Abstract
Neural proliferation in the dentate gyrus (DG) is closely linked with learning and memory, but the transcriptional programming that drives adult proliferation remains incompletely understood. Our lab previously elucidated the critical role of the transcription factor ΔFosB in the dorsal hippocampus (dHPC) in learning and memory, and the FosB gene has been suggested to play a role in neuronal proliferation. However, the subregion-specific and potentially cell-autonomous role of dHPC ΔFosB in neurogenesis-dependent learning has not been studied. Here, we crossed neurotensin receptor-2 (NtsR2) Cre mice, which express Cre within the subgranular zone (SGZ) of dHPC DG, with floxed FosB mice to show that knockout of ΔFosB in hippocampal SGZ neurons reduces antidepressant-induced neurogenesis and impedes hippocampus-dependent learning in the novel object recognition task. Taken together, these data indicate that FosB gene expression in SGZ is necessary for both hippocampal neurogenesis and memory formation.
Keywords: FosB, NtsR2, Hippocampus, Neurogenesis, Learning, Memory
Introduction:
As learning and memory are thought to develop through repeated activation of discrete networks of neurons, activity-dependent immediate early genes (IEGs) are critical mediators of learning and memory processes. Indeed, many studies have linked various IEGs to the creation and expression of memories (Liu X et al., 2014;Minatohara K et al., 2015;Ramirez S, 2018;Tonegawa S et al., 2015), including IEG transcription factors that orchestrate the sweeping changes in gene expression underlying the formation of stable engrams central to learning. As the process of learning and memory consolidation can take place over timescales of up to days or weeks, mechanisms controlling gene expression over these timescales, including epigenetics (Duke CG et al., 2017;Kyrke-Smith M and Williams JM, 2018;Leighton LJ et al., 2018), have become a key area of study in the learning field. The IEG ΔFosB, a transcription factor produced by the FosB gene, is unique in its stability, with a half-life in vivo of around eight days (Carle TL et al., 2007;Nishijima T et al., 2013;Ulery-Reynolds PG et al., 2009), allowing it to accumulate in neurons and regulate gene expression after repeated stimulation (Nestler EJ, 2012). Moreover, hippocampal ΔFosB is critical for learning (Eagle AL et al., 2015), though its mechanism(s) of action in hippocampal function are not fully understood.
ΔFosB function has been implicated in learned rewarding and social behaviors. For example, transcriptional silencing of ΔFosB in the nucleus accumbens (NAc) impairs experience-induced facilitation of sexual behavior (Pitchers KK et al., 2010) and NAc ΔFosB is necessary for learning association of spatial context with positive reinforcers, like cocaine (Robison AJ et al., 2013). ΔFosB is induced by the antidepressant fluoxetine in the NAc (Vialou V et al., 2010;Vialou V et al., 2015) and its function there is critical for antidepressant effects on social interaction (Vialou V et al., 2010). Fluoxetine also induces ΔFosB in the dentate gyrus (DG) of the dHPC (Vialou V et al., 2015), and its antidepressant efficacy is dependent on neurogenesis in the DG (Malberg JE et al., 2000;Santarelli L et al., 2003), so probing the link between ΔFosB and neurogenesis may provide new insights into learning and antidepressant function.
Mice lacking expression of the FosB gene in all tissues from conception (FosB knockout mice) have a variety of hippocampal malformations, including thinning of the DG granular cell layer, and display reduced hippocampal neurogenesis (Yutsudo N et al., 2013). However, such global, germ-line FosB knockout cannot indicate the specific contribution of hippocampal neurogenesis to behavior. Our group has shown that ΔFosB regulation of transcription in dorsal hippocampus (dHPC) is necessary for spatial memory (Eagle AL et al., 2015). However, these studies used a viral method that inhibited ΔFosB activity in fully differentiated CA1 and DG neurons of dHPC, and thus did not address a role for ΔFosB-driven neurogenesis in learning. Moreover, it is critical to consider how specific hippocampal subregions may be involved in learning and memory. Of particular note is the subgranular zone (SGZ) of the DG, an important site of neurogenesis in the brain. SGZ neurogenesis has been tied to learning and memory in the context of spatial learning tasks (Epp JR et al., 2016), stress-induced behaviors (Hill AS et al., 2015;Lagace DC et al., 2010), and fear (Seo D-o et al., 2015). We therefore used NtsR2-Cre mice, in which hippocampal Cre expression is confined to the SGZ, to test the hypothesis that knockout of FosB gene products specifically in the SGZ of mice inhibits neurogenesis and impairs performance in learning and memory-related tasks.
