathy and MMP9 mAb may offer new therapeutic approaches for the treatment of CIPN.
Keywords: Chemotherapy-induced peripheral neuropathy, paclitaxel, neuropathic pain, monoclonal antibody therapy, matrix metalloproteinase 9
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common adverse effect associated with anticancer drugs.45 Nearly 70% of cancer patients experience neuropathic pain symptoms due to the administration of anticancer drugs such as platinum compounds, proteasomes inhibitors, and antitubulins (e.g., vinca alkaloids and taxanes).11,59 Paclitaxel is the frontline chemotherapeutic agent used to treat many solid tumors, but it is also associated with a high incidence of CIPN leading to dose reduction or termination of an otherwise potential life-saving treatment.20,40,45 Moreover, CIPN often becomes a chronic condition affecting rehabilitation, productivity, and quality of life in cancer survivors.20 Unfortunately, there are no effective agents or clinical protocols to successfully prevent and reverse CIPN. Although mechanisms of CIPN remain incompletely known, there is growing preclinical evidence that paclitaxel administration increases sensitivity to mechanical and cold stimuli (mechanical and cold allodynia) targeting peripheral sensory neurons in the dorsal root ganglia (DRGs), as well as leads to oxidative stress, neuroinflammation and results in degeneration of intraepidermal nerve fiber (IENF) density.2,7,8,11,18
Matrix metalloproteinases (MMPs) belong to a large family of zinc-dependent endopeptidases, which are widely correlated with oxidative stress and neuroinflammation in various neurodegenerative diseases.69 MMPs also play important roles in the development and maintenance of neuropathic pain, including mechanical allodynia.33 Recent studies have demonstrated that MMP2 and MMP9 are activated in DRGs leading to neuroinflammation and neuropathic pain.30,33,39 MMP2 and MMP9 are activated by other MMPs.69 For instance, MMP2 is activated by MMP14, and MMP9 is activated by plasminogen/plasmin and MMP3.29 Interestingly, transcriptional levels of MMP3 were significantly upregulated in DRGs of paclitaxel-treated rats.52 In addition, the activity of MMPs is regulated by endogenous tissue inhibitors of metalloproteases (TIMPs), which have been demonstrated to be effective in suppressing neuropathic pain after nerve injury.29
Although MMPs and TIMPs are emerging as new therapeutic targets in cancer, neurodegenerative diseases and chronic pain,29,56,62 initial drug discovery efforts targeting these proteases have all failed in clinical trials due to their lack in specific inhibition and insufficient knowledge about their complex regulation in pathological conditions.27,62 However, recent advances made in understanding the pathological role of various MMPs and the appearance of new specific MMP targeting approaches, including monoclonal antibodies, have created new opportunities for an efficient therapeutic use of MMP inhibition.
Therefore, we have explored the effects of paclitaxel on the expression of various MMPs and TIMPs, as well as the use of a monoclonal antibody to alleviate CIPN. We have found a significant up-regulation of MMP2 and MMP9, and down-regulation of TIMP1 in DRGs of paclitaxel-treated mice. In line with this finding, local delivery of recombinant TIMP1 prevented and reversed mechanical allodynia. Most importantly, we have characterized, for the first time, the therapeutic effects of a MMP9 monoclonal antibody (MMP9 mAb) for the prevention of paclitaxel-induced mechanical allodynia in male and female mice, as well as the attenuation of oxidative stress, neuroinflammation and degeneration of IENF typically associated with CIPN. These findings are important because an humanized MMP9 antibody is already in advanced clinical trials for colitis and cancer treatment, and may provide a safe opportunity for a fast repurposing of this antibody as the first FDA-approved therapeutic for the treatment of both cancer and CIPN.
Methods
Animals and CIPN model
Male and female CD1 mice (8–10 weeks) from Charles River were used as indicated for behavioral and biochemical experiments. Mice were housed four per cage at 22 ± 0.5°C under a controlled 12 hours light/dark cycle with free access to food and water. To produce CIPN, mice were treated with paclitaxel as previously described.40 Briefly, 6 mg/mL stock paclitaxel (Sigma-Aldrich, St. Louis, Mo) was diluted with Cremophor EL and 95% dehydrated ethanol (1:1 ratio, Sigma-Aldrich) and given at a dosage of 2 mg/kg diluted in saline intraperitoneally every other day for a total of 4 injections (days 0, 2, 4, and 6 with a final cumulative dose of 8 mg/kg). Control animals received an equivalent volume of the vehicle with proportional amounts of Cremophor EL and 95% dehydrated ethanol diluted in saline. Signs of peripheral neuropathy with a similar phenotype to that in patients have been validated by multiple investigators in this non–tumor-bearing animal model, including a time-dependent development of mechanical allodynia. As detailed in Suppl. Fig 1, paclitaxel and the effect of various drugs are generally evaluated up to 14 days, when the animals are scarified and tissues harvested. All efforts were made to minimize animal suffering, reduce the number of animals used, and use alternatives to in vivo techniques, in accordance with the International Association for the Study of Pain, the National Institutes of Health Office of Laboratory Animal Welfare Guide for the Care and Use of Laboratory Animals, and adhered to animal welfare guidelines established by the University of Cincinnati Institutional Animal Care and Use Committee.
Drugs and drug administration
We purchased the recombinant mouse TIMP-1 (cat# 593702) from BioLegend (San Diego, CA) and the MMP9 mouse monoclonal antibody raised against a partially purified MMP-9 of human origin (6–6B, cat# sc-12759), as well as its mouse IgG control (cat# sc-2025) from Santa Cruz Biotechnology (Dallas, TX). These drugs were administrated intrathecally (i.t.) to deliver reagent into cerebral spinal fluid and DRG tissues to inhibit MMP9 function, as described previously.25 A valid spinal puncture was confirmed by a reflexive tail flick after the needle entry into subarachnoid space.
Von Frey test for mechanical allodynia
Mechanical allodynia as a readout for CIPN was assessed as the hind paw withdrawal response to von Frey hair stimulation using the up-and-down method, as previously described.14 Briefly, the mice were first acclimatized (1 hour) in individual clear Plexiglas boxes on an elevated wire mesh platform to facilitate access to the plantar surface of the hind paws. Subsequently, a series of von Frey hairs (0.02, 0.07, 0.16, 0.4, 0.6, 1.0, and 1.4 g; Stoelting CO., Wood Dale, IL) were applied perpendicular to the plantar surface of hindpaw. A test began with the application of the 0.6 g hair. A positive response was defined as a clear paw withdrawal or shaking. Whenever a positive response occurred, the next lower hair was applied, and whenever a negative response occurred, the next higher hair was applied. The testing consisted of 6 stimulus, and the pattern of response was converted to a 50% von Frey threshold, using the method described previously,16 by an investigator blinded to treatment until the end of the experiment.
Cold plantar test for cold allodynia
The cold plantar assay was used to evaluate noxious cold (Brenner et al., 2015). Briefly, animals were first placed individually into clear acrylic containers separated by black opaque dividers that were set on top of 3/160 borosilicate glass (Stemmerich Inc, St. Louis, MO) and allowed to acclimate for 20 minutes before testing. A dry ice pellet was applied to the hind paw through the glass and the time until hind paw withdrawal was recorded at 5-minute intervals per mice, alternating paws, for a total of 3 trials, and the mean withdrawal latency was calculated. Withdrawal latencies were evaluated before and 1 to 14 days after the paclitaxel first injection.
