Abstract
B cell immunoglobulin (Ig) repertoire composition shapes immune responses. The generation of Ig diversity begins with Ig variable region exon assembly from gene segments, random inter-segment junction sequence diversity, and combinations of Ig heavy and light chain. This generates vast preemptive sequence free dom in early developing B lineage cell Ig genes that can anticipate a great diversity of threats. This freedom is met with large restrictions that ultimately define the naïve (i.e. preimmune) Ig repertoire. Activation-induced somatic hypermutation (SHM), which further diversifies Ig V regions, is also met with strong selection that shapes Ig affinity maturation. While individual repertoire features, such as affinity for self and competition for foreign antigen, are known to drive selection, the selection filters themselves may be subject to regulation. Large sequence freedom coupled with strong selection for each diversification process provides flexibility for demand-driven regulation to dynamically balance antigen recognition capacities and associated autoimmune risks according to hostneeds.
Graphical abstract
Introduction
A diverse repertoire of antibodies contributes to immunity against a vast number of potential pathogenic threats. Antibodies diversify through two distinct pathways, which can be described as primary and secondary diversification. Primary diversification involves combinatorial assembly of Immunoglobulin (Ig) heavy (H) and light (L) chain variable region (V) exons during B cell development from small gene segments to form the antigen recognition piece of the B cell receptor (BCR), initially expressed as IgM on immature B cells. The second diversification pathway involves somatic hypermutation (SHM) of V exons and affinity-based selection of activated B cells in germinal centers (GCs). Clones with mutated V exons that encode higher affinity Ig win limiting cognate T cell help, leading to antibody affinity maturation [1]. The primary and secondary diversification systems collaborate to provide protective antibody responses. In addition to providing an immediate wave of innate-like, low affinity antibodies in response to infectious challenge, the primary (i.e. pre-immune) repertoire is the substrate upon which initial pathogen recognition takes place to initiate secondary antibody evolution toward development of high affinity antibodies and protective humoral memory responses. In this regard, the GC system is thought to only ripen antibodies that engage in chance recognition of antigen provided by the anticipatory pre-immune Ig repertoire.
Dependence on robust representation of anti-pathogen specificities in the primary repertoire can be a problem, as in cases where the unmutated germline ancestors of antibodies with potential to become highly protective can be low affinity and/or poorly represented in the pre-immune Ig repertoire. This is the case with some classes of broadly neutralizing antibodies (bnAbs) to HIV-1 [2,3]. As a consequence, naïve B cells with bnAb potential are often at a competitive disadvantage to nonneutralizing, strain-specific specificities that dominate in abundance or affinity within primary Ig repertoires. These problems have intensified awareness of knowledge gaps regarding what regulates primary repertoire architecture and what the requirements are for B cells to enter the SHM and affinity maturation process.
While all the recent advances in this area cannot be given due treatment here, we discuss a few recent findings related to the structure of the primary Ig repertoire in terms of its determination and plasticity and its interface with entry into the SHM diversification system. We also speculate on a model of plasticity built into the Ig repertoire system upon which demand-driven regulation can operate according to host needs for naive, as well as experienced Ig repertoires.
Structure of the primary Ig repertoire
While deep sequencing studies have enabled unprecedented advances in understanding sequence structure and clonal dynamics of Ig repertoires, technical limitations keep a full understanding of its true binding capacity beyond our grasp. Studies quantifying the frequencies of naïve B cells able to bind selected antigens in mice have revealed an important feature that is not predicted by general textbook immunological knowledge—namely, that frequencies of naïve B cells that can bind a given antigen is reasonably consistent between individuals.
Binding data from several recent studies shows concordance between individuals within genetically inbred strains. For example, C57BL/6 mice that are naïve to phycoerythrin (PE) or allophycocyanin (APC) were found to have about 20,000 PE-specific naïve B cells (1 in 5,000) and 4,000 APC-specific (1 in 25,000) naive B cells by flow cytometry [4]. In contrast, BALB/c mice are reported to have 1,400 (1 in 71,000) PE-specific naive B cells while harboring a similar number of APC-specific naive B cells as B6 mice [5*]. ELISA evaluation of single clone cultures showed naïve B cell frequencies for Bacillus anthracis protective antigen (PA) and influenza hemagglutinin (HA) are 1 in 8,000, and 1 in 18,000, respectively [6**]. The frequency of naïve B cells specific for the small molecule hapten 4-hydroxy-3-nitrophenyl acetyl (NP) was reported at 1 in 4,000 by flow cytometry [7]. Through single B cell sorting and Ig sequencing, naïve B cell precursors of the VRC01-class of broadly neutralizing anti-HIV-1 antibodies in humans have recently been determined to be present at a frequency of 1 in 2,400,000 B cells with reasonable consistency between individuals [8].
