Abstract
OBJECTIVE.
Whether diabetes mellitus (DM) is associated with increased risk of knee osteoarthritis is uncertain. We evaluated associations of DM and biomarkers of abnormal glucose metabolism with incident radiographic knee osteoarthritis (RKOA), controlling for body mass index (BMI).
METHODS.
Participants (mean age 60.6±7.8 years; mean BMI 29.1±4.9 kg/m2) were from the Multicenter Osteoarthritis Study (MOST) study and did not have RKOA at baseline (Kellgren and Lawrence [KL] grade <2 bilaterally). A random sample (n=987) stratified by BMI was selected. Baseline serum fasting glucose and insulin resistance (homeostasis model of assessment [HOMA-IR]) were measured. Participants were categorized as having DM based on self-report, use of medication or fasting glucose ≥126 mg/dL. Incident RKOA (KL grade ≥ 2 or knee replacement) was assessed at 3 follow up visits (30, 60, 84 months). Knee level pooled logistic regression analysis was performed to obtain odds ratios (95% CI) for associations of DM status and biomarkers of abnormal glucose metabolism with incident RKOA.
RESULTS.
After adjustment for BMI, the odds of incident RKOA were not associated with baseline DM status nor with levels of fasting glucose and HOMA-IR overall and in men. In women, HOMA-IR was inversely associated with odds of incident RKOA (adjusted OR 0.80, 95% CI 0.69–0.94; p=0.005).
CONCLUSION.
DM and higher levels of biomarkers of abnormal glucose metabolism were not associated with increased odds of incident RKOA after adjusting for BMI in this cohort overall. A possible protective association of higher HOMA-IR with incident RKOA in women deserves further investigation.
Osteoarthritis (OA) and diabetes mellitus (DM) are common among older adults in the U.S. Knee OA causes structural changes in the joint, resulting in pain and stiffness, and is a prominent cause of global disability (1). It is estimated that 16% of adults 45 years and older develop symptomatic knee OA in the United States (2, 3). By 2018, the prevalence of knee OA is projected to reach 35% in obese individuals 60–64 years old (4). Approximately 9.3% of the population has DM in the United States, and DM is even more prevalent among older adults (25.9% of adults 65 years and older) (5). Moreover, by 2050, 55% of adults in this age group are expected to be diagnosed with DM. Type 2 DM (DM2) is more common and comprises 90–95% of all DM cases (6).
Previous epidemiological studies have suggested that OA is associated with elevated fasting glucose (7, 8) and is highly prevalent among those with DM (9–13). Proposed etiologies include advanced glycation end products, which reduce cartilage integrity (14, 15). Additionally, inflammation could contribute to changes in cartilage metabolism and integrity (14), as well as neuromuscular impairment (as a result of symmetric sensory polyneuropathy and autonomic neuropathy from long-standing DM), which could lead to muscle weakness and joint laxity (15).
However, there are few studies specifically of the association of DM and knee OA, and results have been conflicting (16, 17). Some studies of DM and knee OA have not adjusted for body mass index (BMI), even though obesity (high BMI) increases mechanical load on the knee and is a strong risk factor for both DM and OA (16–20). A meta-analysis of six studies found an increased risk of knee OA in persons with DM, but a majority of these studies did not adjust for BMI or obesity (17). A second meta-analysis of seven studies that adjusted for BMI found a small, but significant, increase in risk of OA, but this analysis pooled results from studies of knee, hip and hand OA (16). In addition, both meta-analyses pooled results from cross-sectional and prospective studies and included studies with joint replacement as the outcome, so the temporal relationship between DM and OA is uncertain (16, 17). The potential for reverse causality in cross-sectional studies (i.e., OA contributing to an increased risk of DM) has been shown (17, 21), indicating that prospective studies of the association of DM and abnormalities of glucose metabolism with the subsequent development of OA are needed to understand the contribution of DM to the risk of OA. One prospective study (22) found an increased subsequent risk of either knee or hip replacement (a surrogate for severe OA) in patients with DM after adjusting for BMI. It remains unknown whether the presence of DM precedes development of OA when arthroplasty is the outcome.
The finding of an association between DM and OA after adjustment for BMI in some studies suggests that other aspects of DM, including metabolic abnormalities, may contribute to the risk of OA development. However, prospective studies have not found an association between hyperglycemia and either the risk of hip or knee replacement (23) or the risk of incident knee OA (24) after adjustment for BMI. A recent systematic review of abnormal glucose metabolism as a risk factor for OA reported that, of five longitudinal studies related to knee OA which adjusted for obesity, three studies reported no association, one reported a reduction in OA risk, and one reported an increase in OA risk (25). Additional prospective studies of DM and abnormalities of glucose metabolism are needed to fully elucidate their potential role in the development of OA in specific joints.