Experimental Procedures:
Animals and Genotyping: 76 adult male and female mice (>8 weeks) were included in these studies. Neurotensin receptor-2 IRES-Cre (NtsR2cre/+) mice lacking the frt-flanked blocking cassette were crossed with Cre-inducible RosaeGFP-L10a mice, so that any cells expressing NtsR2/Cre are permanently marked with GFP (Woodworth HL et al., 2018). NtsR2cre/+ mice were crossed with floxed FosB (FosBlox/lox) mice (Ohnishi YN et al., 2017) to generate progeny with intact FosB (NtsR2+/+;FosBlox/lox) and those lacking FosB in NtsR2 cells (NtsR2Cre/+;FosBlox/lox). Mice were genotyped using standard PCR with the following primers:
IRES-Cre forward: 5’ – GGACGTGGTTTTCCTTTGAA – 3’
IRES-Cre reverse: 5’ – AGGCAAATTTTGGTGTACGG – 3’
- Rosa26EGFP-L10a:
- mutant forward: 5’ – TCTACAAATGTGGTAGATCCAGGC – 3’
- wild type forward: 5’ – GAGGGGAGTGTTGCAATACC – 3’
- common reverse: 5’ – CAGATGACTACCTATCCTCCC – 3’
FB loxPu sequence: 5’ – GCT GAA GGA GAT GGG TAA CAG – 3’
LIPz sequence: 5’ – AAG CCT GGT GTG ATG GTG A – 3’
LNEo1 sequence: 5’ – AGA GCG AGG GAA GCG TCT ACC TA – 3’.
Adult mice were group housed 4–5 per cage in a 12 h light/dark cycle and provided ad libitum food and water. In some cases, a wheel was provided for ad libitum wheel running (see results below). All experiments were approved by the Institutional Animal Care and Use Committee at Michigan State University and performed in accordance with AAALAC and NIH guidelines.
Immunocytochemistry: 12 mice were transcardially perfused with cold PBS followed by 10% formalin. Brains were post-fixed for 24 hours in 10% formalin, cryopreserved in 30% sucrose, and sliced into 35μm sections. Immunofluorescence was performed using the following primary antibodies: Anti-FosB (FosB 5G4, 1:1000, rb, #2251S, Cell Signaling Technologies), Anti-NeuN (1:1000, ms, MAB377 Millipore), Anti-GFP (1:1000, gt, ab5450, Abcam or ms, A11120, Invitrogen), Anti-BrdU (1:1000, rat, MCA2060, Bio-Rad), and Anti-Doublecortin (1:1000, gt, sc-8066, Santa Cruz). The following corresponding secondary antibodies were then used: Donkey anti-rabbit Cy3 (1:200, 711-165-152, Jackson Immunoresearch), Donkey anti-goat Ig bitotin (1:200, 705-065-147 Jackson Immunoresearch), Donkey anti-goat Alexa Fluor 488 (1:200, 705-545-147 Jackson Immunoresearch), Donkey anti-goat Alexa Fluor 568 (1:200, A11057 Invitrogen), Donkey anti-mouse Cy3 (1:200, 715-165-150 Jackson Immunoresearch), Donkey anti-mouse Alexa Fluor 488 (1:200, 715-545-150 Jackson Immunoresearch), Donkey anti-rat Cy3 (1:200, 712-165-150 Jackson Immunoresearch). BrdU-positive cells were quantified on an Olympus FluoView 1000 filter-based laser scanning confocal microscope by a blinded experimenter. Pseduo-3D image was generated by compiling a z-stack of 40 0.5 micrometer slices using the Olympus FluoView 1000 software.
Open Field: Open field was performed essentially as previously described (Eagle AL et al., 2015). 64 mice underwent 60 minutes of habituation and were placed into an empty arena for one hour under red light conditions. Activity was recorded with a digital CCD camera connected to a computer running automated video tracking software package (Clever Sys). Time spent within the center of the box (the center starts approximately 9.5cm from the edge of the wall) and distance moved were measured as anxiety-like and locomotor behaviors.
Elevated Plus Maze (EPM): EPM was performed essentially as previously described (Eagle AL et al., 2015). In brief, 64 mice underwent 60 minutes of habituation, were placed in the center of the maze, and allowed to roam for 5 minutes under red light conditions. The amount of time spent in the open arms was assessed as a measure of anxiety. Mice that fell from the arena were not excluded from analysis.
Novel Object Recognition (NOR): NOR was assessed using a 3-day paradigm as described previously (Eagle AL et al., 2015). Briefly, 64 mice were habituated for 60 minutes under red light every day, and then placed into the open-field (OF) apparatus for one hour (Day 1). 24 hours later, mice were exposed to two identical objects placed in opposite corners of the open-field (OF) box and allowed to explore the apparatus for 30 min (Day 2). Twenty-four hours later, mice were tested for NOR. One object was removed and replaced with a dissimilar object, and mice were allowed to freely explore the apparatus for 5 min (Day 3).