Adhesive removal test for tactile hyposensitivity behavior
In order to examine the effect of paclitaxel on sensory function, we used a modification of the adhesive removal test. Briefly, a round adhesive patch (3/16” Teeny Touch-Spots, USA Scientific INC. Ocalo, FL) was placed on the plantar surface of the right hind paw. The animal performance in a testing cage (20×20×13 cm3) was recorded in 15 min. The time until the mouse started to shake its paw or bring the paw to the mouth was recorded as a measure of the latency until the mouse noticed the presence of the adhesive patch on the paw, as previously described.6,47
Quantitative real-time RT-PCR (qPCR)
Mice were terminally anesthetized with isoflurane and lumbar DRG (L3–L5) or lumbar spinal cords (L3–L5) were rapidly removed 14 days after paclitaxel treatment. Total RNA was extracted using Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA), which amount and quality were assessed by SimpliNano UV-Vis Spectrophotometer (General Electric, Bosoton, MA), and then converted into cDNA using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Thermo Fisher Scientific, Waltham, MA). Specific primers for MMPs and TIMPs as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were obtained from PrimerBank.64 Primer sequences are depicted in Suppl. Table 1. qPCR was performed on a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific) using PowerUp SYBR™ Green Master Mix (Thermo Fisher Scientific). All samples were analyzed at least in duplicate and normalized by GAPDH expression. The relative expression ratio per condition was calculated based on the method described by Pfaffl et al.5,54
Cell immunohistochemical analysis
DRG cell cultures and immunohistochemistry were carried out as described previously.44 Briefly, mice were terminally anesthetized with isoflurane, and lumbar, thoracic and cervical DRGs were bilaterally removed aseptically from 8-week old mice. Removed DRGs were incubated in papain (60 U, cat# P3125, Sigma-Aldrich) for 20 min at 37°C followed by collagenase (1 mg/ml, cat# C6885, Sigma-Aldrich) for another 20 min at 37°C. After DRG cells were mechanically dissociated, they were cultured in Dulbecco’s Minimal Essential Medium (10% fetal bovine serum) on coverslips percolated with a cell and tissue adhesive (Cell-Tak, cat# CB-40240, Thermo Fisher Scientific) for 24 h at 37°C, with 5% carbon dioxide. DRG cultures were then incubated with paclitaxel (300 nM) or vehicle control for 24 h. For immunohistochemistry, DRG cultures were fixed with 4% paraformaldehyde for 20 min and incubated with MMP-9 (rabbit, 1:500, cat# AB19016, Sigma-Aldrich) and neurons stained with Beta-Tubulin III (mouse, 1:1000, cat# ab78078, Abcam, Cambridge, MA) overnight at 4°C, followed by incubation with the secondary antibody anti-rabbit Alexa Fluor® 546 and anti-mouse Alexa Fluor® 488 (both 1:1000, Thermo Fisher Scientific) for 1 h at room temperature. Quantification of images from multiple optical field of DRG cultures, selected at random, were captured under an Olympus BX63 fluorescent microscope using cellSens imaging acquisition software (Olympus, Center Valley, PA). All image capture and intensity quantification were performed comparing samples from all experimental groups, prepared with the same staining solutions, then measured using identical display parameters.
Tissue immunohistochemical analysis
Deeply anesthetized mice were perfused through the left ventricle with PBS, followed by 4% paraformaldehyde in PBS (PFA solution) and lumbar DRGs (L3-L5) and 3-mm2 skin biopsies from the plantar surface of the hind paws were collected 14 days after paclitaxel treatment. All tissues were post-fixed in PFA solution overnight and subsequently transferred into 30% sucrose in PBS for 24h. For MMP9 quantification, DRG tissues were sliced into 12 μm sections and placed on slides, which were then blocked for 1h at room temperature with 1% BSA with 0.2% Triton X-100 in PBS (BSA solution). Subsequently, sections were incubated with MMP9 primary antibody (rabbit, 1:500, cat# AB19016, Sigma-Aldrich) overnight at 4°C, followed by incubation with the secondary antibody anti-rabbit Alexa Fluor® 546 (1:1000, Thermo Fisher Scientific) for 1 h at room temperature. DAPI (4’,6-diamidino-2-phenylindole, Thermo Fisher Scientific) was used for counterstaining. For the quantification of intradermal neuronal fibers (IENFs), skin tissues were sliced into 40 μm sections and collected in PBS solution for free floating staining. Subsequently, sections were blocked for 1h at room temperature in the BSA solution, incubated with primary antibodies against the pan-neuronal marker PGP9.5 (rabbit, 1:500, cat# Z511601–2, Agilent, Santa Clara, CA) and the anti-collagen IV (goat, 1:500, cat# 1340–01, Southern Biotech, Birmingham, AL) overnight at 4°C, and followed by incubation with the secondary antibodies anti-rabbit Alexa Fluor® 488 and anti-goat Alexa Fluor® 555 (both secondary antibodies at 1:1000, Thermo Fisher Scientific) for 1 h at room temperature. Quantification of images from multiple sections of each DRG or skin tissue, selected at random, were captured under an Olympus BX63 fluorescent microscope using cellSens imaging acquisition software (Olympus, Center Valley, PA). For MMP9, all image capture and intensity quantification were performed comparing samples from all experimental groups, prepared with the same staining solutions, then measured using identical display parameters. For IENFs, nerve fibers that crossed the collagen-stained dermal/epidermal junction into the epidermis were counted and density was determined as the total number of fibers/length of epidermis (IENFs/cm).
Measurement of oxidative stress
To measure oxidative stress we used dihydroethidium (DHE, Sigma-Aldrich), a cell-permeable oxidative fluorescent dye, which proportionally reacts with intracellular reactive oxygen species.63 On day 14 after the injection of paclitaxel, 10 μl of DHE (10 μM in PBS) was delivered intrathecally and after 1 h mice were perfused with PBS, followed by 4% paraformaldehyde in PBS (PFA solution) and lumbar DRGs (L3-L5) were collected. DRGs were then post-fixed in PFA during 2 hours and subsequently transferred into 30% sucrose in PBS for 24h. DRG tissues were sliced into 12 μm sections, placed on slides and counterstained with DAPI. The intensity of DHE fluorescence was quantified under an Olympus BX63 fluorescent microscope using cellSens imaging. All images captured from multiple sections of each DRG tissue selected at random were performed comparing samples from all experimental groups, prepared with the same staining solutions, then measured using identical display parameters.
Statistical analysis
All data were expressed as mean ± SEM. Biochemical data were analyzed using Student t test or 1-way analysis of variance (ANOVA) followed by Dunn post hoc test. Two-way repeated measured ANOVA was used to analyze multiple group data with multiple time points with Bonferroni post hoc test to determine on which days experimental groups differed. The criterion for statistical significance was set at P < 0.05.