In light of these previous reports, we evaluated the association of DM and of biomarkers of abnormal glucose metabolism with incident radiographic knee osteoarthritis (RKOA) of the tibiofemoral (TF) joint among participants in the Multicenter Osteoarthritis Study (MOST). We hypothesized that the presence of DM, as well as hyperglycemia and elevated insulin resistance in participants with and without DM, would be associated with increased odds of incident RKOA, independent of BMI.
MATERIALS AND METHODS
Study Participants
We utilized data from MOST, which is an National Institutes of Health (NIH)-funded, community-based longitudinal cohort study of risk factors for knee OA in 3,026 men and women age 50–79 years, with or at high risk for knee OA. Eligibility for MOST included overweight or obesity (defined as weighing more than the Framingham Study medium weight for age and sex-specific group), history of knee injury that made it difficult to walk for at least one week, prior knee surgery or frequent knee pain (26, 27). The MOST clinical centers are located at the University of Alabama at Birmingham and the University of Iowa; participants were recruited from the surrounding areas. Subjects with bilateral total knee replacement (TKR), those unable to walk without assistance or who were unlikely to participate in follow-up examinations at baseline were excluded from MOST. Weight-bearing posteroanterior and lateral knee radiographs were acquired during baseline, 30-, 60- and 84-month clinic visits.
In compliance with the Helsinki Declaration, the protocol for the MOST Study was approved by Institutional Review Boards at Boston University, the University of Iowa, the University of Alabama at Birmingham and the University of California, San Francisco.
Sample Selection
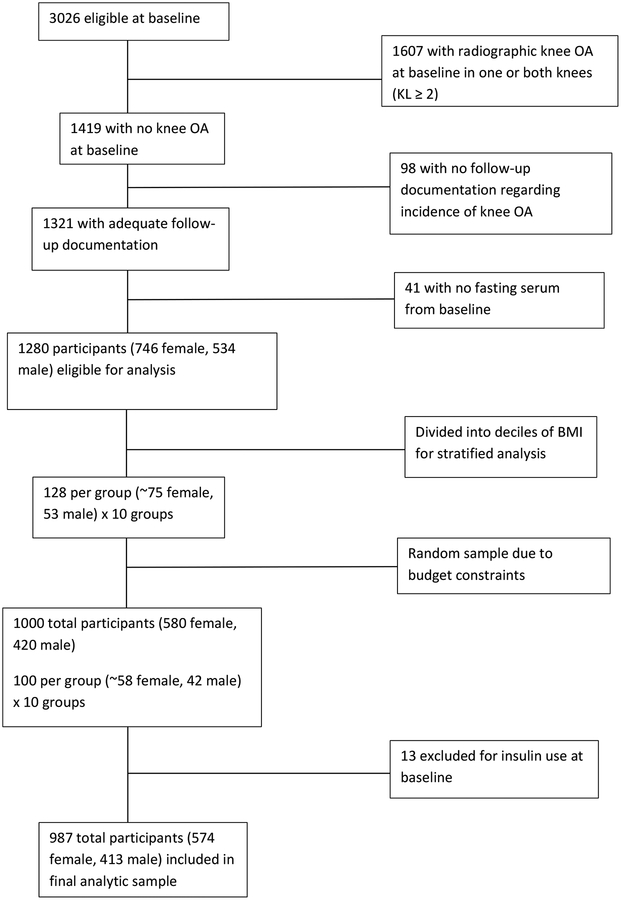
To be eligible for the present analysis, MOST participants had to have two native knees free of osteoarthritis at baseline (Kellgren and Lawrence [KL] grade 0 or 1 in native knees and no KR), knee radiographs at baseline and one or more follow-up visits, and archived baseline fasting serum available. Because of a limited budget for assays, we were unable to include all eligible participants (n=1,280). We therefore selected a random sample of 1000 from the eligible participants. The sample was stratified by decile of BMI (i.e., 100 participants [58 women and 42 men] were randomly selected from each decile of BMI). We stratified on BMI because control for this variable is essential to determine if DM is associated with OA, independent of BMI. As others have noted (28, 29), residual bias can occur if relationships with a continuous confounder are not properly modeled and a linear relationship is incorrectly assumed. Strategies to deal with nonlinear relationships, which are not uncommon in epidemiological studies of exposure-outcome associations, include ensuring that the data are spread across a wide range, as well as the use of restricted splines. Our sampling approach therefore resulted in a more even distribution of BMI across the entire range of values, which increases the precision of the adjusted odds ratios but does not add bias to the estimates of association. It also allowed us to better estimate the shape of the relationship of BMI with exposure and outcome using splines, with the goal of increased accuracy and precision of the BMI adjusted odds ratios. However, we found that the use of splines did not change the adjusted results so we did not use splines in the main analyses. The BMI groups (deciles) were used only for the selection of participants. Of these 1000 participants, 13 were excluded after we determined that they were insulin users at baseline. The final sample size for the present analysis was 987 (Figure 1).
