Abstract
Enterococci are colonizers of the mammalian gastrointestinal tract (GIT) and normally live in healthy association with their human host. However, enterococci are also major causes of healthcare-acquired infections, prompting the US Centers for Disease Control and Prevention to declare vancomycin-resistant enterococci (VRE) a serious threat to public health. Because of both intrinsic and acquired antibiotic resistance, enterococci proliferate in the GIT during antibiotic therapy, leading to dissemination and disease. The recognition that colonization of the GIT is a pre-requisite for enterococcal infections has prompted research to study mechanisms used by enterococci to colonize this niche. This review discusses major findings of recent research to understand GIT colonization by enterococci using diverse experimental models, each of which exhibits unique strengths. This work has revealed enterococcal transcriptional reprogramming in the GIT, contributions of specific enterococcal genes encoded by the core genome to GIT colonization, the impact of genome plasticity, and roles for intra- and inter-species interactions in modulation of GIT colonization.
Introduction
Bacteria seeking to colonize the mammalian GIT face formidable challenges from both the host and from competing microbes. These include constant expulsion of intestinal contents via peristalsis, assault by antimicrobials produced by the host and bacterial competitors, and intense competition for nutrients by the trillions of bacteria co-inhabiting the GIT. Thus, it seems likely that bacteria adept at stably inhabiting the GIT have evolved sophisticated mechanisms to overcome these challenges.
Through millions of years of evolution, enterococci have become highly adapted colonizers of the digestive tract [1]. The success of enterococci as intestinal commensals is reflected by the fact that they colonize the digestive organs of diverse organisms ranging from insects to humans [2,3]. Although enterococci are core members of the healthy human GIT microbial consortium, they only represent a minority (<1%) of the intestinal microbiota [1]. Upon antibiotic therapy of the host, however, enterococci exploit their intrinsic and acquired antibiotic resistance to proliferate in the GIT [4], leading to domination of the GIT community and dissemination to extra-intestinal organs where they may cause life-threatening infections [5]. Hence, enterococci lead a dual lifestyle, in which their proficiency at GIT colonization positions them to effectively exploit ecological dysbiosis resulting from iatrogenic intervention, facilitating a transition to a pathogenic state. Consistent with this, enterococci are among the leading causes of hospital acquired infections [6,7]. Hence, understanding the mechanisms enterococci use to colonize the GIT will likely suggest innovative new therapeutic strategies or targets with the potential to “decolonize” the GIT and prevent enterococcal infections. Additionally, given that enterococci are such successful GIT commensals, it seems likely that some of the colonization strategies used by enterococci may be shared with other commensals. Investigating enterococcal colonization may therefore reveal common mechanisms that shape assembly of the GIT microbiota. However, the genetic and molecular basis for GIT colonization by enterococci remains poorly understood. Most studies of GIT colonization to date have focused on the clinically relevant enterococci, Enterococcus faecalis (Ef) and Enterococcus faecium (Efm).
Three types of experimental mouse models have been used to investigate GIT colonization by enterococci. The first involves pre-treatment of mice with antibiotics to deplete their GIT microbiota and reduce colonization resistance, enabling robust enterococcal colonization of the GIT. This model thus mimics the phenomenon of antibiotic-induced enterococcal proliferation that facilitates pathogenesis in humans. The second strategy exploits germ-free animals for GIT colonization. Enterococci are among the first organisms to colonize the newborn’s GIT [8,9], so the use of germ-free animals may mimic colonization dynamics of the neonatal GIT. More recently, an experimental model that achieves colonization of the antibiotic-naïve GIT in conventional mice was developed [10], enabling investigation of the factors that promote colonization of the unperturbed GIT. Below we summarize insights into enterococcal GIT colonization that have emerged from studies using each of these models.