Statistics: Analyses of one independent variable were performed using PRISM 8.0 (GraphPad Software) using fixed-effect models and treating all samples as independent. Assumptions of normality and equal variance were tested using a D’Agostino & Pearson test and variance ratio test, respectively. If the dependent variable met these criteria, comparisons were tested using unpaired student’s t-tests, or were otherwise tested with the nonparametric Mann-Whitney U test. Analyses of two independent variables, including Immunohistochemistry quantifications and NOR data, were analyzed with Levene’s and Shapiro-Wilks tests for homogeneity of variance and normality, respectively, in in SPSS 25.0, followed by 2×2 factorial ANOVA (IHCs) or Repeated Measures 2×2 ANOVA (NOR) if appropriate; otherwise, an extension of the nonparametric Kruskal-Wallis test was applied. In cases of interaction between variables, multiple comparisons were tested using Sidak’s post-hoc tests. A cutoff of alpha=0.05 was used in all analyses.
Results:
NtsR2-Cre;GFP mice identify cells in the dentate gyrus subgranular zone
Previous reports indicate that NtsR2 is sparsely expressed in adult mouse brain, and this expression is predominantly in glia (Mazella J et al., 1996;Nouel D et al., 1999;Sarret P et al., 1998;Woodworth HL et al., 2018). Additionally, adult NtsR2Cre/+,GFP mice exhibit robust GFP-expression in cells of the SGZ of the DG and in the pyramidal layer of CA3 in the adult dHPC (Fig 1). However,the NtsRCre/+,GFP transgenic line permanently expresses GFP independent of current NtsR2 expression and NtsR2 expression is thought to be phasic in new cells, peaking in P5–P15 (Lepee-Lorgeoux I et al., 1999). As the SGZ is a region enriched in neuroprogenitor cells, we sought to determine the developmental status of the GFP-positive cells. We performed immunohistochemistry for NeuN and Doublecortin (DCX), markers of mature and immature neurons, respectively in two animals (Fig 1A and B). We noted that in the CA3 pyramidal neurons, there was extensive colabeling with NeuN but not DCX, indicating that many of these GFP-positive cells were mature neurons. However, in the SGZ, there was significant overlap between DCX- and GFP-positive cells (Fig 1B), indicating that NtsR2Cre/+,GFP expresses in neuroprogenitor cells in this region.
Figure 1: NtsR2-Cre expresses in the DG and CA3 of the dHPC.
A: 4x and 40x images showing that NtsR2-positive cells colocalize with NeuN in DG and CA3. B: 4x and 40x images showing that NtsR2-positive cells colocalize with doublecortin (DCX) in the DG, but this staining is absent in the CA3.
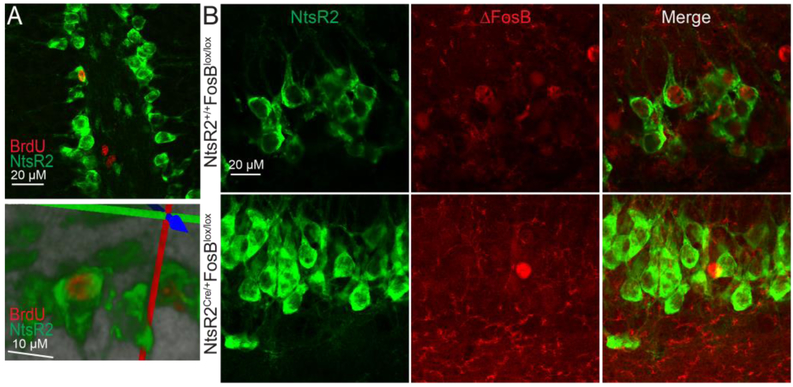
To determine whether these NtsR2-GFP labeled cells of the SGZ are indeed neuroprogenitor cells undergoing division, we used BrdU labeling to mark newly divided cells. We injected adult male NtsR2Cre/+,GFP mice with 50mg/kg BrdU daily for five days, during which they had ad libitum access to a running wheel, as wheel running promotes hippocampal neurogenesis in rodents (van Praag H et al., 1999). Subsequent immunohistochemistry revealed distinct BrdU-staining in nuclei of some NtsR2-GFP cells of the SGZ (Fig 2A), indicating that some of the NtsR2Cre/+,GFP cells had undergone mitosis in the previous five days. Additional immunostaining revealed that ΔFosB was present in some NtsR2Cre/+,GFP cells. However, NtsR2Cre/+,GFP;FosBlox/lox mice did not exhibit ΔFosB immunoreactivity in GFP-positive SGZ cells (Fig 2B). Taken collectively, these data indicate that NtsR2Cre/+,GFP marks multiple cell types in the dHPC, including newly dividing cells in the SGZ, and that some of these cells express FosB gene products that can be knocked out using our Cre/lox approach.