FIGURE 1:
FLOWCHART OF STUDY SAMPLE SELECTION
KL, Kellgren & Lawrence
OA, osteoarthritis
Measurements
Participants were categorized as having DM at baseline based on the presence of at least one of the following: self-reported diagnosis of DM (“Do you have diabetes or high blood sugar?”), use of anti-diabetic medications (e.g., meglitinides, metformin, sulfonylureas, thiazolidinediones) determined by review of all prescription medications taken in the past 30 days, or a fasting glucose of ≥ 126 mg/dL.
Weight (kg) and height (mm) were measured by standard medical beam balance and wall mounted Harpenden stadiometer, respectively (30). Weight and height measurements were used to calculate BMI (kg/m2). Self-reported health status was determined via the SF-12 Health Survey (26). Self-reported physical activity was assessed at baseline with the Physical Activity Scale for the Elderly (PASE) (31).
Fasting glucose (Unical DxC 800 Auto-analyzer (Beckman Coulter, Fullerton, CA, USA) and free insulin (Abbott Architect i1000SR immunoassay analyzer, Abbot Diagnostics, Santa Clara, CA, USA) levels at baseline were measured on all subjects. Coefficients of variation ranged from 1.4–5.6% and 2.4–2.9%, respectively. These assays were performed at the University of California, Davis Medical Center (UCDMC) clinical lab in Sacramento, CA, USA. Insulin resistance was determined with the homeostasis model of assessment – insulin resistance (HOMA-IR) formula, which is calculated as: (fasting glucose concentration [mg/dl] × fasting insulin concentration [μU/ml])/405. HOMA-IR values have been validated as surrogate markers of insulin resistance and correlate well with insulin resistance values determined by euglycemic-insulin clamp or the frequently sampled IV glucose tolerance test (32).
Posteroanterior weight bearing knee radiographs were obtained using a Synaflexer frame (Synarc, San Francisco, CA, USA) to create a standardized knee position. All radiographs for each participant were read together in known order by a team of experienced readers at a central location (Boston University). Readers were blind to the DM status and all clinical characteristics of the participants. Each of two readers read all radiographs from all the participants. Cases of disagreement were addressed by adjudication with a third reader (33, 34). Self-reported KR was confirmed by radiographs and/or medical records. For the purposes of this knee-level investigation, knees with incident tibiofemoral RKOA were identified separately at each visit from baseline to 84 months. A knee was defined as having this endpoint if there was at least one follow-up radiograph showing RKOA (KL grade 2 or greater) or if there had been a TKR at the time of the visit. The status of every knee was followed from baseline to 84 months. Once the criteria for incident RKOA were met, the knee was considered to have RKOA for the remainder of the study. Additionally, physical examinations of joints occurred at baseline and 30 months (26).
Statistical Analysis
Descriptive characteristics were analyzed by analysis of variance (ANOVA) for continuous variables and chi-squared test for categorical variables. Incidence of RKOA between baseline and the 84-month follow-up were evaluated as cumulative incidence.
In this study, events were discovered at clinic visits when knee radiographs were acquired, and we used pooled logistic regression to conduct a discrete time survival analysis (consecutive clinic visit at 30m, 60m or 84m). To conduct such an analysis, the data were transformed to multiple records per knee, one record per visit. If knee status was unknown (e.g., if the visit was missed or if the visit was done without imaging), the outcome was considered to be missing. Once an event occurred, all subsequent records (visits) for the affected knee were dropped from the analysis (i.e., each incidence was counted only once). Data collection continued for the other knee.
Knee level pooled logistic regression analysis (35, 36) was performed to obtain odds ratios and 95% CIs for incidence of RKOA predicted by baseline DM status, baseline fasting glucose and HOMA-IR. Generalized estimating equations were used to account for within-person correlations (between knees). Models were adjusted for age, gender, race, clinic site, visit (time to event; this variable allowed for the rate of events to change over time) and time-dependent BMI at each visit. Formal interactions for exposure*sex, as well as exposure*BMI, were examined. Missing observations (2.4% in this study) were dropped from the analysis.