Consistent binding frequencies between individuals is not necessarily expected, at least for large complex antigens, because despite findings that V gene usage frequencies are consistent between individuals when examined with adequate sequence depth [9,10], V gene segments only encode for 2 of the 3 complementarity determining regions (CDRs) that make up the majority of the antigen binding surface of the antibody.Junctions between assembled gene segments that make up V region exons generate the CDR3 region, which has been argued to encode for diversity sufficient for most antibody specificities [11]. A substantial contribution of stochastically-generated CDR3 to Ig repertoire binding capacity would predict large variability between individuals. The consistency of antigen binding frequencies in naïve B cell populations between individuals could be due to germ-line encoded CDR1 and 2 playing a larger role than anticipated. Alternatively, Ig developmental selection filters may mold Ig repertoires to roughly predetermined architectures, offering opportunities for regulatory control to adapt the protective utility of Ig repertoires according to host needs. Understanding how baseline Ig repertoires are shaped is important because antigen recognition frequencies significantly influence immune responses, including immunodominance [12*] quality of the initial response [13**,14] as well as the fate characteristics of the memory and recall responses [5*].
What shapes Ig repertoires?
It has been known for decades that selection plays a major role in shaping the Ig repertoire as the original V gene segment usage frequencies selected for V(D)J recombination differ from usage frequencies found in mature naïve B cells [15]. As discussed in a recent review [16], tolerance control processes negatively select out Ig specificities with strong self-reactive potential. Positive selection also occurs, but the nature of the antigens driving positive selection, and where and how these processes take places are not fully defined [17]. The innate-like B1 cell repertoire is relatively oligoclonal enriched in specificities for self and common components of bacteria [18]. The B1 Ig repertoire undergoes age-dependent shifts that occur independently of microbiota, possibly through timed exposures to self-antigens [19]. The B2 repertoire is more diverse, but also appears to contain a positive selection process for targets that presumably would provide beneficial specificities [17].
While self-ligands play a role in B cell selection, recent studies have shown that symbiotic microbes influence both the primary Ig and memory IgM repertoires in conventional B2 cells. Germ-free mice colonized with microbial symbionts at weaning age developed increased bacterial symbiont recognition frequencies in naïve follicular B cells with an IgM+IgD+CD73−CD80− phenotype. This occurs in a T cell-independent manner and early in weaning age [20**]. Frequencies of B cells that recognize symbiotic microbes were notably high. Limiting dilution analysis found a frequency of approximately 1/58 naïve follicular B cells in germ-free mice reactive to intestinal bacteria that increased to approximately 1/33 after conventionalization [20**]. How microbial symbionts lead to enrichment of naïve cells is not fully understood. equencing studies indicated that some Ig repertoire changes were already apparent in naïve transitional B cells, which represent new bone marrow emigrants—indicating that microbial colonization may influence early B cell selection. In addition, microbial symbionts have also been shown to influence the Ig repertoire via B cell receptor editing for a small amount of B cells that develop in the intestinal lamina propria during weaning age [9].
An AID reporter mouse model, in which memory cells are labeled, revealed that microbial symbionts also influence baseline IgM memory cell repertoires in unimmunized mice [21**]. In this system, cells become and remain YFP+ when exposed simultaneously to AID and tamoxifen. Reactivity to gut luminal antigens by YFP+ memory cells was enriched in conventional, but not germ-free mice [21**]. YFP+ memory cells expressed IgM+CD73+CD80+ and their accumulation occurred for several months and was dependent upon T cells and toll-like receptor signaling pathways [21**].Colonization with microbiota was also required for the induction of systemic IgA concentrations in serum [22].
In terms of functional consequence, the influence of symbiotic microbes on the primary and memory IgM repertoires led to increased anti-bacterial vaccine responses in both cases [20**,21**]. Microbe-dependent IgA in the serum was shown to protect against microbial sepsis due to symbiont-dependent anti-bacterial plasma cells that can cross-react to bacteria more generally [22]. The functional impact of symbiotic microbes on Ig repertoires has been shown to have important implications for antibody immunodominance in humans. An example of this is the finding that accumulation of memory B cells specific for symbiotic microbes was shown to influence immunodominance toward non-neutralizing gp41 epitopes on the HIV-1 envelope protein complex [23*].