Nutritional adaptation
The genomes of enterococci lack the machinery for biosynthesis of several amino acids and vitamins [1,11]. Enterococci thus rely on acquisition of nutrients from their environment to outcompete other microbes. Comparative analysis of Efm clinical and commensal isolates revealed that a four-gene cluster, encoding a putative mannose/fructose/sorbose family phosphotransferase system (PTS), is enriched in clinical Efm isolates from human and veterinary infections [12], suggesting this PTS promotes colonization of the antibiotic-perturbed GIT. PTSs are specialized, substrate-specific transport systems that mediate carbohydrate uptake. Deletion of ptsD, which encodes the predicted transporter, impaired colonization of the GIT in antibiotic-perturbed mice [12]. Paradoxically, elimination of the ability of an Ef strain to use ethanolamine (an abundant nutrient in the GIT) enabled the ethanolamine-deficient mutant to outcompete an otherwise wild-type strain in the antibiotic-perturbed mouse GIT [13]. Although the underlying mechanisms remain to be elucidated, these studies highlight the importance of nutrient acquisition in colonization of the GIT.
Investigation of the enterococcal transcriptional response during GIT colonization revealed additional evidence of nutritional adaptation. Lindenstrauß et al. inoculated the GIT of germ-free mice with Ef and analyzed the enterococcal transcriptome by RNA-seq [14], revealing that the majority of induced genes are involved in nutrient transport or metabolism (e.g. multiple PTSs, and genes for glycerol metabolism). The results also revealed downregulation of the notable virulence proteins SprE (a serine protease) and the GelE metalloprotease. These findings suggest that, upon introduction to the sterile GIT, enterococci repress virulence gene expression and rely on import of nutrients present in this environment to persist.
To assess changes in Ef gene expression during colonization of the unperturbed GIT, a previously described Recombinase-based In Vitro Expression Technology (RIVET) system [15] was applied in the antibiotic-free colonization model [10]. The screen identified 114 Ef promoters that were induced in the GIT (unpublished data). Similar to the Lindenstrauß study [14], genes involved in nutrient transport and energy metabolism were the major functional categories identified as induced in the GIT.
Genome plasticity and antimicrobial production
Comparative genomics of Efm isolates from diverse sources revealed a phylogenetic split into 2 clades – a hospital-associated clade (clade A) and a community-associated clade (clade B), which exhibit substantial differences in the content of their genomes [16–18]. Clade B strains outcompete clade A strains when co-cultured in vitro and when competed with each other in an antibiotic-treated mouse GIT colonization model [19]. Although this study did not identify the specific gene(s) responsible, it is clear that differences in genome content can influence competitive fitness of enterococcal lineages to impact GIT colonization.
The presence of chromosomally integrated bacteriophages is variable among enterococcal isolates. Duerkop and coworkers [20] reported that a particular composite bacteriophage found in Ef V583 (a VRE isolate) confers a competitive advantage over Ef lacking the phage during colonization of the antibiotic-treated mouse GIT. Lysogens of an Ef strain that previously lacked the phage (resistant to phage infection) no longer exhibited a competitive defect, indicating that phage production in the GIT can impact the dynamics of enterococcal colonization in the GIT.
Naturally occurring plasmids contribute to the plasticity of enterococcal genomes and thus the evolution of hospital-adapted multidrug-resistant enterococci [21]. In addition, plasmids can influence GIT colonization fitness. For example, a study by Rice and colleagues [22] found that acquisition of a plasmid carrying a hyaluronidase gene enhances GIT colonization by Efm in an antibiotic-treated mouse model, and this trait was transferable to other Efm strains; however, the specific plasmid gene(s) conferring this phenotype were not identified. Gilmore and co-workers [23] reported inhibition of VRE by commensal enterococci in an ex vivo culture system through a mechanism in which commensal enterococci induced lethal cross-talk between mobile genetic elements resident in the VRE. These findings suggest that accretion of mobile elements (typically encoding antibiotic resistance) in multi-drug resistant enterococcal isolates render them incompatible with commensal enterococci, suggesting by extension that VRE occupy distinct niches in the GIT from commensal enterococci.