Sensitivity analyses were also performed. We first examined whether excluding participants with newly reported DM (n=54) would influence the results of the analyses. Secondly, we performed the analyses in whites only (n=849) in order to remove the influence of the race. Additionally, we ran further sensitivity analyses using time-dependent DM (a combination of baseline DM and incident self-report of DM at a subsequent visit) and DM medication use, and specifically metformin use, as primary exposures. We also adjusted models for metformin use (i.e., in this sensitivity analysis, metformin use was a covariate rather than a primary exposure). Lastly, we examined the effect of physical activity by adjusting models for PASE score. All analyses were performed using SAS v9.4.
RESULTS
Among the 987 participants (mean age 60.6±7.8 years, 58% female, mean BMI 29.1±4.9 kg/m2), 94 (9.5%) had DM at baseline. Compared to participants without DM, participants with DM were more likely to be male, non-white and reside in Alabama. Among whites residing in Iowa, the prevalence of DM was 7.1% (5.8% in women and 9.0% in men). Among whites in Alabama, the prevalence of DM was 8.4% (7.2% in women and 10.1% in men). The prevalence of DM in blacks residing in Alabama was 23.3% (16.7% in women and 31.5% in men). There were only three black participants residing in Iowa; all were men and none had DM.
Of the 94 participants classified as having DM at baseline, 56 were taking DM medications (including 42 taking metformin), and 56 had elevated fasting glucose. Nineteen participants were classified as having DM based on self-report only, 2 had DM based on medications only, and 19 had DM based on fasting glucose levels only.
As expected, participants with DM had significantly higher fasting glucose, fasting insulin and HOMA-IR compared to participants without DM at baseline (Table 1). Additionally, participants with DM had a higher BMI and were less likely to consider their health status to be “excellent,” “very good” or “good” compared to participants without DM (Table 1).
TABLE 1: Descriptive characteristics at baseline,
Mean (SD) or n (%)
| No baseline DM (n=893) | Baseline DM (n=94) |
P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 60.6 (7.8) | 60.7 (8.0) | 0.877 |
| Sex (female) | 531 (59.5%) | 43 (45.7%) | 0.010 |
| Race (white) | 784 (87.8%) | 65 (69.1%) | <.0001 |
| Clinic (UAB) | 425 (47.6%) | 59 (62.8%) | 0.005 |
| Metabolic parameters | |||
| Fasting blood glucose (mg/dL) | 95.4 (9.2) | 141.7 (45.9) | <.0001 |
| Fasting insulin (muU/mL) | 9.0 (7.6) | 14.6 (10.2) | <.0001 |
| HOMA-IR | 2.2 (1.9) | 5.0 (3.7) | <.0001 |
| Anthropometric characteristics | |||
| Weight (kg) | 83.0 (15.8) | 95.9 (19.0) | <.0001 |
| Height (m) | 1.7 (0.08) | 1.7 (0.09) | 0.1105 |
| BMI (kg/m2) | 28.7 (4.7) | 32.5 (5.2) | <.0001 |
| General functional status | |||
| Health status (self-assessment) | |||
| Excellent/Very Good/Good | 840 (94.1%) | 73 (77.7%) | <.0001 |
| Fair/Poor | 53 (5.9%) | 21 (22.3%) | |
| PASE (activity score) | 181.5 (89.3) | 197.2 (98.3) | 0.1088 |
BMI, body mass index; DM, diabetes mellitus; HOMA-IR, homeostasis model of assessment – insulin resistance; PASE, physical activity scale for the elderly; UAB, University of Alabama at Birmingham
In the cohort of 987 participants, 1972 knees were included in this analysis. Two participants did not return for follow up visits but reported by telephone contact a KR between the 60-month and 84-month visits. There were 165 (2.4%) records with a missing interim visit, and there were 365 knees (18%) that did not have 7 years of follow up.
There were 409 knees with incident RKOA. Between baseline and the 84-month follow-up visit, the cumulative incidence of RKOA for all knees was 23.3%; the cumulative incidence was slightly higher for knees with DM compared to knees without DM (24.0% versus 23.2%; Table 2).
TABLE 2.