Results
Regulation of MMP signaling in paclitaxel-treated mice
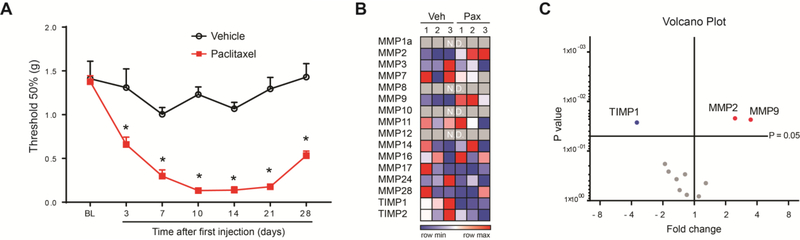
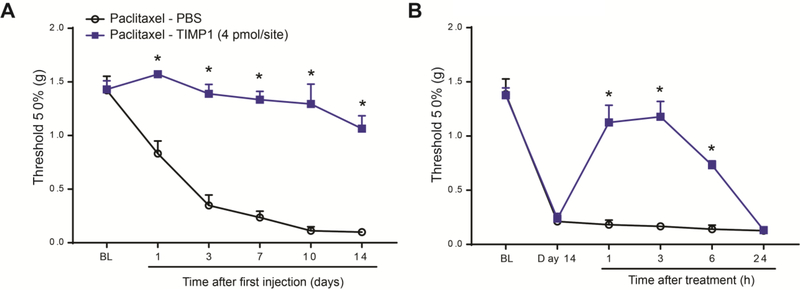
Paclitaxel is one of the most effective chemotherapeutic drugs, but it is associated with the development of CIPN symptoms, such as persistent shooting, stabbing, or burning pain, and most frequently loss of sensation or “numbness”.45 Although mice treated with the chemotherapeutic agent cisplatin manifested significant sensorimotor deficits47 using the well-characterized adhesive removal test,6 which may be indicative of “numbness”, we did not observe similar deficits in mice treated with paclitaxel (Suppl. Fig 2). However, it is well reported that paclitaxel-treated animals have increased sensitivity to mechanical stimuli (mechanical allodynia), and this increased sensitivity is commonly used as a read out for the development of CIPN.21,36,40,68 Therefore, we assessed mechanical sensitivity in paclitaxel-treated mice using von Frey hairs, and observed a significant and robust mechanical allodynia by day 14 after the first injection of paclitaxel (Fig 1A). Previous studies showed that the transcriptional changes of various MMPs and TIMPs in DRGs play critical roles for the development and maintenance of neuropathic pain.30,33,39,52 Therefore, we screened by qPCR the transcriptional regulations of multiple MMPs and TIMPs in DRG tissues at day 14 after paclitaxel treatment (Fig 1B). Of the 16 MMP and TIMP transcripts tested, 12 transcripts were expressed in DRGs and 3 transcripts were significantly regulated in paclitaxel-treated mice compared to vehicle-treated mice (Fig 1C). Specifically, we found that MMP2 and MMP9 transcripts were significantly increased, whereas TIMP1 was significantly decreased. It has been reported that recombinant TIMP-1, a general endogenous tissue inhibitor of MMPs, when given early after traumatic injury, was highly effective in reducing mechanical allodynia.33 Consistently with this report and its downregulation after paclitaxel, repeated administration of the recombinant protein TIMP1 (i.t., 4 pmol in 5 μl in PBS, every other day for 6 days) significantly prevented the development of paclitaxel-induced mechanical allodynia (Fig 2A). TIMP1 was also effective in reversing mechanical allodynia when we delayed its administration until day 14 after the first injection of paclitaxel (Fig 2B). Together these data clearly indicate the regulation MMPs in DRG tissues and the involvement of MMP signaling in the development and maintenance of mechanical allodynia in paclitaxel-treated mice.
Figure 1.
Time course of paclitaxel-induced mechanical allodynia and transcriptional of MMP signaling in DRGs. (A) Time course of mechanical withdrawal threshold (in grams) for vehicle- (black open circles) and paclitaxel-treated mice (filled red squares). Paclitaxel (2 mg/kg, i.p.) or vehicle was administered to mice on day 0, 2, 4 and 6. BL = baseline prior to treatment. Statistical analysis was performed using 2-way repeated measures analysis of variance, followed by Bonferroni’s post hoc test (n=6 male mice/group). *P<0.05 compared to vehicle. (B) Heat map of the expression of 14 MMPs and a TIMPs in DRGs of vehicle- and paclitaxel-treated mice (n=3 male mice/group) at day 14 of chemotherapy. (C) Volcano plot representing the fold change and the p-values of 12 transcripts that are expressed in DRGs tissue of paclitaxel- compared to vehicle-treated mice (Multiple t test, fold change p=0.05, n=3 male mice/group).
Figure 2.
Paclitaxel-induced mechanical allodynia is prevented and reversed by recombinant TIMP1. (A) TIMP1 (4 pmol/site, i.t.) prevents paclitaxel-induced mechanical allodynia up to 14 days after the first paclitaxel injection. TIMP1 or PBS were delivered every other day form day −1 to day 7. (B) Paclitaxel-induced mechanical allodynia is significantly reversed up to 6 h by a single intrathecal administration of TIMP1 (4 pmol/site) delivered at day 14 after chemotherapy. BL = baseline prior to treatment. Statistical analysis was performed using 2-way repeated measures analysis of variance, followed by Bonferroni’s post hoc test (n=5–6 male mice/group). *P<0.05 compared to PBS.
Paclitaxel-induced increase of MMP9 in vivo and in vitro
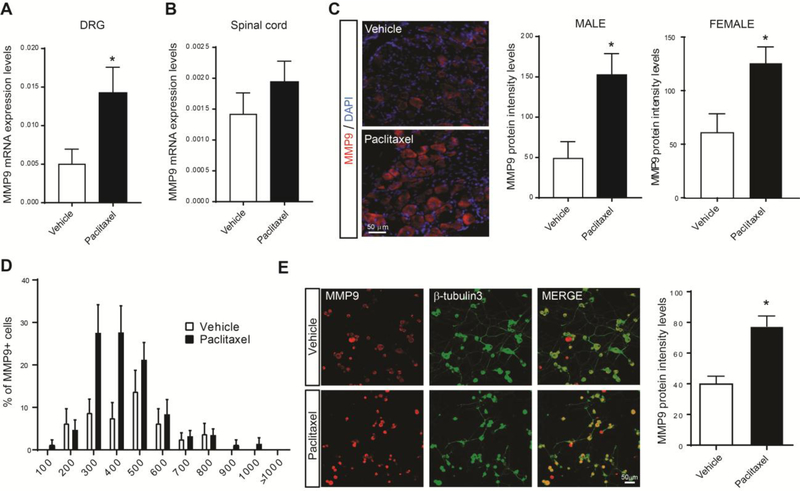
While TIMP1 inhibits most MMPs, it is a predominant and strong inhibitor of MMP9.10 Therefore, we confirmed the transcriptional change of MMP9 (~ 3 fold increase) in DRGs of a new and larger cohort of mice treated with paclitaxel (Fig 3A). To note, no significant increase of MMP9 was observed in spinal cord tissue after paclitaxel treatment (Fig 3B). To further validate the regulation of MMP9, we also performed immunohistochemistry to investigate MMP9 protein expression patterns and levels in DRG tissues and DRG cultured cells. MMP9 protein was significantly increased in DRG neurons in male and female mice at day 14 after paclitaxel treatment (Fig 3C), and predominately expressed in small- to medium-sized sensory neurons in male mice (Fig 3D). Remarkably, paclitaxel (300 nM, 24h) directly and significantly increased MMP9 in cultured DRG neurons (Fig 3E). These results indicate that MMP9 is quickly induced and maintained in DRG neurons after paclitaxel exposure. Can MMP9 represent a new therapeutic target for CIPN?