Similar to other microbial ecosystems, commensals in the GIT enhance their competitive fitness by producing antimicrobials to inhibit the growth of competing bacteria [24]. A subset of these antimicrobials are bacteriocins, often genetically encoded on conjugative plasmids. Analyzing colonization of the unperturbed mouse GIT, Kommineni et al. [10] observed that the bacteriocin-encoding plasmid pPD1 enhances the ability of Ef to colonize the GIT. Carriage of pPD1 allowed newly introduced Ef strains to displace preexisting enterococcal populations, including VRE. Importantly, the pPD1-mediated colonization advantage required the resident bacteriocin synthesis operon. Thus, this study demonstrated a role for naturally occurring plasmids in promoting GIT colonization, and provided evidence that plasmid-encoded bacteriocins could potentially be leveraged in therapeutic strategies intended to reduce the GIT burden of multidrug resistant enterococci [25].
Cell envelope integrity and antimicrobial resistance
A recent study by Lebreton and colleagues [26] tracing the evolution of the Enterococcus genus argues that the success of these organisms as GIT commensals is tightly linked to the evolution of a robust cell envelope. This study found that the origins of the Enterococcus genus coincided with the terrestrialization of their hosts, and that this transition required the development of a hardened cell envelope to facilitate survival under harsh physico-chemical conditions encountered during transmission from host to host on land. Given that many intestinally produced antimicrobial agents target the cell envelope, this hardened cell envelope also contributes to antimicrobial resistance, suggesting that maintenance of envelope integrity is a key determinant of GIT colonization. In E. faecalis, IreK, a transmembrane Ser/Thr kinase in the PASTA kinase protein family, is critical for cell envelope integrity and resistance towards antimicrobials that target the cell wall [27,28]. Deletion of ireK in Ef resulted in a profound GIT colonization defect in antibiotic-naïve mice [29], consistent with the hypothesis that maintenance of enterococcal cell envelope integrity is a key driver of efficient GIT colonization. However, resistance to individual intestinal antimicrobials is not, in itself, critical for GIT colonization, as Ef mutants singularly defective in resistance to either bile acids or lysozyme retained the ability to colonize [29].
The enterococcal polysaccharide antigen (Epa) is a rhamnose-containing cell-wall polysaccharide whose functions are not fully understood. Epa was shown to be important for cell shape, resistance to phage-induced lysis, biofilm formation and virulence in mice [30]. Although a core epa locus is conserved across enterococci, the full epa locus varies in organization and gene content among strains, suggesting biochemical variation of the polysaccharide [17]. Analysis of the genome content of a subset of virulent Ef clinical isolates revealed the enrichment of epaX (an “accessory” epa gene) in these hospital-associated Ef isolates. Deletion of epaX altered the composition of Epa polysaccharide, increased susceptibility to the bile acid cholate, and impaired GIT colonization in antibiotic-treated mice, suggesting that strain-specific biochemical features of Epa can influence colonization of the GIT [31].
Biofilm formation and physical interactions with the intestinal environment
Peristalsis poses a challenge to intestinal colonization. To overcome this challenge, one hypothesis proposes that commensals have developed mechanisms to adhere to the mucus layer [32,33]. The turnover rate of the mucus layer is slower than the peristalsis-driven transit time for intestinal contents, suggesting that mucus binding would be advantageous during intestinal colonization [34]. In addition, some researchers proposed that commensals associate with mucosal structures as part of biofilms [32,33,35]. Microscopic examination of intestinal sections from germ-free mice colonized with Ef revealed biofilm microcolonies throughout the GIT [36]. Ef microcolonies were found to abut the intestinal epithelial cells, indicating that these structures form at the base of the inner mucus layer. It is unclear if this phenomenon is specific to the monoassociation mouse model, or if it can be generalized to mice colonized with a complex GIT community. Others have reported the presence of bacteria adjacent to the epithelium at a limited number of sites along the GIT, although not specifically in the form of a biofilm [37,38]. Whether enterococcal microcolonies persist in the adult microbiota remains unknown; if so, these structures could represent a reservoir from which enterococci could continuously seed the GIT lumen. Formation of biofilms in the GIT by enterococci could also have important implications on the ability of these organisms to exchange genetic material and tolerate antibiotics in this environment.