Knee-level incidence of radiographic knee osteoarthritis (RKOA) during 84 month follow-up
| Study sample N=1972 knees |
No baseline DM N=1785 knees |
Baseline DM N=187 knees |
|
|---|---|---|---|
| Number of knees with incident RKOA | 409 | 368 | 41 |
| Cumulative incidence (%): | |||
| At 30 months | 6.5 | 6.4 | 8.0 |
| At 60 months | 17.2 | 17.1 | 17.5 |
| At 84 months | 23.3 | 23.2 | 24.0 |
DM, diabetes mellitus
The odds ratios of incident RKOA by baseline DM status, baseline fasting glucose and HOMA-IR are presented in Table 3. Baseline fasting glucose and HOMA-IR were associated with increased odds of incident RKOA in the unadjusted model, as well as model adjusted for age, race, clinic site and visit. After adjustment for BMI, these associations were attenuated and lost statistical significance. Fasting insulin values were highly correlated with HOMA-IR by design. As expected, the risk of incident RKOA by fasting insulin mirrored the results for HOMA-IR and did not reveal any new findings (data not shown).
TABLE 3. Odds ratio for incidence of knee osteoarthritis (OA),
n=987 participants (1972 knees)
| Model | Exposure | Odds Ratio (95% CI) and P for incidence of knee OA | ||
|---|---|---|---|---|
| Unadjusted Model | Adjusted Modela | Adjusted Modelb | ||
| #1 | Baseline DM status | 1.06 (0.72–1.54) 0.778 | 1.05 (0.71–1.54) 0.813 | 0.79 (0.53–1.18) 0.246 |
| #2 | Fasting glucose (per 1 SD) | 1.14 (1.02–1.28) 0.021 | 1.14 (1.01–1.27) 0.031 | 0.98 (0.87–1.11) 0.751 |
| #3 | HOMA-IR (per 1SD) | 1.13 (1.01–1.27) 0.033 | 1.13 (1.00–1.27) 0.042 | 0.89 (0.79–1.01) 0.077 |
Adjusted for sex, age, race, clinic site and visit
Adjusted for sex, age, race, clinic site, visit and BMI (time-dependent)
BMI, body mass index; DM, diabetes mellitus; HOMA-IR, homeostasis model of assessment – insulin resistance
The only formal interaction that neared significance (p<0.05) was between HOMA-IR and sex (p=0.12); sex stratified results for the odds ratio for HOMA-IR and incident RKOA are presented in Table 4. In women, elevated HOMA-IR was associated with lower odds of incident RKOA in the model adjusted for sex, age, race, clinic site, visit and BMI. In men, elevated HOMA-IR was associated with increased odds for RKOA in the model adjusted for sex, age, race, clinic site and visit. However, after adjustment for BMI, this association was attenuated and no longer statistically significant.
TABLE 4.
Odds ratio for incidence of knee osteoarthritis (OA) by HOMA-IR, stratified by sex
| Odds Ratio (95% CI) and P for incidence of knee OA | |||||
|---|---|---|---|---|---|
| Women, n=574 | Men, n=413 | ||||
| Unadjusted Model | Adjusted Modela | Adjusted Modelb | Unadjusted Model | Adjusted Modela | Adjusted Modelb |
| 1.08 (0.94–1.24) 0.303 |
1.05 (0.91–1.22) 0.470 |
0.80 (0.69–0.94) 0.005 |
1.28 (1.05–1.56) 0.015 |
1.30 (1.07–1.58) 0.007 |
1.09 (0.89–1.33) 0.424 |
Adjusted for sex, age, race, clinic site and visit
Adjusted for sex, age, race, clinic site, visit and BMI (time-dependent)
BMI, body mass index; DM, diabetes mellitus; HOMA-IR, homeostasis model of assessment – insulin resistance
We repeated all analyses using participant-level incidence of RKOA as the outcome. There were no noteworthy differences between results at the participant level and results at the knee level (data not shown). A total of 54 participants self-reported a diagnosis of DM between baseline and the 84-month follow up visit, but excluding these participants from the non-DM group did not substantially change overall results (Supplementary Table 1). Among white participants overall, there were no associations between baseline DM status, fasting glucose or HOMA-IR and odds of incident RKOA in any of the models (Supplementary Table 2). Sensitivity analyses using time-dependent DM as the exposure and PASE score as a covariate did not substantially alter our main findings (data not shown). Metformin usage by itself was associated with a nonsignificant protective effect (OR: 0.69; 95% CI 0.43–1.09, p=0.11) for RKOA, but when included as a covariate in models with HOMA-IR, the protective effect of the HOMA-IR in women was unchanged and remained significant (p=0.007).
DISCUSSION
This prospective analysis among older women and men with an elevated risk of developing knee OA contributes to the literature by focusing specifically on the associations between DM status (as defined by self-report, use of anti-diabetic medications or fasting glucose), and biomarkers of abnormal glucose metabolism, with odds of subsequently developing incident RKOA. Contrary to our hypothesis, after adjustment for BMI (a strong risk factor for both DM and knee OA (16–20)) we did not find increased odds of incident RKOA in participants with DM nor in participants with higher baseline levels of fasting glucose and HOMA-IR. Surprisingly, after adjustment for BMI, we observed a protective association of higher levels of HOMA-IR with the odds of incident RKOA in women. This protective effect was not explained by use of diabetic medications in those with insulin resistance.