The influence of both tolerance to self and symbiotic microbes in affecting baseline Ig repertoires and disease outcomes is illustrated in the example of antibodies against the Galα1–3Galβ1-(3)4GlcNAc - (α-gal) glycan. Some bacteria, and most mammals produce α-gal due to the action of the UDP:galactose:β-galactoside-α1,3-galactosyltransferase gene (1,3 GT). A notable mammalian exception to α-gal production is old world primates, including humans, due to inactivation of 1,3 GT. Tolerance-related negative selection downregulates anti-α-gal antibodies in α-gal-producing mammals, but they are allowed to accumulate in the absence of functional 1,3 GT through a mechanism that is dependent upon α-gal-producing microbial symbionts [24]. Because Anti-α-gal antibody levels correlate with protection from malaria in endemic regions [24,25], malaria was likely a driving force for evolutionary loss of 1,3 GT in old world primates [24,26]. Gut colonization with E. Coli O86:B7, an α-Gal-producing bacterial strain, induced anti-α-Gal antibodies in α1,3Gt−/− mice, and this was associated with reduction in Plasmodium infection [24]. Colonization with E. coli K12 bacteria, which do not express α-Gal, neither induced anti-α-Gal IgM antibodies, nor did it not block Plasmodium infection. How anti-symbiotic bacterial glycoforms are induced remains to be fully elucidated, but may include some of the pathways described above. Notably, while inhibition of Plasmodium was dependent upon anti-α-Gal IgM antibodies, it was not dependent upon somatic Ig mutation [24], indicating that either naïve and/or memory B cell anti-microbiota Ig enrichment pathways may contribute.
High Polyreactivity in Immature Ig Repertoires: Bug or Feature?
While many antibodies appear to be highly specific for a unique antigen-binding site, polyreactive antibodies can recognize multiple structurally unrelated antigens. Polyreactive antibodies are often self-reactive. In a sense, it is intuitive that an antibody reactive to multiple unrelated targets is more likely to include self-antigens among those targets. There may also be a contribution of technical bias arising from the use of self-antigen among the panels used to test polyreactivity in studies.
The primary repertoire in developing bone marrow B cells is composed of Ig specificities that are extraordinarily polyreactive and self-reactive. Self/polyreactivity comprises up to well over half of early B lineage Ig repertoire in humans [27], a surprisingly high level thought to be due to inadvertent byproducts of agnostic Ig diversification. However, there are autoreactive VH gene segments encoded in the genome that have stood the test of evolutionary time. An example in humans is VH4–34, which recognizes human erythrocyte surface antigens [28] and symbiotic microbes [29]. Mouse studies have uncovered examples of V gene segments that, not only have a high degree of intrinsic self-reactivity, they are preferentially utilized during V(D)J recombination in early B lineage development [30,31], indicating that self/polyreactivity in the pre-selected immature Ig repertoire is a deliberate feature. As B cells mature, tolerance check points suppress polyreactive and self-reactive specificities, such that they ultimately comprise 20–30% [32] as measured by binding studies of cloned soluble antibodies. In addition, a BCR signaling reporter mouse indicated that most mature naïve B cells exhibit some degree of self-ligand recognition capacity across a broad range of signal strength [33].
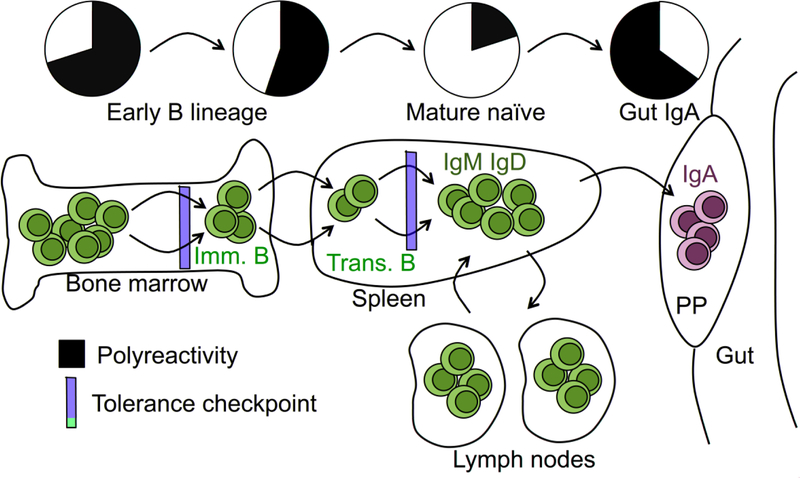
While self/polyreactive antibodies are usually considered unfavorable for host health, they can offer the benefit of providing superior defense capabilities in the context of recognition, neutralization, and clearance of pathogens [34,35]. In this regard, the greater part of antibodies that are broadly neutralizing for challenging vaccine targets, such as HIV-1 and influenza, are inherently polyreactive [36–39]. Thus, B cell tolerance filters can be seen, paradoxically, as a barrier to host health under certain infectious conditions [40*]. In addition, polyreactivity was reported to be enriched among IgA B cells, suggesting that increased polyreactivity may be beneficial in the gut environment [41*] (Fig. 1). Thus, despite the potential cost of self-reactivity, the immune system may draw upon polyreactive antibodies in times of need, implying a potential role for dynamic regulation of tolerance filter stringency.