Using antibiotic-treated mouse models, several studies have identified genes that influence enterococcal biofilm formation in vitro as contributors to GIT colonization. Deletion of ebrB, which encodes an AraC family transcriptional regulator required for biofilm formation, results in a modest decrease in GIT colonization by Efm [39]. EbrB was also found to be required for expression of Esp, a cell wall anchored protein required for biofilm formation. Previous studies had indicated that Esp, although enriched among clinical Ef and Efm isolates, does not influence intestinal colonization by either organism [40,41], suggesting that the contributions of EbrB to GIT colonization is independent of its regulation of Esp.
Disruption of the bop (biofilm on plastic) locus, which contains putative maltose metabolism genes, results in a moderate delay in GIT colonization by Ef [42]. bop genes influence biofilm formation, but their specific effect depends on growth conditions. Mutants lacking the bopABC genes form more biofilms than wild type cells in the presence of glucose, while they are unable to produce biofilms in the presence of maltose. Although the reason for these opposing phenotypes remains unclear, this study suggests that the ability to form biofilms can influence GIT colonization by enterococci, and by extension that the nature of available nutrients may impact biofilm-mediated colonization.
Sortase A (SrtA) is a membrane-associated enzyme that mediates anchoring of surface proteins to the enterococcal cell wall [43] and promotes biofilm formation [44,45]. Inspection of the genome of Ef OG1RF revealed that it encodes 21 predicted sortase-dependent cell wall-anchored proteins, including cell-surface adhesins such as Ace (mediates binding to extracellular matrix proteins [46]) and the Ebp pilus (a polymeric structure previously shown to mediate biofilm formation and adhesion to various host extracellular matrix proteins [47,48]). Hypothesizing that SrtA-dependent surface proteins could be important for binding to host molecules in the GIT, we found that an Ef mutant lacking SrtA was defective in adherence to mucin in vitro, and this defect could be traced specifically to loss of a combination of Ace and Ebp (unpublished data). Using the antibiotic-naïve mouse GIT colonization model, we found that enterococcal mutants (both Ef and Efm) lacking sortase were impaired at GIT colonization as well (unpublished data). Together, these data suggest that enterococci use their surface proteins to facilitate retention in the mucus layer during GIT colonization.
Microbiota and bacterial competition
The proliferation of GIT enterococci that occurs during antibiotic therapy reflects the elimination of microbial components that regulate the enterococcal population, either directly or through their interaction with the host mucosal immune system [5,49–51]. The longstanding view is that enterococci proliferate because of increased availability of nutrients and physical niche(s) in the context of a depleted microbiota. Recent research has provided a more detailed understanding of this phenomenon. In hospitalized patients placed on antibiotic therapy, colonization with bacteria belonging to the Barnesiella genus has a protective effect against domination by VRE [52]. Detailed analysis of microbial taxa associated with elimination of VRE in mice revealed that cooperative relationships between Clostridum bolteae and Blautia producta drive clearance of VRE [53]. The presence of C. bolteae promotes colonization of B. producta, which directly suppresses the proliferation of VRE, although the specific mechanism by which this occurs is unclear. This data indicates that enterococcal populations in the GIT can be directly modulated by the presence of other members of the microbiota, although the extent to which this phenomenon can be generalized across commensal enterococcal lineages, and across hosts with diverse GIT communities, remains unknown.