Figure 3.
MMP9 transcriptional and protein increases in DRGs of paclitaxel-treated mice. (A) qRT-PCR analysis showing mRNA expression levels of MMP9 in DRGs, and (B) spinal cord (SC) of paclitaxel- vs. vehicle-treated mice at day 14 after first treatment (n=6 male mice/group). (C) Representative staining of MMP9 protein in DRG tissues of vehicle- and paclitaxel-treated male and female mice and quantification of MMP9 signal intensity after 14 days (n=3–5 male and female mice/group). (D) Size frequency distribution (percentage) of MMP-9-positive neurons in vehicle- and paclitaxel-treated mice after 14 days (n=5 male mice/group) (E) Representative staining of MMP9 protein in cultured DRG neurons counterstained with the neuronal marker β-tubilin-3 and treated with vehicle or paclitaxel (300nM), and quantification of MMP9 signal intensity 24 h after treatment (n=4 treated culture/group). Statistical analysis was performed using t-test. *p<0.05 compare to vehicle group.
Effect of MMP9 monoclonal antibody on paclitaxel-induced mechanical allodynia
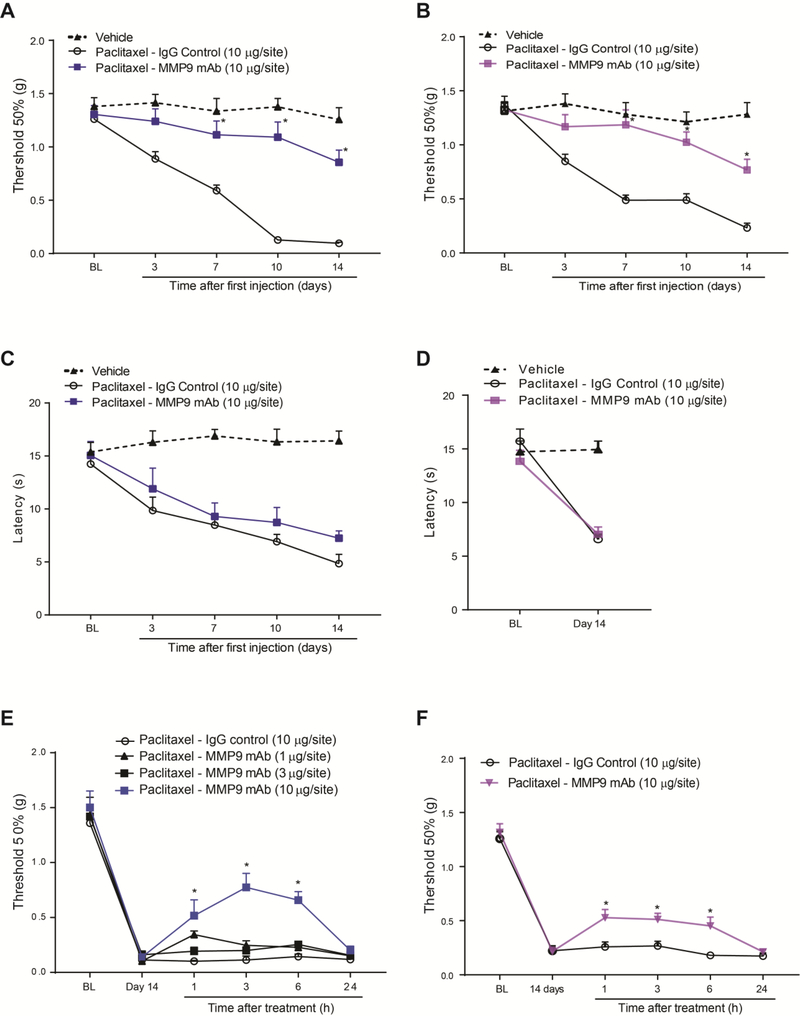
Therapeutic antibodies have been successfully used to treat cancer and immune diseases by targeting receptors or secreted proteins.13,66 Targeting MMP9 to treat CIPN is advantageous because this protease is highly inducible and a target for cancer treatment, and indeed a selective humanized monoclonal antibody is now in clinical trials for cancer treatment.1,29,48 In line with this clinical trials and as a proof of concept, we characterized the therapeutic effects in paclitaxel-treated mice of the monoclonal antibody targeting the human and mouse MMP-9 (MMP9 mAb, clone 6–6B), a previously well-characterized neutralizing antibody.9,51,55 Similar to TIMP1 effect, repeated administration of MMP9 mAb (i.t., 10 μg in 5 μl in PBS, every other day for 6 days) significantly prevented the development of paclitaxel-induced mechanical allodynia in male and female mice (Fig 4A, B). However, MMP9 mAb was not able to prevent paclitaxel-induced cold allodynia (Fig 4C, D). Remarkably, delayed injection of MMP9 mAb, was also effective to dose-dependently reverse the established mechanical allodynia at day 14 in male mice, and the analgesic effect for the higher dose lasted up to 6 h after the treatment (Fig 4E). Similar to male, MMP9 mAb significantly reversed the mechanical allodynia at day 14 in female mice for up to 6 h after the treatment (Fig 4F). These results show that the specific targeting of MMP9 by a monoclonal antibody efficiently alleviate paclitaxel-induced mechanical allodynia in male and female mice and may represent a new potential approach to treat CIPN.
Figure 4.
Paclitaxel-induced mechanical allodynia is prevented and partially reversed by the MMP9 monoclonal antibody (MMP9 mAb, clone 6–6B). MMP-9 antibody (MMP9 mAb, 10 μg/site, i.t.) prevents paclitaxel-induced mechanical allodynia in male (A) and female (B) mice up to 14 days after the first paclitaxel injection compared to IgG control (10 μg/site, i.t.). (C, D) However, MMP-9 antibody (MMP9 mAb, 10 μg/site, i.t.) does not prevents paclitaxel-induced cold allodynia. MMP9 antibody significantly reverses the established paclitaxel-induced mechanical allodynia in male (E) and female (F) mice at day 14 of chemotherapy. BL = baseline prior to treatment. Statistical analysis was performed using 2-way repeated measures analysis of variance, followed by Bonferroni’s post hoc test (n=5–6 male and female mice/group). *P<0.05 compared to the paclitaxel – IgG control group.