Figure 1. Polyreactivity in the B cell system.
Schematic of the B cell development and literature-based representations of general locations of tolerance checkpoints and polyreactivity of associated B cell subsets. Shown are two B cell tolerance checkpoints. Tolerance checkpoints in the bone marrow are designated central tolerance, and those in the periphery are designated as such. Immature (imm.), transitional (trans.), mature naïve (IgM IgD), and IgA B cells (green) are indicated.
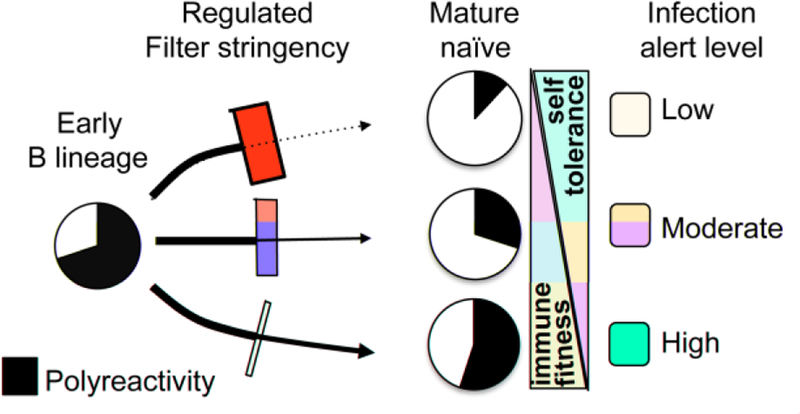
We speculate that high polyreactivity of freshly assembled bone marrow Ig repertoires (i.e. pre-tolerance filtered) may be a proactive storehouse of innateness, instead of solely an undesirable byproduct of agnostic Ig diversification. Polyreactive-loaded progenitors enable the recognition capacity of the BCR repertoire to swiftly expand in conditions of stress, such as that of a persisting infection, by loosening of the tolerance filter, versus reshaping recognition capacity de novo. This would allow the system to dynamically tune tolerance filters to provide levels of Ig polyreactivity to the optimal place on the cost/benefit scale to adapt to changing host conditions (Fig. 2).
Figure 2. Physiologic control of B cell tolerance filter stringency may be a demand-driven process.
Schematic representation of our hypothesis that plasticity of B cell developmental checkpoint stringency is an adaptive feature, balancing risks of infection and autoimmunity to host needs.
An analogy is a factory conveyer belt meant for product inspection and quality control of newly made products. BCRs/antibodies are deliberately manufactured in a form that maximizes recognition capacity (i.e. polyreactive form) and pass through screening for removal by tolerance checkpoint workers at various points along the way. In the absence of high-level infectious threats, careful and tight screening produces a relatively limited, but high tolerance repertoire sufficient to maintain low-threat host:pathogen homeostasis. A stronger inflammatory stimulus, such as chronic infection or barrier breach, could swiftly be met by an increase in Ig recognition capacity by simple cost-efficient mechanisms, such as altering the environment to conditions where selection against polyreactivity is less stringent. In the analogy, increasing conveyer belt speed or slowing removal could accomplish this. These or other mechanisms may quickly limit tolerance checks whose work is to remove already-present polyreactivity. The implication is that certain inflammatory signals may be a contributing upstream cause of deliberate crescendos in pre-immune Ig polyreactivity. This may be a contributory etiology for the observation that a variety of inflammatory conditions are associated with polyreactivity [34], and opens the possibility that, in terms of autoimmune disease, self-reactive Igs may be both chicken and egg.
Collaboration of the Diversification Systems
SHM and associated B cell selection can both ripen Ig affinities toward foreign antigen while redeeming promising Ig specificities from autoreactivity. SHM-mediated Ig redemption has been shown to be able to occur with swifter kinetics compared to affinity ripening toward foreign antigens [42**]. Redemption before ripening allows the retention of self-reactive clones in the primary Ig repertoire [42**], thus maximizing the recognition capacity of the Ig system. SHM and the GC selection system may also pressure Ig towards less polyreactivity due to improved competitive fitness of B cells that can concentrate peptide from a single entity to present to limiting cognate T cells. A very broad net can therefore be cast before affinity focusing.