Our findings contrast with some previous studies of DM and DM-related factors and knee OA but are consistent with other studies. At least two meta-analyses have suggested an overall positive, though modest, association between the presence of DM and OA (16, 17). However, these analyses have important limitations, which include combining studies of knee, hip and hand joints, combining studies using different definitions of OA (radiographic, arthroplasty and clinical diagnosis) and pooling results from cross-sectional and prospective studies. Many of the included studies were cross-sectional and/or did not adjust for obesity (16, 17), a strong risk factor for both DM and knee OA (18–20) and important potential confounding factor. To our knowledge, there are few prospective studies of DM and knee OA outcomes, and none of incident radiographic knee OA, the focus of the present study. A recent prospective study (13) found, after BMI adjustment, that increased radiographic progression of existing knee OA was associated with baseline DM in men, but not women. Whether DM preceded the onset of knee OA in this study is not known.
Several cohort studies have explored associations between DM-related variables, including metabolic factors, and knee OA (22, 37–39). Yoshimura and colleagues analyzed impaired glucose tolerance [IGT], based on elevated serum hemoglobin A1c, and the three-year incidence and progression of RKOA in a cohort of Japanese men and women. They found that IGT was associated with an increased incidence of RKOA, but not with the progression of RKOA, after adjusting for obesity and other components of the metabolic syndrome (37). In contrast, in a recent study of components of metabolic syndrome and the incidence of both RKOA and symptomatic knee OA, Niu et al. found no association between elevated fasting glucose and these knee OA outcomes (24). Similarly, in two prospective studies with knee replacement as the outcome, after adjusting for BMI, there was no association between elevated fasting glucose and knee OA (23, 38). The findings of the present analysis, along with the conflicting reports in the literature, illustrate the complexity of determining whether an independent relationship between DM and OA exists after accounting for the potentially confounding role of obesity.
Our observation of an inverse association between HOMA-IR and odds of RKOA incidence in women was unexpected and possibly spurious but was not explained by use of diabetic medications, or metformin in particular, in those with insulin resistance. We also observed a potential, though not significant, protective effect of metformin usage on incident RKOA. This aligns with recent reports (39–41). Further investigation of potential protective effects of DM and DM medications is warranted.
The association of obesity and DM with OA has suggested the existence of a metabolic syndrome-associated osteoarthritis phenotype, with potential etiologic roles in joint damage for a variety of systemic and local metabolic abnormalities, including impaired glucose metabolism, hypertension, dyslipidemia, inflammation, oxidative stress and advanced glycation end products (15, 42). Future studies should address associations of DM and OA within the broader context of metabolic OA phenotypes.
This study has several strengths, including the utilization of a prospective design to study incident RKOA while eliminating the potential for reverse causality, a well-characterized, community acquired cohort and repeated follow-up over 7 years for development of RKOA. We did not rely solely on self-report to identify participants with DM but also based DM status on use of prescription anti-diabetic medications and fasting glucose levels.
Our study also has several limitations. It is possible that some participants may have given unreliable information about fasting (e.g., overstating the number of hours they had been fasting before the blood draw) and could have been falsely classified as having DM due to elevated glucose levels). Since the study is prospective, this type of misclassification is likely to be nondifferential with respect to incident OA, which could influence the odds ratios toward the null (i.e., no association). Data on DM type were not collected in the present study. It is likely that the majority of participants with DM in the present analysis had DM2 since DM2 is more prevalent (6) and insulin users were excluded; however, it is possible that some participants had type 1 DM (DM1). The definition of knee OA in this study was based on radiographic findings only (43). Because our sample included only those without radiographic OA at baseline, there were just 14 knees with incident symptomatic OA (the combination of radiographic OA and frequent knee pain), which is too few for a separate analysis. Results may differ for incident symptomatic OA. Furthermore, this analysis was limited to tibiofemoral OA; findings may differ when taking patellofermoral or whole knee OA into account. DM has also been associated with reduced levels of osteophyte formation, possibly due to diminished availability of insulin at the cellular level or diabetic microvascular disease attenuating proliferation (44). Potential protective effects on RKOA incidence in this study may reflect the importance of osteophytes in radiographic definitions of OA. Additionally, the MOST cohort was selected to have an elevated risk for incident RKOA and had a high BMI at baseline. (6). Thus, results of the present study may have limited generalizability since our study population does not reflect the incidence of RKOA or prevalence of DM in the general population. Finally, it is possible that our sample size was too small to detect associations between the exposure variables and RKOA; in particular, the wide confidence intervals for the baseline DM models in Table 3 suggest a certain degree of imprecision.