Effect of MMP9 monoclonal antibody on paclitaxel-induced oxidative stress
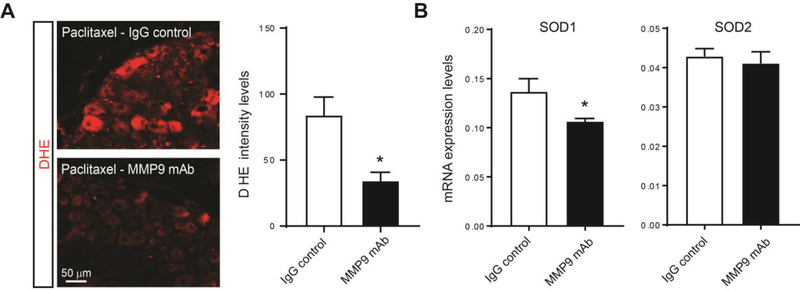
Multiple studies have reported that chemotherapeutic agents, including paclitaxel, induce increases in oxidative stress in DRG neurons that may contribute to the development of CIPN.8,18 Therefore, we examined the effect of MMP9 mAb on oxidative stress by measuring the fluorescence intensity of dihydroethidium (DHE), a cell-permeable dye that proportionally reacts with and quantify ROS levels.63 We found that at day 14 of paclitaxel treatment, there was a significant reduction in DHE fluorescence of mice injected with MMP9 mAb compared to the ones injected with an IgG control (Fig 5A), indicating a decrease in the production of ROS. Several endogenous antioxidant enzymes are induced in oxidative stress conditions to control ROS to prevent further damage.18,32 In neurons, the superoxide dismutase (SOD) enzymes SOD1 and 2 act as a first line of defense against mitochondrial electron transport chain-derived oxidative stress.26 It has been previously reported a significant increase in SOD1 activity, but not SOD2 in DRG after paclitaxel.18 Interestingly, we showed that MMP9 mAb significantly reduced the expression of SOD1 mRNA in paclitaxel-treated mice (Fig 5B). Together, these results strongly indicate that MMP9 monoclonal antibodies may prevent oxidative stress in CIPN.
Figure 5.
Decrease of paclitaxel-induced ROS production by the MMP9 monoclonal antibody. (A) Representative images of dihydroethidium (DHE) fluorescence in DRG sections of mice treated with MMP9 monoclonal antibody (MMP9 mAb) compared to the ones treated with IgG control at day 14 after paclitaxel. Quantification of DHE intensity indicates a significant reduction of the fluorescence signal in animal treated with MMP9 mAb (n=4 male mice/group). (B) Transcriptional expression levels of superoxide dismutase (SOD) enzymes SOD1 and 2 in DRGs of paclitaxel- vs. vehicle-treated mice after 14 days (n=6 male mice/group). Statistical analysis was performed using t-test. *p<0.05 compare to Ig G control.
Effect of MMP9 monoclonal antibody on paclitaxel-induced neuroinflammation
Several lines of evidence also have shown the contribution of neuroinflammation to CIPN and inhibition of proinflammatory cytokines have been demonstrated to prevent mechanical allodynia induced by paclitaxel.36,37,43,71 Interestingly, MMP9 has been shown previously to increase the activity of macrophages and satellite glial cells.3,33 However, glial fibrillary acidic protein (GFAP) and transmembrane protein CD68 transcripts that are respectively used as markers for activation of satellite glial cell and macrophage were unaltered in mice injected with the MMP9 mAb compared to the ones injected with the IgG control at day 14 of paclitaxel administration (Fig 6A and B). MMP9 has been also shown to participate to the increase and maturation of proinflammatory cytokines.69 Transcriptional analysis of proinflammatory cytokines of the same samples revealed that MMP9 antibody significantly decreased the expression of IL-6 and TNFα (Fig 6C), two proinflammatory cytokines previously involved in CIPN.67,73 To note, the transcriptional expression of the proalgesic inducible nitric oxide synthetase (NOS2) was also decreased by MMP9 mAb treatment (Fig 6C). These findings provide evidence that MMP9 monoclonal antibodies may suppress the transcription of proinflammatory cytokines, as well as NOS2, that are involved in the pathogenesis of CIPN.
Figure 6.
Decrease of paclitaxel-induced pro-inflammatory cytokines by the MMP-9 monoclonal antibody (MMp9 mAb). Transcriptional expression levels assessed by qPCR of (A) the satellite glial cell marker GFAP, and (B) the macrophage markers IBA1 and CD68. (C) Transcriptional expression levels of the pro-inflammatory cytokines IL-1β, IL-6 and TNFα, as well as the proalgesic NOS2. Student’s t test measured differences mRNA expression levels in DRGs 14 day after paclitaxel in mice treated with IgG control or MMP9 Ab (n=5–6 male mice/group). *p<0.05 compare with Ig G control group.
Effect of MMP9 monoclonal antibody on paclitaxel-induced IENF loss
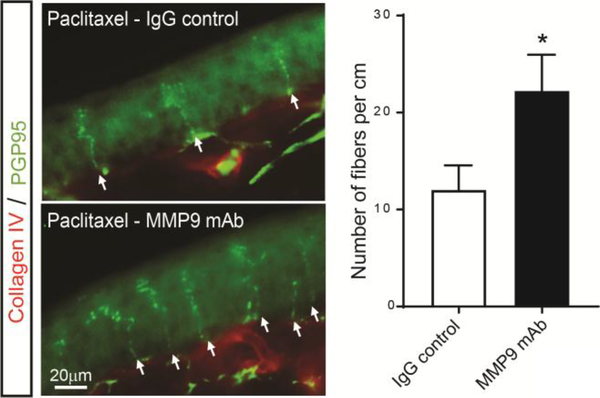
Finally, paclitaxel treatment has been previously shown to induce a loss of IENFs, and this phenomenon is believed to be associated with oxidative stress and neuroinflammation.2,7,58,71 Therefore, we analyzed the potential protective effect of the MMP9 mAb on the loss of IENFs in the hind paws at day 14 of paclitaxel-treated mice. The number of nerve fibers entering the epidermis was significantly increased in mice injected with the MMP9 mAb when compared with vehicle the IgG control (Fig 7). This data demonstrates that MMP9 monoclonal antibodies may protect against paclitaxel-induced IENF loss, which is consider a hallmark of CIPN.24,49
Figure 7.
Prevention of paclitaxel-induced IENF loss by the MMP-9 monoclonal antibody. Representative images of plantar skin sections stained with PGP9.5 (green) and collagen IV (red) 14 day after paclitaxel in mice treated with IgG control or MMP9 mAb. The number of fibers per centimeter were significantly higher in sections from mice treated with MMP9 mAb. Statistical analysis was performed using t-test (n=4 male mice/group). *p<0.05 compare with Ig G control group.
Discussion
Chemotherapy-induced peripheral neuropathy affects as many as 70% of patients treated with chemotherapeutic drugs, including paclitaxel. However, there are no clinical treatments to prevent and/or reverse CIPN. In the present study, we investigated the molecular and functional regulation of MMPs in DRGs in the paclitaxel murine model of CIPN. In particular, we showed the up-regulation of MMP9 after paclitaxel exposure and identified, for the first time, the MMP-9 monoclonal antibody (MMP9, mAb, clone 6–6B) as a potential therapeutic approach for CIPN. Administration of the MMP9 mAb not only completely prevented and reversed mechanical allodynia in male and female mice, but also protected against oxidative stress, neuroinflammation, and the loss of IENFs in male mice treated with paclitaxel.