How much Ig affinity ripening is possible from SHM and GC selection? In other words, what is the minimum affinity required for entry into the SHM-mediated Ig evolution process? Recent binding studies of B cells activated for SHM-mediated diversification reveal that a surprisingly substantial proportion of B cells have undetectable affinities for immunizing antigens by standard in vitro measures. This was shown by measurements of affinities from GC B cells to immunizing antigen [6**,43**], as well as assessing B cells undergoing SHM-mediated affinity maturation in an extra follicular context upon challenge with Salmonella typhimurium [44**]. In the latter study, diminished IgG responses were found when the BCR repertoire was artificially restricted, highlighting the importance of a diverse repertoire [44**]. In addition, antibodies with detectable salmonella antigen binding had more V region mutations compared to the great majority that had no detectable binding, and this binding was lost when reactive Igs were reverted back to germline configuration—consistent with the proposed model of in vivo recognition by B cells whose binding ability cannot be detected [44**]. Thus, under certain immunization or infectious conditions, many B cells may recognize antigen with initial binding affinities that are undetectable by conventional assays, which typically involve in vitro binding assays with soluble antibody.
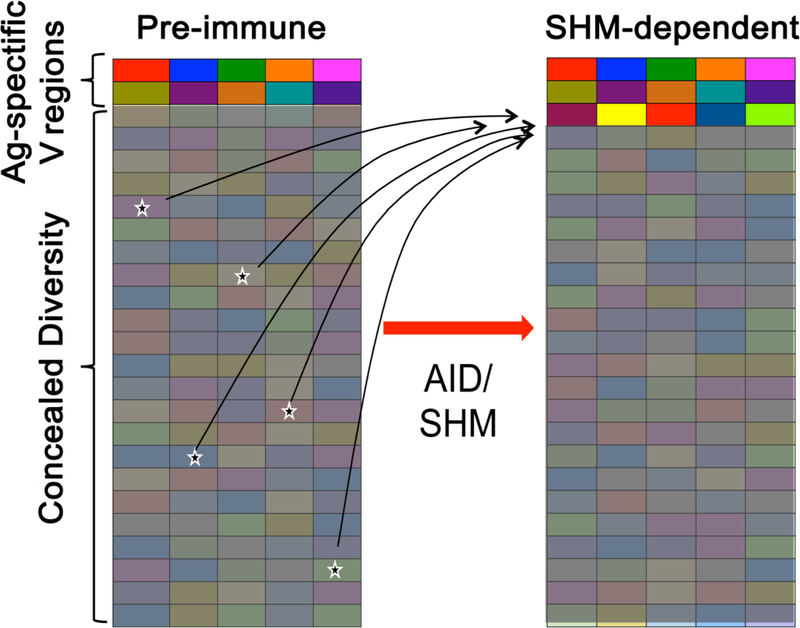
These examples shed light on the collaboration between the primary and SHM diversification systems. They illuminate the ability of the SHM system to do some heavier-than-expected lifting in the antigen-recognition process, enhancing some Igs from very low—in some cases, undetectable—to high affinity. This raises the possibility that the SHM system may do more than just ripen Ig affinities already present in the preimmune repertoire (Fig. 3).
Figure 3. Acquisition of de novo recognition capacities from concealed Ig diversity.
Schematic representation of an implication the discovery that non-cognate B cells can be activated and undergo SHM. Shown is a hypothetical scenario where new antigen recognition capacities could be acquired from concealed Ig diversity (i.e. not initially recognized by immunizing antigen) through SHM. Colored boxes represent Ig variable (V) region specificities. Thin curved arrows represent affinity maturation pathways of some V region specificities (asterisked) in B cells that fail to initially recognize antigen, but gain de novo recognition capacity through non-specific B cell activation-induced SHM. Shaded areas represent B gene segments that do not bind a given antigen, but may have recognition potential.
How is it that Ig with undetectable affinity can be activated to evolve through SHM? Mechanisms include the concept that GC B cells can bind immunizing antigen with affinities which are biologically relevant in vivo, but undetectable with usual in vitro assays. This is supported by robust, albeit indirect, evidence [43**,44**]. A non-mutually-exclusive alternative model is that some B cells could be specific for ‘dark antigen’, described to be an unknown antigen (presumably linked to immunizing antigen), or non-native form of immunizing antigen [6**]. A third non-mutually exclusive pathway is non-specific activation and SHM-mediated diversification of non-cognate B cells,whichunder special conditions, may generate new specificities not otherwise present in the primary repertoire [45**]. This was explored using a monoclonal B cell system in which BCRs were shown in multiple ways not to functionally engage with antigen. The study demonstrated that B cells harbor an intrinsic permissiveness to participate in SHM-mediated diversification, which can lead to Ig evolution toward de novo recognition of multiple distinct epitopes in the absence of B cell competition and with abundant T cell help [45**]. Thus, B cell entry into the SHM-mediated Ig evolution system may not be governed by a particular BCR affinity requirement for antigen. This is consistent with the notion that, with regard to receiving SHM-inducing cell help, it’s not how much antigen a B cell has,but whether it has more than it’s neighbors. Non-specific antigen uptake by basic mechanisms outside of BCR involvement [46] may be the baseline sufficiency to qualify for activated cell help absent neighbors with BCR-mediated antigen recognition ability. While it remains to be seen whether new antigen recognition can occur through SHM in physiologic settings, low levels of non-cognate B cells have been shown to undergo SHM-mediated diversification in a polyclonal B and T cell setting [45**].