In this cohort of older adults with a high risk of developing knee OA, DM and higher levels of biomarkers of abnormal glucose metabolism were not associated with increased odds of incident RKOA after adjusting for BMI. We found evidence suggesting that these associations may differ by sex. In particular, a protective association of higher levels of insulin resistance (HOMA-IR) with odds of incident RKOA after BMI adjustment in women, but not men, deserves further investigation.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
Osteoarthritis (OA) and diabetes mellitus (DM) are two of the most prevalent diseases worldwide.
Meta-analyses have suggested positive associations of DM and OA, though limitations of previous studies include heterogeneity in definitions of OA and DM and lack of adjustment for body mass index (BMI) in some studies. In this prospective study, we examined associations of DM status and biomarkers of abnormal glucose metabolism specifically with incident radiographic knee OA (RKOA).
Among older women and men with a high risk of developing knee OA, DM and biomarkers of abnormal glucose metabolism were not associated with odds of incident RKOA after adjustment for BMI.
A possible protective association in women of greater insulin resistance (HOMA-IR) with lower odds of incident RKOA (after BMI adjustment) deserves further investigation.
Acknowledgement
The authors thank Dr. Charles McCulloch at UCSF for his critical review of this analysis and manuscript.
FUNDING: National Institutes of Health. Grant numbers: Felson - AG18820; Lewis - AG18947; Nevitt - AG19069. The authors report no conflicts of interest.
REFERENCES
- 1.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. [DOI] [PubMed] [Google Scholar]
- 2.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol 2007;34:172–80. [PubMed] [Google Scholar]
- 3.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum 2009;60:3546–53. [DOI] [PubMed] [Google Scholar]
- 4.Holt HL, Katz JN, Reichmann WM, Gerlovin H, Wright EA, Hunter DJ, et al. Forecasting the burden of advanced knee osteoarthritis over a 10-year period in a cohort of 60–64 year-old US adults. Osteoarthritis Cartilage 2011;19:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Diabetes Fact Sheet: National Diabetes Statistics Report, 2014. Atlanta, GA: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 6.Caspersen CJ, Thomas GD, Boseman LA, Beckles GL, Albright AL. Aging, diabetes, and the public health system in the United States. Am J Public Health 2012;102:1482–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimmino MA, Cutolo M. Plasma glucose concentration in symptomatic osteoarthritis: a clinical and epidemiological survey. Clin Exp Rheumatol 1990;8:251–7. [PubMed] [Google Scholar]
- 8.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol 1995;22:1118–23. [PubMed] [Google Scholar]
- 9.Waine H, Nevinny D, Rosenthal J, Joffe IB. Association of osteoarthritis and diabetes mellitus. Tufts Folia Med 1961;7:13–9. [PubMed] [Google Scholar]
- 10.Sturmer T, Brenner H, Brenner RE, Gunther KP. Non-insulin dependent diabetes mellitus (NIDDM) and patterns of osteoarthritis. The Ulm osteoarthritis study. Scand J Rheumatol 2001;30:169–71. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YJ, Imperatore G, Caspersen CJ, Gregg EW, Albright AL, Helmick CG. Prevalence of diagnosed arthritis and arthritis-attributable activity limitation among adults with and without diagnosed diabetes: United States, 2008–2010. Diabetes Care 2012;35:1686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanchek N, Gersing AS, Schwaiger BJ, Nevitt MC, Neumann J, Joseph GB, et al. Association of diabetes mellitus and biochemical knee cartilage composition assessed by T2 relaxation time measurements: Data from the osteoarthritis initiative. J Magn Reson Imaging 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eymard F, Parsons C, Edwards MH, Petit-Dop F, Reginster JY, Bruyere O, et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis Cartilage 2015;23:851–9. [DOI] [PubMed] [Google Scholar]
- 14.Laiguillon MC, Courties A, Houard X, Auclair M, Sautet A, Capeau J, et al. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthritis Cartilage 2015;23:1513–22. [DOI] [PubMed] [Google Scholar]
- 15.Berenbaum F Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann Rheum Dis 2011;70:1354–6. [DOI] [PubMed] [Google Scholar]
- 16.Williams MF, London DA, Husni EM, Navaneethan S, Kashyap SR. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis. J Diabetes Complications 2016;30:944–50. [DOI] [PubMed] [Google Scholar]
- 17.Louati K, Vidal C, Berenbaum F, Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 2015;1:e000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee R, Kean WF. Obesity and knee osteoarthritis. Inflammopharmacology 2012;20:53–8. [DOI] [PubMed] [Google Scholar]