Figure 2: NtsR2-Cre expresses in newly-dividing SGZ progenitor cells, some of which express FosB.
A: Some NtsR2-Cre cells are stained with BrdU in the SGZ of the DG (100x, top), and this is confirmed by 3D reconstruction from confocal imaging (bottom). B: NtsR2-Cre cells also express with ΔFosB in the SGZ in WT animals. This staining is absent in floxed FosB animals.
FosB in the SGZ is critical for induced neurogenesis
It has been previously suggested that the FosB gene is critical for adult hippocampal neurogenesis, as germline FosB knockout mice show reduced BrdU staining in response to kainic acid (Yutsudo N et al., 2013). Moreover, multiple antidepressants induce hippocampal neurogenesis, and FosB gene products are critical for the behavioral effects of antidepressants like fluoxetine (Robison AJ et al., 2014;Vialou V et al., 2010). To examine if FosB gene expression in the hippocampal SGZ is necessary for fluoxetine-induced hippocampal neurogenesis, we studied NtsR2Cre/+,GFP mice crossed onto the FosBlox/lox line, providing developmental FosB knockout in a subset of hippocampal cells including the SGZ. 4 NtsR2Cre/+,GFP;FosBlox/lox mice (referred to as Cre-positive) and 5 littermates lacking Cre (referred to as WT) were injected i.p with fluoxetine (2mg/kg) daily and BrdU (50mg/kg) every 3rd day for 18 days to both induce neurogenesis and mark dividing cells, respectively (Fig 3A). Mice were sacrificed 24 hours after the last injection (Fig 3A) and brains were then immunolabeled for BrdU and DCX, (Fig 3B and C). Cre-positive animals showed reduced BrdU staining compared to WT animals (Fig 3D), with main effect of genotype on BrdU labeled cells and dorsoventral axis of the brain (dorsal vs ventral HPC) (H(1)=4.218; p=0.039 and H(1)=7.25; p=0.007, respectively), without interaction between the two variables (H(1)=0.206; p=0.649). DCX staining was significantly reduced in the vHPC compared to dHPC (Fig 3E). We found a main effect of the dorsoventral axis, but not genotype (F(1,13)=29.64, p=0.0001 and F(1,13)=2.832, p=0.1162, respectively), with a trend for an interaction between the genotype and dorsoventral axis (F(1,13)=4.634, p=0.0507). These indicate that mitotic division, but not differentiation into a neuronal lineage, are reduced with FosB SGZ knockout, and that these effects are exaggerated in the dHPC.
Figure 3: Genetic Knockout of FosB in SGZ reduces neurogenesis.
A: Timeline of experiment. Representative images of BrdU (B) and doublecortin (C) staining in the dorsal HPC of WT or Cre-positive animals after 18 days of daily fluoxetine. D and E: Cre-positive animals have significantly fewer BrdU ((a):p=0.039 for effect of Cre; (b):p=0.007 for dHPC vs vHPC) with no effect on DCX positive cells ((#):p=0.0507 for interaction between genotype and dorsoventral axis; (a):p=0.0001 for dHPC vs vHPC).
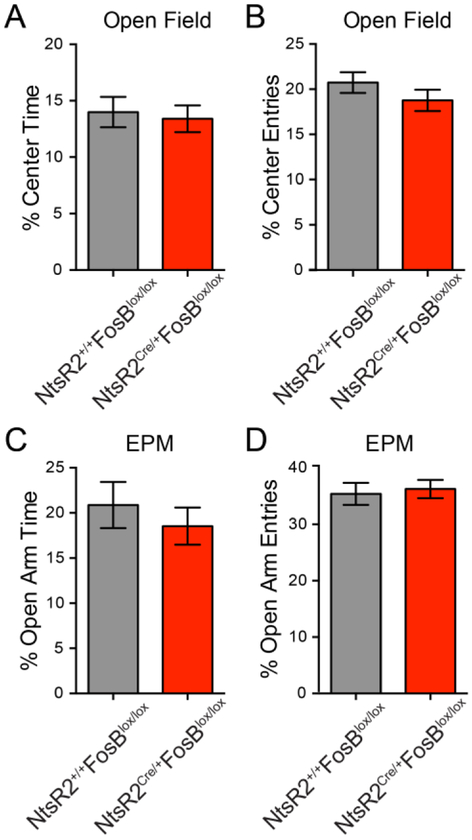
FosB KO in the SGZ does not alter basal anxiety behaviors
Enhanced neurogenesis is thought to be one of the mechanisms behind antidepressant drug effects, and neurogenesis has been linked to abnormalities in anxiety behaviors (Hill AS et al., 2015;Revest JM et al., 2009;Vialou V et al., 2015). Therefore, 30 adult NtsR2Cre/+,GFP;FosBlox/lox mice and 35 NtsR2+/+,GFP;FosBlox/lox littermates were tested in both the open-field and the elevated plus maze (EPM). Despite the developmental knockout, there were no differences between these two genotypes in the percent of total time spent in the center of the open field arena, nor the percent of center entries (Fig 4A and B; group medians of 13.05 and 12.13 Mann Whitney U=503.5, p=0.93 and t(62)=1.186, p=0.24). In EPM, there was no difference in percentage of open arm time or percentage of open arm entries between genotypes (Fig 4C and D; t(52)=0.719, p=0.475 and t(52)=0.344, p=.732). Taken together, these data suggest that FosB SGZ knockout causes no changes in anxiety-like behavior.