The fiercely competitive GC environment under typical experimental conditions renders non-specifically-activated B cells extremely unlikely to survive in the presence of other cells with some BCR-mediated affinity for antigen. However, an SHM-mediated somatic Ig evolution system flexible enough to generate new specificities to immunizing antigen would be beneficial in situations where Ig specificities to immunologically important epitopes may not be represented in the primary repertoire. If the primary repertoire provides ineffective antibody profiles (e.g. to non-neutralizing epitopes), immunodominance shifts over time through antibody feedback/epitope masking [47], iteratively exhausting available repertoire options for some difficult infections. Such a response would be inherently demand-driven (i.e., effective antibody responses would resolve lymphoid inflammation). Persistent pathogen-driven lymphoid inflammation that exhausts available Ig recognition solutions could still be met by real-time stochastic SHM-mediated diversification of Ig genes. At least with respect to actionable lg recognition, this can be analogous to the last soldier standing, armed with no weapon other than a hyperspeed version of one of nature’s most powerful innovations—evolution.
Concluding Remarks
Here we discussed how emerging data might support new perspectives on how Ig repertoires are shaped. In addition to being receptive to symbiont influences, we suggest that B cell biological plasticity allows both Ig diversification systems access to concealed diversity to regulate the sensitivity and specificity of humoral responses. For the naïve Ig repertoire—access to deliberately-produced immature B cell polyreactivity, enabling swift adaption of Ig recognition capacity. For the SHM-dependent Ig repertoire—de novo epitope recognition through non-cognate B cell mutation, resulting in expanded diversity not otherwise functionally available from the primary Ig repertoire. Future studies promise to challenge these perspectives, and in so doing will advance understanding of the depth, flexibility, and utility of humoral immune responses.
Highlights.
Ig tolerance checkpoints themselves may be subject to regulation
Microbial symbionts influence naïve and experienced Ig repertoires
Early B lineage polyreactivity may be a deliberately-abundant storehouse of
Somatic hypermutation may expand Ig diversity beyond naïve boundaries
Acknowledgements
This work was supported by the National Institutes of Health grants AI121394, AI1113217, and AI137940 (to D.R.W.). D.R.W. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Victora GD, Nussenzweig MC: Germinal centers. Annu Rev Immunol 2012, 30:429–457. [DOI] [PubMed] [Google Scholar]
- 2.Kelsoe G, Haynes BF: Host controls of HIV broadly neutralizing antibody development. Immunol Rev 2017, 275:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Victora GD, Mouquet H: What Are the Primary Limitations in B-Cell Affinity Maturation, and How Much Affinity Maturation Can We Drivewith Vaccination? Lessons from the Antibody Response to HIV-1. Cold Spring Harb Perspect Biol 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK: Different B cell populations mediate early and late memory during an endogenous immune response. Science 2011, 331:1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pape KA, Maul RW, Dileepan T, Paustian AS, Gearhart PJ, Jenkins MK: Naive B Cells with High-Avidity Germline-Encoded Antigen Receptors Produce Persistent IgM(+) and Transient IgG(+) Memory B Cells. Immunity 2018, 48:1135–1143 e1134.(.)This paper showed that a single VH gene segment can confer high-affinity antigen binding and influence B cell fate.
- 6.Kuraoka M, Schmidt AG, Nojima T, Feng F, Watanabe A, Kitamura D, Harrison SC, Kepler TB, Kelsoe G: Complex Antigens Drive Permissive Clonal Selection in Germinal Centers. Immunity 2016, 44:542–552.(..)This study found that a large fraction of GC B cells do not show detectable binding to native antigen and proposes the concept of dark antigen.