- 19.Gray N, Picone G, Sloan F, Yashkin A. Relation between BMI and diabetes mellitus and its complications among US older adults. South Med J 2015;108:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford AG, Cote C, Couto J, Daskiran M, Gunnarsson C, Haas K, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity Electronic Medical Record database. Popul Health Manag 2010;13:151–61. [DOI] [PubMed] [Google Scholar]
- 21.Rahman MM, Cibere J, Anis AH, Goldsmith CH, Kopec JA. Risk of Type 2 diabetes among osteoarthritis patients in a prospective longitudinal study. Int J Rheumatol 2014;2014:620920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schett G, Kleyer A, Perricone C, Sahinbegovic E, Iagnocco A, Zwerina J, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engstrom G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage 2009;17:168–73. [DOI] [PubMed] [Google Scholar]
- 24.Niu J, Clancy M, Aliabadi P, Vasan R, Felson DT. Metabolic Syndrome, Its Components, and Knee Osteoarthritis: The Framingham Osteoarthritis Study. Arthritis Rheumatol 2017;69:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson LP, Fairley JL, Papandony MC, Hussain SM, Cicuttini FM, Wluka AE. Is abnormal glucose tolerance or diabetes a risk factor for knee, hip, or hand osteoarthritis? A systematic review. Semin Arthritis Rheum 2018. [DOI] [PubMed] [Google Scholar]
- 26.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The Multicenter Osteoarthritis Study: opportunities for rehabilitation research. PM R 2013;5:647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal NA, Torner JC, Felson DT, Niu J, Sharma L, Lewis CE, et al. Knee extensor strength does not protect against incident knee symptoms at 30 months in the multicenter knee osteoarthritis (MOST) cohort. PM R 2009;1:459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groenwold RH, Klungel OH, Altman DG, van der Graaf Y, Hoes AW, Moons KG. Adjustment for continuous confounders: an example of how to prevent residual confounding. Cmaj 2013;185:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May S, Bigelow C. Modeling nonlinear dose-response relationships in epidemiologic studies: statistical approaches and practical challenges. Dose Response 2006;3:474–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal NA, Felson DT, Torner JC, Zhu Y, Curtis JR, Niu J, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil 2007;88:988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153–62. [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 33.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol 2008;35:2047–54. [PMC free article] [PubMed] [Google Scholar]
- 34.Felson DT, Niu J, Yang T, Torner J, Lewis CE, Aliabadi P, et al. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis Cartilage 2013;21:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horton DB, Haynes K, Denburg MR, Thacker MM, Rose CD, Putt ME, et al. Oral glucocorticoid use and osteonecrosis in children and adults with chronic inflammatory diseases: a population-based cohort study. BMJ Open 2017;7:e016788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med 1990;9:1501–15. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura N, Muraki S, Oka H, Tanaka S, Kawaguchi H, Nakamura K, et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012;20:1217–26. [DOI] [PubMed] [Google Scholar]
- 38.Hellevik AI, Johnsen MB, Langhammer A, Baste V, Furnes O, Storheim K, et al. Metabolic syndrome as a risk factor for total hip or knee replacement due to primary osteoarthritis: a prospective cohort study (the HUNT study and the Norwegian Arthroplasty Register). Clin Epidemiol 2018;10:83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shirinsky IV, Shirinsky VS. Effects of medication-treated diabetes on incidence and progression of knee osteoarthritis: a longitudinal analysis of the Osteoarthritis Initiative data. Rheumatol Int 2017;37:983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnett LA, Jordan KP, Edwards JJ, van der Windt DA. Does metformin protect against osteoarthritis? An electronic health record cohort study. Prim Health Care Res Dev 2017:1–6. [DOI] [PubMed] [Google Scholar]
- 41.Lu CH, Chung CH, Lee CH, Hsieh CH, Hung YJ, Lin FH, et al. Combination COX-2 inhibitor and metformin attenuate rate of joint replacement in osteoarthritis with diabetes: A nationwide, retrospective, matched-cohort study in Taiwan. PLoS One 2018;13:e0191242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dell’Isola A, Allan R, Smith SL, Marreiros SS, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskelet Disord 2016;17:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 44.Horn CA, Bradley JD, Brandt KD, Kreipke DL, Slowman SD, Kalasinski LA. Impairment of osteophyte formation in hyperglycemic patients with type II diabetes mellitus and knee osteoarthritis. Arthritis Rheum 1992;35:336–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.