Figure 4: Genetic knockout of FosB in SGZ does not alter basal anxiety behaviors.
FosB SGZ knockout caused differences in the percentage of total time spent (A), nor in the percentage of entries (B) into the center of the open field (p=0.93 and p=0.24, respectively). There were also no differences in the percentage of total time spent (C) or percentage of entries (D) into the open arms of the elevated plus maze (p=0.475 and p=0.732, respectively).
FosB SGZ Knockout impairs learning
Reductions in neurogenesis have been linked to abnormalities in hippocampus-dependent learning (Akers KG et al., 2014;Deng W et al., 2010;Epp JR et al., 2016;Niibori Y et al., 2012;Zhao C et al., 2008). Therefore, 29 adult NtsR2Cre/+,GFP;FosBlox/lox mice and 35 wild-type littermates also underwent novel object recognition (NOR; Fig 5A) to test for deficits in a hippocampal-dependent task. During the acclimatization phase, Cre-positive mice and wild-type littermates showed no difference in locomotor behavior (Fig 5B; t(62)=0.96, p=0.342). As long term memory is dependent upon protein synthesis (Kogan JH et al., 2000), and FosB gene products are transcription factors, a 24 hour timepoint was chosen to test long-term memory as opposed to a more immediate timepoint which results from non-genomic actions (Goelet P et al., 1986). 24 hours after familiarization with two identical objects, wildtype mice spent significantly longer interacting with a novel object (Fig 5C; F(1,60)=9.086, p=0.0038, followed by Sidak post hoc test) compared to Cre-positive mice, with main effects of Cre decreasing total exploration time (F(1,60)=8.363, p=0.0053) and time around novel objects (F(1,60)=5.908, p=0.0181). Thus, FosB SGZ knockout induces a deficit in hippocampus-dependent learning.
Figure 5: Genetic knockout of FosB in SGZ reduces hippocampus-dependent memory.
A: Schematic of the NOR task. B: Cre-positive and WT littermates show no differences in locomotor behaviors in the acclimatization phase. C: Cre-positive mice display reduced time spent with a novel object compared to WT littermates (*:p=0.0038).
Discussion:
Here we crossed NtsR2Cre/+,GFP mice onto the FosBlox/lox background to investigate the role of FosB in hippocampal SGZ cells. NtsR2 labeling has previously indicated glia and has not been explicitly characterized on neurons throughout the adult brain (Lepee-Lorgeoux I et al., 1999;Woodworth HL et al., 2018). Nevertheless, NtsR2 has reported in the dentate gyrus of both rodents (Lepee-Lorgeoux I et al., 1999) and primates (Kohler C et al., 1987). As the scope of this study was to explore subregion specific effects on learning and memory, the NtsRCre/+,GFP line allowed us to limit Cre-mediated deletion of FosB to a discrete cell population in the hippocampus to investigate how the FosB gene affected both neurogenesis and learning.
Previous studies of hippocampal ΔFosB could not address neither subregion nor cell type specificity, as they employed either germline whole body knockouts, local viral effects, or correlations (Kurushima H et al., 2005;Niu H et al., 2018;Yutsudo N et al., 2013). Here we show that specifically knocking out FosB in a subset of DG and CA3 cells overlapping strongly with the SGZ caused both reduced cellular proliferation in the SGZ and learning deficits, without altering basal anxiety or locomotor behavior. Changes in neurogenesis have been causally linked to stress-induced behaviors and antidepressant efficacy (Anacker C et al., 2018; Hill AS et al., 2015; Santarelli L et al., 2003), while fluoxetine increases memory retrieval and cognitive flexibility processes in hippocampal dependent tasks such as the T-maze, NOR, and the Barnes maze, but not memory acquisition (Flood JF and Cherkin A, 1987;Marwari S and Dawe GS, 2018; Yi JH et al., 2018). Importantly fluoxetine induces neurogenesis concurrently with increased long-term memory performance (Ibi D et al., 2008; Marwari S and Dawe GS, 2018). Our studies suggest that FosB gene products may be a molecular mechanism that underlies these two effects. This is intriguing as standard treatment with SSRIs requires several weeks for efficacy, pointing to long-term changes in the brain mediating antidepressant responses as opposed to acute actions at serotonin receptors. Although the timeline for antidepressant response is consistent with the timeline for neuronal maturation (Lucassen PJ et al., 2010;Zhao C,Deng W and Gage FH, 2008), the specific molecular mechanisms which underlie neuronal division, survival, and maturation are incompletely understood.