The MMP family has over 24 family members in mammals, which endogenous activity is controlled by four TIMPs.35 Previous transcriptional, biochemical, pharmacological and transgenic studies have clearly indicated that MMPs have pathological roles in cancer, inflammation and pain.29,62 Here, we showed that the transcriptional up-regulation of the gelatinases MMP2 and MMP9, as well as the transcriptional down-regulation of TIMP1 at day 14 of chemotherapy. Rescue of TIMP1 expression by injection of its recombinant protein significantly prevented and reversed CIPN mechanical allodynia. This effects may due to the inhibition of MMP2 and MMP9. In particular, we have confirmed the up-regulation of MMP9 protein in DRG neurons of male and female mice at day 14 of chemotherapy, and shown the therapeutic effects of the MMP9 mAb for the prevention of paclitaxel-induced mechanical allodynia in male and female mice. Increases of MMP2 and MMP9 protein were previously described following a nerve injury, and similar to our behavioral data the mechanical allodynia associated with the injury was significantly reduced in MMP9 knockout mice or mice treated with recombinant protein TIMP1.33 Nerve injury induced a transient up-regulation of MMP9 in DRG neurons consistently with its role in preventing neuropathic pain after nerve injury and chemotherapy. However, we observed a significant up-regulation of MMP9 at day 14 of chemotherapy and MMP9 inhibition at this time point significantly reduced mechanical allodynia, suggesting a functional role of MMP9 not only in the development but also in the progression of CIPN. This discrepancy of expression and function of MMP9 between nerve injury and CIPN may be intrinsic to the pain models and different underlying mechanisms. For instance, CIPN induces a slower but continuous neuronal damage due to the multiple injections of paclitaxel compared to the drastic but temporarily limited nerve injury. It also interesting to point out that MMP9 activation is regulated by MMP3.29 Although we did not find a transcriptional change in MMP3 at day 14 of chemotherapy, microarray analysis of DRG tissues have previously revealed an increase of MMP3 in CIPN conditions but not after nerve injury.4,15,52
Despite MMP9 inhibition significantly reversed mechanical allodynia, TIMP1 seems to be more effective. This may result from the broad inhibition by TIMP1 of various MMPs,27 which may also include the inhibition of the paclitaxel-induced MMP2. Delayed but persistent upregulation of MMP2 was found in DRG satellite glial cells and spinal cord astrocytes after nerve injury.33 Both satellite glial cells and astrocytes have been shown to contribute to CIPN.65,72 Inhibition of MMP2 could have the potential to treat established mechanical allodynia after nerve injury and chemotherapy. It may be warranted to test whether TIMP1 and MMP2 inhibition efficiently attenuate CIPN and in particular neuropathic pain symptoms that are unaltered by the MMP9 monoclonal antibody, such as cold allodynia. However, it is important to consider that MMPs also have normal physiological functions, and so use of TIMP1 that lack selectivity or targeting MMP2 that is constitutively expressed in many tissues may cause undesirable side effects.29,53 MMP9 is an appealing therapeutic target because it is highly inducible and a specific monoclonal antibody against MMP9 is already in clinical trials.1,29,48
Andecaliximab (GS-5745), a humanized anti-MMP9 monoclonal antibody, is presently in Phase 3 for the treatment of gastric cancer and ulcerative colitis.1,48,57 These trials have reported encouraging safety, tolerability and pharmacokinetics of this antibody, suggesting that Andecaliximab may serve as a two pronged therapeutic approach, on one hand to fight tumor progression and on the other hand to reduce CIPN.
Before clinical translation of MMP9 monoclonal antibodies can be considered for the treatment of CIPN, further studies are needed to define their actions with other chemotherapeutic agents and their different routes of administration (many of them are given intravenously, whereas we have used i.t. injections). However, our results seem to suggest that these antibodies may act on several mechanisms and symptoms that are shared between different agents and delivery routes. Although the mechanisms of CIPN remain incompletely known, there is growing evidence that paclitaxel administration targets peripheral sensory neurons, leading to maladaptive changes in oxidative stress, neuroinflammation, as well as loss of IENFs.2,7,8,11,18 Remarkably, our data show that MMP9 mAb significantly prevents these maladaptive changes.
We have demonstrated that MMP9 mAb protect against paclitaxel-induced oxidative stress (i.e. increase of ROS). ROS are significantly increased in DRG neurons after paclitaxel,18 and behavioral studies with pharmacological scavenging agents have shown to be a casual factors in CIPN.17,19,21 However, it is worth noting that the same scavenging agents can also disrupt oxidants that are important intracellular signaling molecules for the maintenance of homeostasis and protective responses to various stresses,32 which may explain the largely discouraging results obtained in regards to the clinical treatment of pain.12,23 This may be true for the MMP9 mAb, since treatment with this antibody resulted in a decreased expression of the antioxidant SOD1 after paclitaxel. However, we believe that this decrease is a consequence of a reduced production of ROS, and therefore a reduced engagement of the antioxidant defenses.41 Induction of MMP9 by oxidative stress has been reported after virus infection, stroke, and spinal cord injury.34,38,70 However, how the MMP9 mAb can vice versa reduce ROS and oxidative stress is unclear. Indeed, we observed an increase of MMP9 mostly in small-sized DRG neurons, whereas DHE fluorescence seems equally distributed in small- and large-sized DRG neurons. This observation may indicate that oxidative stress and MMP9 may be indirectly linked by additional mechanisms such as neuroinflammation.3,28,50
MMP9 and MMPs in general are important players in neuroinflammation. MMP9 controls the infiltration and activation of macrophages in abdominal aortic aneurysm.22 Infiltration and activation of macrophages has been also reported to contribute to CIPN and their depletion in DRGs prevented the development of paclitaxel-induced mechanical allodynia.71 To note minocycline, a macrophages and partially MMP9 inhibitor,29 also prevented mechanical allodynia in paclitaxel-treated animals.42 Interestingly, we showed that MMP9 mAb did not regulate any of the macrophage markers for proliferation and activation (i.e. IBA1 and CD68, respectively). Previously, we have reported that MMP9 mediates neuron–SGC interaction in DRGs after acute morphine treatment, which can mask morphine analgesia by the release of IL-1β from satellite glial cells.3 Accordingly, MMP9 is both sufficient and required for eliciting persistent mechanical allodynia via pro-inflammatory IL-1β signaling in mice.33 However, both satellite glial cell activation marker GFAP or IL-1β transcripts were unchanged by MMP9 mAb treatment. To note, MMPs can cleave other substrates such as growth factors, chemokines and other cytokines to regulate varied aspects of inflammation and immunity.29,53 Therefore, we explored the effect of MMP9 mAb on other proinflammatory mediators and found that this antibody significantly decreased the expression of proinflammatory cytokines IL-6 and TNFα, two cytokines previously involved in CIPN,46,61 as well as the expression levels of the proalgesic NOS2 transcripts. Although further studies are required to properly characterize the actions of the MMP9 monoclonal antibodies on neuroinflammation, these results suggests that the analgesic effect of this antibody may due at least in part to the reduction of pro-inflammatory mediators.
Loss of IENFs is associated with chemotherapy treatment as measured by a decrease in the density of nerve fibers entering into the epidermis. Loss of IENFs may result from increases in oxidative stress and neuroinflammation in DRG tissues.2,7,58,71 Here, we reported that MMP9 mAb reduced ROS, IL-6 and TNFα as well as IENF density after paclitaxel treatment. Although the direct causal link between the loss of IENFs and chronic pain is unclear,31 this loss is an hallmark of neuropathic pain observed in nearly every peripheral neuropathies and its recovery with the resolution of neuropathic pain. Therefore, the preservation of these IENFs by the MMP9 mAb may be linked to its analgesic effect and MMP9 mAb may represent a therapeutic approach for other peripheral neuropathies.