Conclusion
Although much work remains to truly understand the mechanisms by which enterococci establish and maintain colonization of the mammalian GIT, the initial studies summarized here represent important initial steps towards that goal. These findings begin to paint an emerging picture of what is likely to be a sophisticated, multifactorial strategy employed by enterococci to ensure they maintain a foothold in the highly competitive environment of the GIT. Already we have evidence that enterococcal cell surface proteins, maintenance of cell envelope integrity, adaptation to available nutrient sources, and potentially formation of biofilms in vivo play key roles in promoting GIT colonization. Most of these functions are mediated largely by genes encoded in the core enterococcal genome, which is consistent with the concept that enterococci have evolved for millennia as GIT commensals, and remain ubiquitous GIT colonizers today. Hence, future investigations into the roles of core genes during GIT colonization are likely to be informative as we seek to understand the forces that shape enterococcal-microbiota-host interactions and dynamics. The role of genome plasticity as an important driver of GIT colonization should not be overlooked, however. Already we have evidence indicating that, for example, acquisition of accessory genes can alter the Epa surface polysaccharide with consequences for GIT colonization, and that various types of mobile genetic elements can also impact colonization. It is possible that such elements in the accessory genome are especially important for colonization in the dysbiotic setting of the antibiotic-treated GIT, although more comparative studies in antibiotic-naïve mice are necessary to definitively establish such a conclusion. Lastly, acquisition of the capacity to produce bacteriocins can provide a substantial colonization advantage sufficient to displace established enterococcal lineages from the GIT, a significant observation with obvious potential to be developed into new therapeutic strategies with the goal of preventing infections by antibiotic-resistant enterococci in the future.
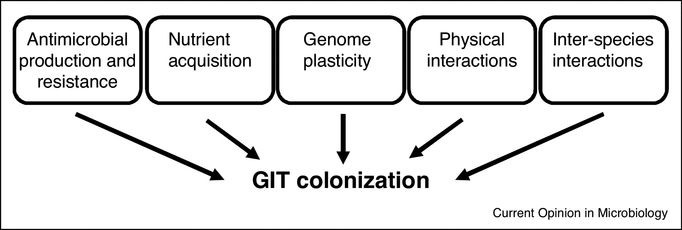
Figure 1. Multifactorial strategy for enterococcal GIT colonization.
Multiple traits encoded by both the core enterococcal genome as well as mobile genetic elements together influence GIT colonization.
Highlights.
Enterococci employ a multifactorial strategy to ensure efficient colonization
Genes in the core enterococcal genome are key drivers of GIT colonization
Genome plasticity may enhance GIT colonization in dysbiotic environments
Intra- and inter-species interactions influence enterococcal GIT colonization
Bacteriocin production is a powerful means to shape enterococcal gut communities
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers AI128219, AI121552, AI132927, GM122503, and GM099526]. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Lebreton F, Willems RJL, Gilmore MS: Enterococcus Diversity, Origins in Nature, and Gut Colonization In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection, edn 2014/03/21. Edited by Gilmore MS, Clewell DB, Ike Y, Shankar N: [Internet]. Massachusetts Eye and Ear Infirmary; 2014. [PubMed] [Google Scholar]
- 2.Martin JD, Mundt JO: Enterococci in insects. Appl Microbiol 1972, 24:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundt JO: Occurrence of enterococci in animals in a wild environment. Appl Microbiol 1963, 11:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. **.Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, HuttonThomas RA, Whalen CC, Bonomo RA, Rice LB: Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000, 343:1925–1932. Seminal study that documented the proliferation of enterococci in the human GIT in response to therapy with antibiotics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. *.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, et al. : Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 2010, 120:4332–4341. Important study documenting the correlation of enterococcal domination of the GIT microbial community with subsequent emergence of enterococcal infection in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmore MS, Lebreton F, van Schaik W: Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr Opin Microbiol 2013, 16:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arias CA, Murray BE: The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012, 10:266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanaro S, Chierici R, Guerrini P, Vigi V: Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 2003, 91:48–55. [DOI] [PubMed] [Google Scholar]