- 7.Weisel FJ, Zuccarino-Catania GV, Chikina, Shlomchik MJ: Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity 2016, 44:116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jardine JG, Kulp DW, Havenar-Daughton C, Sarkar A, Briney B, Sok D, Sesterhenn F, Ereno-Orbea J, Kalyuzhniy O, Deresa I, et al. : HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 2016, 351:1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesemann DR,Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, Panchakshari RA, Rodig SJ, Kepler TB, Alt FW: Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 2013, 501:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greiff V, Menzel U, Miho E, Weber C, Riedel R, Cook S, Valai A, Lopes T, Radbruch A, Winkler TH, et al. : Systems Analysis Reveals High Genetic and Antigen-Driven Predetermination of Antibody Repertoires throughout B Cell Development. Cell Rep 2017, 19:1467–1478. [DOI] [PubMed] [Google Scholar]
- 11.Xu JL, Davis MM: Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 2000, 13:37–45. [DOI] [PubMed] [Google Scholar]
- 12.Angeletti D, Yewdell JW: Understanding and Manipulating Viral Immunity: Antibody Immunodominance Enters Center Stage. Trends Immunol 2018,39:549–561(.)This review comprehensively discusses the potential mechanisms contributing to B cell immunodominance in primary responses.
- 13.Abbott RK, Lee JH, Menis S, Skog P, Rossi M, Ota T, Kulp DW, Bhullar D, Kalyuzhniy O, Havenar-Daughton C, et al. : Precursor Frequency and Affinity Determine B Cell Competitive Fitness in Germinal Centers, Tested with Germline-Targeting HIV Vaccine Immunogens. Immunity 2018, 48:133–146 e136(..)With a VRC01-germline B cell transfer model, the authors assessed the roles of precursor frequency, affinity, and avidity on in vivo B cell expansion, GC recruitment, GC competition, SHM,and memory B cell development.
- 14.Dosenovic P, Kara EE, Pettersson AK, McGuire AT, Gray M, Hartweger H, Thientosapol ES, Stamatatos L, Nussenzweig MC: Anti-HIV-1 B cell responses are dependent on B cell precursor frequency and antigen-binding affinity. Proc Natl Acad Sci S A 2018, 115:4743–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I: Most peripheral B cells in mice are ligand selected. J Exp Med 1991, 173:1357–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nemazee D: Mechanisms of central tolerance for B cells. Nat Rev Immunol 2017, 17:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancro MP, Kearney JF: B cell positive selection: road map to the primary repertoire? J Immunol 2004, 173:15–19. [DOI] [PubMed] [Google Scholar]
- 18.Baumgarth N: B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front Immunol 2016, 7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, Qin G, Mazmanian SK, Han J, Herzenberg LA: Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife 2015, 4:e09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Chaudhary N, Yang N, Granato A, Turner JA, Howard SL, Devereaux C, Zuo T, Shrestha A, Goel RR, et al. : icrobial symbionts regulate the primary Ig repertoire. JExp Med 2018, 215:1397–1415(..)This paper demonstrated that microbial symbionts enrich frequencies of antibacterial specificities within preimmune B cell repertoires in a T cellindependent manner.
- 21.Le Gallou S, Zhou Z, Thai LH, Fritzen R, de Los Aires AV, Megret J, Yu P, Kitamura D, Bille E, Tros F, et al. : A splenic IgM memory subset with antibacterial specificities is sustained from persistent mucosal responses. J Exp Med 2018, 215:2035–2053(..) This study showed that ongoing T cell-dependent activation of B cells in gutassociated lymphoid tissues generates a diversified systemic compartment with long-lasting clonal persistence and protective capacity against systemic bacterial infections.
- 22.Wilmore JR, Gaudette BT, Gomez Atria D, Hashemi T, Jones DD, Gardner CA, Cole SD, Misic AM, Beiting DP, Allman D: Commensal Microbes Induce Serum IgA Responses that Protect against Polymicrobial Sepsis. Cell Host Microbe 2018, 23:302–311 e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams WB, Liao HX, Moody MA, Kepler TB, Alam SM, Gao F, Wiehe K, Trama AM, Jones K, Zhang R, et al. : HIV-1 VACCINES. Diversion of HIV-1 vaccinenduced immunity by gp41-microbiota cross-reactive antibodies. Science 2015, 349:aab1253(.)This paper showed that dominant nonprotective antibody responses towards gp41 after HIV-1 gp140 vaccination are induced by a preexisting pool of gp41-microbiota cross-reactive B cells.