In conclusion, these findings demonstrate, for the first time, the involvement of MMPs in the development and maintenance of paclitaxel-induced neuropathic pain. Importantly, we showed that the MMP9 mAb effectively prevents and reverses the paclitaxel-induced mechanical allodynia in male and female mice. Although this antibody also attenuated several mechanisms underlying CIPN in male mice, further investigations are warranted since it curiously had no effect on paclitaxel-induced cold allodynia, and different mechanisms may cause mechanical allodynia in female mice.60 Further investigations are also warranted to determine how MMP9 monoclonal antibodies eventually interfere with various chemotherapies in killing tumor cells. However, MMP9 inhibition has been extensively studied as antitumor therapeutic approach62 and a humanized MMP9 antibody is currently in clinical trial for this purpose.48 Thus, our preclinical investigation provides a proof of concept for future clinical trials to evaluate whether MMP9 antibodies may also be used for the prevention and treatment of the excruciating side effects of CIPN.
Supplementary Material
Highlights:
1) Paclitaxel-induced peripheral neuropathy in mice is associated with transcriptional changes in MMP signaling. 2) Paclitaxel increased MMP9 protein levels in small-sized DRG neurons. 3) MMP9 antibody prevents paclitaxel-induced oxidative stress, neuroinflammation in DRGs and retraction of intraepidermal nerve fibers. 4) MMP9 antibody prevents and reverses paclitaxel-induced mechanical allodynia in male and female mice.
Perspective:
Chemotherapy-induced peripheral neuropathy (CIPN) remain ineffectively managed in cancer patients potentially leading to the discontinuation of an otherwise life-saving treatment. Here, we demonstrate that a monoclonal antibody targeting the matrix metalloproteinase 9 (MMP9) alleviates neuropathic pain and several mechanisms linked to CIPN. This study is particularly relevant since a humanized MMP9 antibody is already in advanced clinical trials for the treatment of colitis and cancer, and it may be straightforwardly repurposed for the relief of CIPN.
Acknowledgments
Funding statements: This work has been partially funded by the NIH/NINDS R21 NS106264 and by the Gardner Neuroscience Institute Pilot Award 2017 from the University of Cincinnati.
Footnotes
Disclosures:
Conflict of Interests: The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amar S, Fields GB: Potential clinical implications of recent matrix metalloproteinase inhibitor design strategies. Expert Rev Proteomics Informa Healthcare; 12:445–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C: Terminal arbor degeneration - a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci 33:1667–76, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berta T, Liu T, Liu Y-C, Xu Z-Z, Ji R-R: Acute morphine activates satellite glial cells and up-regulates IL-1β in dorsal root ganglia in mice via matrix metalloprotease-9. Mol Pain 8:18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berta T, Perrin FE, Pertin M, Tonello R, Liu Y-C, Chamessian A, Kato AC, Ji R-R, Decosterd I: Gene Expression Profiling of Cutaneous Injured and Non-Injured Nociceptors in SNI Animal Model of Neuropathic Pain. Sci Rep 7:9367, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berta T, Poirot O, Pertin M, Ji RR, Kellenberger S, Decosterd I: Transcriptional and functional profiles of voltage-gated Na+ channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol Cell Neurosci 37:196–208, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, Freret T: The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat Protoc 4:1560–4, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Boyette-Davis J, Xin W, Zhang H, Dougherty PM: Intraepidermal nerve fiber loss corresponds to the development of Taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain 152:308–13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyette-Davis JA, Walters ET, Dougherty PM: Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag 5:285–96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozdagi O, Nagy V, Kwei KT, Huntley GW: In Vivo Roles for Matrix Metalloproteinase-9 in Mature Hippocampal Synaptic Physiology and Plasticity. J Neurophysiol NIH Public Access; 98:334–44, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brew K, Nagase H: The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta - Mol. Cell Res. page 55–71, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carozzi VA, Canta A, Chiorazzi A: Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci. Lett. page 90–107, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Cascella M: Chemotherapy-induced peripheral neuropathy: limitations in current prophylactic strategies and directions for future research. Curr Med Res Opin Taylor & Francis; 33:981–4, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Chan AC, Carter PJ: Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. page 301–16, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL: Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ: Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci 3:16, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon WJ: Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–62, 1980. [DOI] [PubMed] [Google Scholar]
- 17.Doyle T, Chen Z, Muscoli C, Bryant L, Esposito E, Cuzzocrea S, Dagostino C, Ryerse J, Rausaria S, Kamadulski A, Neumann WL, Salvemini D: Targeting the Overproduction of Peroxynitrite for the Prevention and Reversal of Paclitaxel-Induced Neuropathic Pain. J Neurosci 32:6149–60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duggett NA, Griffiths LA, McKenna OE, de Santis V, Yongsanguanchai N, Mokori EB, Flatters SJL: Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 333:13–26, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fidanboylu M, Griffiths LA, Flatters SJL: Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. Baccei ML, editor. PLoS One Public Library of Science; 6:e25212, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flatters SJL, Dougherty PM, Colvin LA: Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. page 737–49, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Flatters SJL, Xiao WH, Bennett GJ: Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci Lett 397:219–23, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong Y, Hart E, Shchurin A, Hoover-Plow J: Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest 118:3012–24, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grace PM, Gaudet AD, Staikopoulos V, Maier SF, Hutchinson MR, Salvemini D, Watkins LR: Nitroxidative Signaling Mechanisms in Pathological Pain. Trends Neurosci 39:862–79, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland NR, Crawford TO, Hauer P, Cornblath DR, Griffin JW, McArthur JC: Small-fiber sensory neuropathies: Clinical course and neuropathology of idiopathic cases. Ann Neurol 44:47–59, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Hylden JL, Wilcox GL: Intrathecal morphine in mice: A new technique. Eur J Pharmacol 67:313–6, 1980. [DOI] [PubMed] [Google Scholar]
- 26.J. E, M. E, F. B, Barnham KJ, Masters CL, Bush AI: Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3:39–46, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Jackson HW, Defamie V, Waterhouse P, Khokha R: TIMPs: versatile extracellular regulators in cancer. Nat Rev Cancer 17:38–53, 2016. [DOI] [PubMed] [Google Scholar]