- 9.Orrhage K, Nord CE: Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr Suppl 1999, 88:47–57. [DOI] [PubMed] [Google Scholar]
- 10. **.Kommineni S, Bretl DJ, Lam V, Chakraborty R, Hayward M, Simpson P, Cao Y, Bousounis P, Kristich CJ, Salzman NH: Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 2015, 526:719–722. First description of a mouse model system enabling stable, long-term enterococcal colonization of the unperturbed GIT, and use of the model to demonstrate that bacteriocin production can provide a sufficiently strong competitive advantage in the GIT to eliminate established VRE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niven CF, Sherman JM: Nutrition of the Enterococci. J Bacteriol 1944, 47:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Top J, de Been M, Bierschenk D, Rogers M, Leendertse M, Bonten MJ, van der Poll T, Willems RJ, van Schaik W: Identification of a genetic determinant in clinical Enterococcus faecium strains that contributes to intestinal colonization during antibiotic treatment. J Infect Dis 2013, 207:1780–1786. [DOI] [PubMed] [Google Scholar]
- 13.Kaval KG, Singh KV, Cruz MR, DebRoy S, Winkler WC, Murray BE, Garsin DA: Loss of Ethanolamine Utilization in Enterococcus faecalis Increases Gastrointestinal Tract Colonization. MBio 2018, 9:pii: e00790–00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindenstrauss AG, Ehrmann MA, Behr J, Landstorfer R, Haller D, Sartor RB, Vogel RF: Transcriptome analysis of Enterococcus faecalis toward its adaption to surviving in the mouse intestinal tract. Arch Microbiol 2014, 196:423–433. [DOI] [PubMed] [Google Scholar]
- 15.Ballering KS, Kristich CJ, Grindle SM, Oromendia A, Beattie DT, Dunny GM: Functional genomics of Enterococcus faecalis: multiple novel genetic determinants for biofilm formation in the core genome. J Bacteriol 2009, 191:2806–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebreton F, van Schaik W, McGuire AM, Godfrey P, Griggs A, Mazumdar V, Corander J, Cheng L, Saif S, Young S, et al. : Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio 2013, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer KL, Godfrey P, Griggs A, Kos VN, Zucker J, Desjardins C, Cerqueira G, Gevers D, Walker S, Wortman J, et al. : Comparative genomics of enterococci: variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. MBio 2012, 3:e00318–00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galloway-Pena J, Roh JH, Latorre M, Qin X, Murray BE: Genomic and SNP analyses demonstrate a distant separation of the hospital and community-associated clades of Enterococcus faecium. PLoS One 2012, 7:e30187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montealegre MC, Singh KV, Murray BE: Gastrointestinal Tract Colonization Dynamics by Different Enterococcus faecium Clades. J Infect Dis 2016, 213:1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. *.Duerkop BA, Clements CV, Rollins D, Rodrigues JL, Hooper LV: A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc Natl Acad Sci U S A 2012, 109:17621–17626. This study described a role for bacteriophages in enterococcal competition in the germfree mouse GIT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer KL, Kos VN, Gilmore MS: Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr Opin Microbiol 2010, 13:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rice LB, Lakticova V, Carias LL, Rudin S, Hutton R, Marshall SH: Transferable capacity for gastrointestinal colonization in Enterococcus faecium in a mouse model. J Infect Dis 2009, 199:342–349. [DOI] [PubMed] [Google Scholar]
- 23.Gilmore MS, Rauch M, Ramsey MM, Himes PR, Varahan S, Manson JM, Lebreton F, Hancock LE: Pheromone killing of multidrug-resistant Enterococcus faecalis V583 by native commensal strains. Proc Natl Acad Sci U S A 2015, 112:7273–7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbing ME, Fuqua C, Parsek MR, Peterson SB: Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 2010, 8:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kommineni S, Kristich CJ, Salzman NH: Harnessing bacteriocin biology as targeted therapy in the GI tract. Gut Microbes 2016, 7:512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. **.Lebreton F, Manson AL, Saavedra JT, Straub TJ, Earl AM, Gilmore MS: Tracing the Enterococci from Paleozoic Origins to the Hospital. Cell 2017, 169:849–861 e813. Insightful