- 24.Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, Regalado A, Cowan PJ, d’Apice AJ, Chong AS, et al. : Gut microbiota elicits a protective immune response against malaria transmission. Cell 2014, 159:1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabezas-Cruz A, Mateos-Hernandez L, Alberdi P, Villar M, Riveau G, Hermann E, Schacht AM, Khalife J, Correia-Neves M, Gortazar C, et al. : Effect of blood type on anti-alpha-Gal immunity and the incidence of infectious diseases. Exp Mol Med 2017, 49:e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop JR, Gagneux P: Evolution of carbohydrate antigens--microbial forces shaping host glycomes? Glycobiology 2007, 17:23R–34R. [DOI] [PubMed] [Google Scholar]
- 27.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC: Predominant autoantibody production by early human B cell precursors. Science 2003, 301:1374–1377. [DOI] [PubMed] [Google Scholar]
- 28.Reed JH, Jackson J, Christ D, Goodnow CC: Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. J Exp Med 2016, 213:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schickel JN, Glauzy S, Ng YS, Chamberlain N, Massad C, Isnardi I, Katz N, Uzel G, Holland SM, Picard C, et al. : Self-reactive VH4–34-expressing IgG B cells recognize commensal bacteria. J Exp Med 2017, 214:1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellon B, Manheimer-Lory A, Monestier M, Moran T, Dimitriu-Bona A, Alt F, Bona C: High frequency of autoantibodies bearing cross-reactive idiotopes among hybridomas using VH7183 genes prepared from normal and autoimmune murine strains. J Clin Invest 1987, 79:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Kearney JF: Generation and function of natural self-reactive B lymphocytes. Semin Immunol 1996, 8:19–27. [DOI] [PubMed] [Google Scholar]
- 32.Meffre E, Wardemann H: B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol 2008, 20:632–638. [DOI] [PubMed] [Google Scholar]
- 33.Zikherman J, Parameswaran R, Weiss A: Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature 2012, 489:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimitrov JD, lanchais C, Roumenina LT, Vassilev TL, Kaveri SV, Lacroix Desmazes S: Antibody polyreactivity in health and disease: statu variabilis. J Immunol 2013, 191:993–999. [DOI] [PubMed] [Google Scholar]
- 35.Schroeder KMS, Agazio A, Strauch PJ, Jones ST, Thompson SB, Harper MS, Pelanda R, Santiago ML, Torres RM: Breaching peripheral tolerance promotes the production of HIV-1-neutralizing antibodies. J Exp Med 2017, 214:2283–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. : Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 2011, 333:1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. : Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 2005, 308:1906–1908. [DOI] [PubMed] [Google Scholar]
- 38.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, et al. : Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med 2015, 7:316ra192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, et al. : Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature 2010, 467:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.chroeder KM, Agazio A, Torres RM: Immunological tolerance as a barrier to protective HIV humoral immunity. Curr Opin Immunol 2017, 47:26–34.(.)This review highlights the autoreactivity of HIV-1 bnAbs and the roles of central tolerance in limiting the development of bnAbs.
- 41.Bunker JJ, Erickson SA, Flynn TM, Henry C, Koval JC, Meisel M, Jabri B, Antonopoulos DA, Wilson PC, Bendelac A: Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 2017, 358.(.)This paper showed that IgA antibodies are rather polyreactive and they recognize microbiotawith innate-like specificity.
- 42.Burnett DL, Langley DB, Schofield P, Hermes JR, Chan TD, Jackson J, Bourne K, Reed JH, Patterson K, Porebski BT, et al. : Germinal center antibody mutation trajectories are determined by rapid self/foreign discrimination. Science 2018, 360:223–226.(..)This study showed that self-reactive B cells follow distinct mutational trajectories to lose self-binding capacity, leading to optimal affinity for foreign antigen.
- 43.Tas JM, Mesin L, Pasqual G, Targ S, Jacobsen JT, Mano YM, Chen CS, Weill JC, Reynaud CA, Browne EP, et al. : Visualizing antibody affinity maturation in germinal centers. Science 2016, 351:1048–1054.(..)By combining imaging and sequencing, the authors analyzed the dynamics of B cell clonal diversity in GCs and find the diversity decreased at wide range of rates during affinity maturation process.
- 44.almonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity 2015, 43:120–131(..)This paper showed that Salmonella infection leads activation of B cells with undetectable affinities and affinity marturation in a non-classic GC pathway.
- 45.Silver J, Zuo T, Chaudhary N, Kumari R, Tong P, Giguere S, Granato A, Donthula R, Devereaux C, Wesemann DR: Stochasticity enables BCR-independent germinal center initiation and antibody affinity maturation. J Exp Med 2018, 215:77–90(..)Using a mouse model whose knock-in BCR does not functionally engage with immunizing antigen, the authors showed that chronic immunization induced antigen-specific serological responses with diverse SHM-mediated antibody affinity maturation pathways and divergent epitope targeting.
- 46.Zhong G, Reis e Sousa C, Germain RN: Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. J Exp Med 1997, 186:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zarnitsyna VI, Ellebedy AH, Davis C, Jacob J, Ahmed R, Antia R: Masking of antigenic epitopes by antibodies shapes the humoral immune response to influenza. Philos Trans R Soc Lond B Biol Sci 2015, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]