Previous work has highlighted that ΔFosB’s long half-life contributes to prolonged effects of gene expression (Carle TL et al., 2007;Ulery-Reynolds PG et al., 2009), and indeed its accumulation in the NAc is critical for resilience to stress and the antidepressant effects of fluoxetine (Robison AJ et al., 2014;Vialou V et al., 2010). In addition, ΔFosB is also induced in the dHPC after stress and several antidepressant treatment regimens including fluoxetine, ketamine, and exercise, and it is necessary in the ventral CA3 for the prophylactic effects of the non-traditional antidepressant ketamine (Mastrodonato A et al., 2018;Nishijima T et al., 2013;Perrotti LI et al., 2004;Vialou V et al., 2015). Our finding that knocking out FosB in a subset of SGZ cells reduced cell proliferation is concordant with the idea that hippocampal FosB gene products, including ΔFosB, support fluoxetine-induced neurogenesis and perhaps adaptive responses to stress.
Hippocampal ΔFosB is also linked to preclinical models of comorbid diseases which have cognitive associated deficits, like Alzheimer’s disease and epilepsy. Germline FosB KO mice have depressive phenotypes and spontaneous seizures (Yutsudo N et al.,2013). In addition, ΔFosB accumulates in the dHPC in response to natural seizures, kainic acid, and pilocarpine models of epilepsy, as well as in Alzheimer’s disease mouse models and patients (Cho KO et al., 2015;Corbett BF et al., 2017;Yutsudo N et al., 2013). This accumulation could be neuroprotective, but general ΔFosB viral overexpression in the dHPC results in impaired cognition, while viral inhibition of ΔFosB impairs cognition in wild type mice but prevents cognitive decline in an Alzheimer’s model (Eagle AL et al., 2015;You JC et al., 2017). Coupled with our finding that SGZ FosB is necessary for normal cognition, this suggests that cell-specific hippocampal ΔFosB is necessary for normal cognition, but aberrant or non-specific ΔFosB may result in cognitive deficits.
The importance of appropriate expression of ΔFosB in normal behavior is reinforced by its unique molecular characteristics. ΔFosB accumulation in the dHPC results in a number of downstream changes in gene expression, including epigenetic alterations at target genes. Of note is histone deacetylation at the Calb1 gene, a calcium binding protein and marker of mature neurons (Corbett BF et al., 2017;LaGamma CT et al., 2018;You JC et al., 2017;You JC et al., 2018), whose regulation by ΔFosB may be critical for the altered neurogenesis we report in FosB SGZ knockout. Alternatively, ΔFosB may directly regulate microglia through C5aR1 and C5aR2 (Nomaru H et al., 2014), and this in turn may elicit the altered neurogenesis we report in FosB SGZ knockout (Rivera PD et al., 2018). Thus, future studies will focus on identifying downstream transcriptional targets of ΔFosB in both glia and neurons to provide insight into mechanisms of hippocampal neurogenesis and potentially uncover novel therapeutic targets for treatment of diseases in which neurogenesis may play a role, such as depression or Alzheimer’s Disease.
HIGHLIGHTS:
The NtsR2 Cre mouse is a useful tool for manipulating cells of the hippocampal SGZ.
The transcription factor ΔFosB is robustly expressed in NtsR2-positive SGZ cells.
SGZ knockout of the FosB gene reduces hippocampal cell proliferation.
SGZ knockout of the FosB gene impedes hippocampus-dependent learning.
Acknowledgements:
We would like to thank Kenneth Moon for outstanding technical assistance. Floxed FosB mice were a generous gift from Dr. Eric Nestler at the Icahn School of Medicine at Mount Sinai. This work was supported by NIMH R01 111604 (to AJR).
Abbreviations:
- BrdU
Bromodeoxyuridine / 5-bromo-2’-deoxyuridine
- DCX
Doublecortin
- DG
Dentate Gyrus
- EPM
Elevated Plus Maze
- GFP
Green Florescent Protein
- HPC
Hippocampus
- IEG
Immediate Early Gene
- NAc
Nucleus Accumbens
- NeuN
Neuronal Nuclei
- NOR
Novel Object Recognition
- NtsR2
Neurotensin Receptor 2
- SGZ
Subgranular Zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no conflicts of interest.