- 28.Ji R-R, Xu Z-Z, Gao Y-J: Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov Nature Research; 13:533–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji R-RR, Xu Z-ZZ, Wang X, Lo EH: Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci 30:336–40, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L, Pan CL, Wang CY, Liu BQ, Han Y, Hu L, Liu L, Yang Y, Qu JW, Liu WT: Selective suppression of the JNK-MMP2/9 signal pathway by tetramethylpyrazine attenuates neuropathic pain in rats. J Neuroinflammation BioMed Central; 14:174, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalliomäki M, Kieseritzky J v, Schmidt R, Hägglöf B, Karlsten R, Sjögren N, Albrecht P, Gee L, Rice F, Wiig M, Schmelz M, Gordh T: Structural and functional differences between neuropathy with and without pain? Exp Neurol Academic Press; 231:199–206, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Kawagishi H, Finkel T: Unraveling the Truth About Antioxidants: ROS and disease: finding the right balance. Nat Med 20:711–3, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki Y, Xu Z-Z, Wang X, Park JY, Zhuang Z-Y, Tan P-H, Gao Y-J, Roy K, Corfas G, Lo EH, Ji R-R: Distinct roles of matrix metalloproteases in the early-and late-phase development of neuropathic pain. Nat Med 14:331–6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly PJ, Morrow JD, Ning MM, Koroshetz W, Lo EH, Terry E, Milne GL, Hubbard J, Lee H, Stevenson E, Lederer M, Furie KL: Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: The Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke 39:100–4, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Khokha R, Murthy A, Weiss A: Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. page 649–65, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, Dougherty PM, Heijnen CJ, Kavelaars A: CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 36:11074–83, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR: Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 21:686–98, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YH, Lai CL, Hsieh SH, Shieh CC, Huang LM, Wu-Hsieh BA: Influenza A virus induction of oxidative stress and MMP-9 is associated with severe lung pathology in a mouse model. Virus Res 178:411–22, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Xu L, Deng X, Jiang C, Pan C, Chen L, Han Y, Dai W, Hu L, Zhang G, Cheng Z, Liu W: N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain 157:1711–23, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM: The Cancer Chemotherapeutic Paclitaxel Increases Human and Rodent Sensory Neuron Responses to TRPV1 by Activation of TLR4. J Neurosci 35:13487–500, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin MT, Beal MF: Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. page 787–95, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Liu CC, Lu N, Cui Y, Yang T, Zhao ZQ, Xin WJ, Liu XG: Prevention of Paclitaxel-induced allodynia by Minocycline: Effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol Pain BioMed Central; 6:1744–8069-6–76, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu XJ, Zhang YL, Liu T, Xu ZZ, Park CK, Berta T, Jiang DH, Ji RR: Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res Nature Publishing Group; 24:1374–7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y-C, Berta T, Liu T, Tan P-H, Ji R-R: Acute morphine induces matrix metalloproteinase-9 up-regulation in primary sensory neurons to mask opioid-induced analgesia in mice. Mol Pain 8:19, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma J, Kavelaars A, Dougherty PM, Heijnen CJ: Beyond Symptomatic Relief for Chemotherapy-Induced Peripheral Neuropathy: Targeting the Source. [DOI] [PMC free article] [PubMed]
- 46.Makker PGS, Duffy SS, Lees JG, Perera CJ, Tonkin RS, Butovsky O, Park SB, Goldstein D, Moalem-Taylor G: Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. Costigan M, editor. PLoS One Public Library of Science; 12:e0170814, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao-Ying QL, Kavelaars A, Krukowski K, Huo XJ, Zhou W, Price TJ, Cleeland C, Heijnen CJ: The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One 9:, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall DC, Lyman SK, McCauley S, Kovalenko M, Spangler R, Liu C, Lee M, O’Sullivan C, Barry-Hamilton V, Ghermazien H, Mikels-Vigdal A, Garcia CA, Jorgensen B, Velayo AC, Wang R, Adamkewicz JI, Smith V: Selective allosteric inhibition of MMP9 is efficacious in preclinical models of ulcerative colitis and colorectal cancer. PLoS One Public Library of Science; 10:e0127063, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW: Epidermal nerve fiber density: Normative reference range and diagnostic efficiency. Arch Neurol 55:1513–20, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB: Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid Redox Signal 20:1126–67, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore BA, Manthey CL, Johnson DL, Bauer AJ: Matrix metalloproteinase-9 inhibition reduces inflammation and improves motility in murine models of postoperative ileus. Gastroenterology NIH Public Access; 141:1283–92, 1292.e14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishida K, Kuchiiwa S, Oiso S, Futagawa T, Masuda S, Takeda Y, Yamada K: Up-regulation of matrix metalloproteinase-3 in the dorsal root ganglion of rats with paclitaxel-induced neuropathy. Cancer Sci 99:1618–25, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parks WC, Parks WC, Wilson CL, Wilson CL, López-Boado YS, López-Boado YS: Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4:617–29, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:45e–45, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radjabi AR, Sawada K, Jagadeeswaran S, Eichbichler A, Kenny HA, Montag A, Bruno K, Lengyel E: Thrombin induces tumor invasion through the induction and association of matrix metalloproteinase-9 and ??1-integrin on the cell surface. J Biol Chem American Society for Biochemistry and Molecular Biology; 283:2822–34, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg GA: Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. page 205–16, 2009. [DOI] [PubMed] [Google Scholar]
- 57.Sandborn WJ, Bhandari BR, Fogel R, Onken J, Yen E, Zhao X, Jiang Z, Ge D, Xin Y, Ye Z, French D, Silverman JA, Kanwar B, Subramanian GM, McHutchison JG, Lee SD, Shackelton LM, Pai RK, Levesque BG, Feagan BG: Randomised clinical trial: A phase 1, dose-ranging study of the anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 versus placebo for ulcerative colitis. Aliment Pharmacol Ther Wiley-Blackwell; 44:157–69, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schappacher KA, Styczynski L, Baccei ML: Early life vincristine exposure evokes mechanical pain hypersensitivity in the developing rat. Pain 158:1647–55, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, Macleod MR, Colvin LA, Fallon M: Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155:2461–70, 2014. [DOI] [PubMed] [Google Scholar]
- 60.Sorge RE, Mapplebeck JCS, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin J-S, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji R-R, Zhang J, Salter MW, Mogil JS: Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci Nature Research; 18:1081–3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Starkweather A, Angela: Increased interleukin-6 activity associated with painful chemotherapy-induced peripheral neuropathy in women after breast cancer treatment. Nurs Res Pract Hindawi; 2010:281–531, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandenbroucke RE, Libert C: Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. page 904–27, 2014. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Fang H, Huang Z, Shang W, Hou T, Cheng A, Cheng H: Imaging ROS signaling in cells and animals. J. Mol. Med. page 917–27, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Spandidos A, Wang H, Seed B: PrimerBank: A PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res Oxford University Press; 40:D1144–9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warwick RA, Hanani M: The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. Eur J Pain (United Kingdom) 17:571–80, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Weiner LM, Surana R, Wang S: Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. page 317–27, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Z, Wang S, Wu I, Mata M, Fink DJ: Activation of TLR-4 to produce tumour necrosis factor-α in neuropathic pain caused by paclitaxel. Eur J Pain (United Kingdom) 19:889–98, 2015. [DOI] [PubMed] [Google Scholar]
- 68.Xu Z-Z, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji R-R: Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 21:1326–31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yong VW: Metalloproteinases: Mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. page 931–44, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Yu F, Kamada H, Niizuma K, Endo H, Chan PH: Induction of MMP-9 Expression and Endothelial Injury by Oxidative Stress after Spinal Cord Injury. J Neurotrauma 25:184–95, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang H, Li Y, De Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM: Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J Pain 17:775–86, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang HH, Yoon SY, Zhang HH, Dougherty PM: Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of paclitaxel-induced painful neuropathy. J Pain 13:293–303, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK: Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflammation. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.