Literature Cited:
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, et al. (2014), Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344:598–602. [DOI] [PubMed] [Google Scholar]
- Anacker C, Luna VM, Stevens GS, Millette A, Shores R, Jimenez JC, Chen B, Hen R (2018), Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 559:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, Kumar A, Nestler EJ (2007), Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. Eur J Neurosci 25:3009–3019. [DOI] [PubMed] [Google Scholar]
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, et al. (2015), Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun 6:6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BF, You JC, Zhang X, Pyfer MS, Tosi U, Iascone DM, Petrof I, Hazra A, et al. (2017), DeltaFosB Regulates Gene Expression and Cognitive Dysfunction in a Mouse Model of Alzheimer’s Disease. Cell Rep 20:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH (2010), New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke CG, Kennedy AJ, Gavin CF, Day JJ, Sweatt JD (2017), Experience-dependent epigenomic reorganization in the hippocampus. Learning & memory (Cold Spring Harbor, NY) 24:278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Gajewski PA, Yang M, Kechner ME, Al Masraf BS, Kennedy PJ, Wang H, Mazei-Robison MS, et al. (2015), Experience-Dependent Induction of Hippocampal DeltaFosB Controls Learning. J Neurosci 35:13773–13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Silva Mera R, Kohler S, Josselyn SA, Frankland PW (2016), Neurogenesis-mediated forgetting minimizes proactive interference. Nat Commun 7:10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Cherkin A (1987), Fluoxetine enhances memory processing in mice. Psychopharmacology 93:36–43. [DOI] [PubMed] [Google Scholar]
- Goelet P, Castellucci VF, Schacher S, Kandel ER (1986), The long and the short of long-term memory--a molecular framework. Nature 322:419–422. [DOI] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R (2015), Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacol 40:2368–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, et al. (2008), Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem 105:921–932. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ (2000), Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10:47–56. [DOI] [PubMed] [Google Scholar]
- Kohler C, Radesater AC, Chan-Palay V (1987), Distribution of neurotensin receptors in the primate hippocampal region: a quantitative autoradiographic study in the monkey and the postmortem human brain. Neurosci Lett 76:145–150. [DOI] [PubMed] [Google Scholar]
- Kurushima H, Ohno M, Miura T, Nakamura TY, Horie H, Kadoya T, Ooboshi H, Kitazono T, et al. (2005), Selective induction of DeltaFosB in the brain after transient forebrain ischemia accompanied by an increased expression of galectin-1, and the implication of DeltaFosB and galectin-1 in neuroprotection and neurogenesis. Cell Death Differ 12:1078–1096. [DOI] [PubMed] [Google Scholar]
- Kyrke-Smith M, Williams JM (2018), Bridging Synaptic and Epigenetic Maintenance Mechanisms of the Engram. Front Mol Neurosci 11:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, et al. (2010), Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. P Natl Acad Sci USA 107:4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGamma CT, Tang WW, Morgan AA, McGowan JC, Brachman RA, Denny CA (2018), Antidepressant but Not Prophylactic Ketamine Administration Alters Calretinin and Calbindin Expression in the Ventral Hippocampus. Front Mol Neurosci 11:404–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton LJ, Zhao Q, Li X, Dai C, Marshall PR, Liu S, Wang Y, Zajaczkowski EL, et al. (2018), A Functional Role for the Epigenetic Regulator ING1 in Activity-induced Gene Expression in Primary Cortical Neurons. Neuroscience 369:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepee-Lorgeoux I, Betancur C, Rostene W, Pelaprat D (1999), Differential ontogenetic patterns of levocabastine-sensitive neurotensin NT2 receptors and of NT1 receptors in the rat brain revealed by in situ hybridization. Dev Brain Res 113:115–131. [DOI] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Redondo RL, Tonegawa S (2014), Identification and Manipulation of Memory Engram Cells. Cold Spring Harb Sym 79:59–65. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czeh B (2010), Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharm 20:1–17. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000), Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwari S, Dawe GS (2018), (R)-fluoxetine enhances cognitive flexibility and hippocampal cell proliferation in mice. J Psychopharmacol 32:441–457. [DOI] [PubMed] [Google Scholar]
- Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, Denny CA (2018), Ventral CA3 Activation Mediates Prophylactic Ketamine Efficacy Against Stress-Induced Depressive-like Behavior. Biol Psychiat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP (1996), Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J Neurosci 16:5613–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minatohara K, Akiyoshi M, Okuno H (2015), Role of Immediate-Early Genes in Synaptic Plasticity and Neuronal Ensembles Underlying the Memory Trace. Front Mol Neurosci 8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2012), Transcriptional Mechanisms of Drug Addiction. Clin Psychopharm Neu 10:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niibori Y, Yu TS, Epp JR, Akers KG, Josselyn SA, Frankland PW (2012), Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun 3:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T, Kawakami M, Kita I (2013), Long-term exercise is a potent trigger for DeltaFosB induction in the hippocampus along the dorso-ventral axis. PloS one 8:e81245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Ding S, Li H, Wei J, Ren C, Wu X, Huma T, Zhang Q (2018), Effect of Long-Term Sodium Salicylate Administration on Learning, Memory, and Neurogenesis in the Rat Hippocampus. BioMed Res Int 2018:7807426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomaru H, Sakumi K, Katogi A, Ohnishi YN, Kajitani K, Tsuchimoto D, Nestler EJ, Nakabeppu Y (2014), Fosb gene products contribute to excitotoxic microglial activation by regulating the expression of complement C5a receptors in microglia. Glia 62:1284–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouel D, Sarret P, Vincent JP, Mazella J, Beaudet A (1999), Pharmacological, molecular and functional characterization of glial neurotensin receptors. Neuroscience 94:1189–1197. [DOI] [PubMed] [Google Scholar]
- Ohnishi YN, Eagle AL, Ohnishi YH, Cahill ME, Wirtz AJ, Robison AJ, Nestler EJ (2017), Generation and validation of a floxed FosB mouse line. bioRxiv:179309. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ (2004), Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci 24:10594–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM (2010), DeltaFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav 9:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez S (2018), Crystallizing a memory. Science 360:1182–1183. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN (2009), Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatr 14:959–967. [DOI] [PubMed] [Google Scholar]
- Rivera PD, Hanamsagar R, Kan MJ, Tran PK, Stewart D, Jo YC, Gunn M, Bilbo SD (2018), Removal of microglial-specific MyD88 signaling alters dentate gyrus doublecortin and enhances opioid addiction-like behaviors. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, Wee S, Koob G, et al. (2013), Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci 33:4295–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C, Turecki G, Tamminga C, et al. (2014), Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacol 39:1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, et al. (2003), Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Sarret P, Beaudet A, Vincent JP, Mazella J (1998), Regional and cellular distribution of low affinity neurotensin receptor mRNA in adult and developing mouse brain. J Comp Neurol 394:344–356. [PubMed] [Google Scholar]
- Seo D-o, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR (2015), Adult Hippocampal Neurogenesis Modulates Fear Learning through Associative and Nonassociative Mechanisms. J Neurosci 35:11330–11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, Redondo R (2015), Memory Engram Cells Have Come of Age. Neuron 87:918–931. [DOI] [PubMed] [Google Scholar]
- Ulery-Reynolds PG, Castillo MA, Vialou V, Russo SJ, Nestler EJ (2009), Phosphorylation of DeltaFosB mediates its stability in vivo. Neuroscience 158:369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (1999), Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270. [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ 3rd, et al. (2010), DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Thibault M, Kaska S, Cooper S, Gajewski P, Eagle A, Mazei-Robison M, Nestler EJ, et al. (2015), Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology 99:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth HL, Perez-Bonilla PA, Beekly BG, Lewis TJ, Leinninger GM (2018), Identification of Neurotensin Receptor Expressing Cells in the Ventral Tegmental Area across the Lifespan. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Zhang J, Ko SY, Kwon H, Jeon SJ, Park SJ, Jung J, Kim BC, et al. (2018), Fluoxetine Inhibits Natural Decay of Long-Term Memory via Akt/GSK-3beta Signaling. Mol Neurobiol 55:7453–7462. [DOI] [PubMed] [Google Scholar]
- You JC, Muralidharan K, Park JW, Petrof I, Pyfer MS, Corbett BF, LaFrancois JJ, Zheng Y, et al. (2017), Epigenetic suppression of hippocampal calbindin-D28k by DeltaFosB drives seizure-related cognitive deficits. Nat Med 23:1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JC, Stephens GS, Fu CH, Zhang X, Liu Y, Chin J (2018), Genome-wide profiling reveals functional diversification of FosB gene targets in the hippocampus of an Alzheimer’s disease mouse model. PloS one 13:e0192508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutsudo N, Kamada T, Kajitani K, Nomaru H, Katogi A, Ohnishi YH, Ohnishi YN, Takase K, et al. (2013), fosB-null mice display impaired adult hippocampal neurogenesis and spontaneous epilepsy with depressive behavior. Neuropsychopharmacol 38:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH (2008), Mechanisms and Functional Implications of Adult Neurogenesis. Cell 132:645–660. [DOI] [PubMed] [Google Scholar]