study examining the evolutionary history of the genus Enterococcus and providing plausible explanations for the emergence of the phenotypic traits that enable robust GIT colonization by enterococci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristich CJ, Wells CL, Dunny GM: A eukaryotic-type Ser/Thr kinase in Enterococcus faecalis mediates antimicrobial resistance and intestinal persistence. Proc Natl Acad Sci U S A 2007, 104:3508–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristich CJ, Little JL, Hall CL, Hoff JS: Reciprocal regulation of cephalosporin resistance in Enterococcus faecalis. MBio 2011, 2:e00199–00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banla IL, Kommineni S, Hayward M, Rodrigues M, Palmer KL, Salzman NH, Kristich CJ: Modulators of Enterococcus faecalis Cell Envelope Integrity and Antimicrobial Resistance Influence Stable Colonization of the Mammalian Gastrointestinal Tract. Infect Immun 2018, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng F, Singh KV, Bourgogne A, Zeng J, Murray BE: Further characterization of the epa gene cluster and Epa polysaccharides of Enterococcus faecalis. Infect Immun 2009, 77:3759–37S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rigottier-Gois L, Madec C, Navickas A, Matos RC, Akary-Lepage E, Mistou MY, Serror P: The surface rhamnopolysaccharide epa of Enterococcus faecalis is a key determinant of intestinal colonization. J Infect Dis 2015, 211:62–71. [DOI] [PubMed] [Google Scholar]
- 32.Smith HF, Fisher RE, Everett ML, Thomas AD, Bollinger RR, Parker W: Comparative anatomy and phylogenetic distribution of the mammalian cecal appendix. J Evol Biol 2009, 22:1984–1999. [DOI] [PubMed] [Google Scholar]
- 33.Sonnenburg JL, Angenent LT, Gordon JI: Getting a grip on things: how do communities of bacterial symbionts become established in our intestine? Nat Immunol 2004, 5:569–573. [DOI] [PubMed] [Google Scholar]
- 34.Lebeer S, Vanderleyden J, De Keersmaecker SC: Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 2008, 72:728–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bollinger RR, Barbas AS, Bush EL, Lin SS, Parker W: Biofilms in the normal human large bowel: fact rather than fiction. Gut 2007, 56:1481–1482. [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes AMT, Dale JL, Chen Y, Manias DA, Greenwood Quaintance KE, Karau MK, Kashyap PC, Patel R, Wells CL, Dunny GM: Enterococcus faecalis readily colonizes the entire gastrointestinal tract and forms biofilms in a germ-free mouse model. Virulence 2017, 8:282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekirov I, Russell SL, Antunes LC, Finlay BB: Gut microbiota in health and disease. Physiol Rev 2010, 90:859–904. [DOI] [PubMed] [Google Scholar]
- 38.Swidsinski A, Loening-Baucke V, Lochs H, Hale LP: Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol 2005, 11:1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Top J, Paganelli FL, Zhang X, van Schaik W, Leavis HL, van Luit-Asbroek M, van der Poll T, Leendertse M, Bonten MJ, Willems RJ: The Enterococcus faecium enterococcal biofilm regulator, EbrB, regulates the esp operon and is implicated in biofilm formation and intestinal colonization. PLoS One 2013, 8:e65224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pultz NJ, Shankar N, Baghdayan AS, Donskey CJ: Enterococcal surface protein Esp does not facilitate intestinal colonization or translocation of Enterococcus faecalis in clindamycin-treated mice. FEMS Microbiol Lett 2005, 242:217–219. [DOI] [PubMed] [Google Scholar]
- 41.Heikens E, Leendertse M, Wijnands LM, van Luit-Asbroek M, Bonten MJ, van der Poll T, Willems RJ: Enterococcal surface protein Esp is not essential for cell adhesion and intestinal colonization of Enterococcus faecium in mice. BMC Microbiol 2009, 9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Creti R, Koch S, Fabretti F, Baldassarri L, Huebner J: Enterococcal colonization of the gastro-intestinal tract: role of biofilm and environmental oligosaccharides. BMC Microbiol 2006, 6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazmanian SK, Liu G, Ton-That H, Schneewind O: Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 1999, 285:760–763. [DOI] [PubMed] [Google Scholar]
- 44.Guiton PS, Hung CS, Kline KA, Roth R, Kau AL, Hayes E, Heuser J, Dodson KW, Caparon MG, Hultgren SJ: Contribution of autolysin and Sortase A during Enterococcus faecalis DNA-dependent biofilm development. Infect Immun 2009, 77:3626–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kristich CJ, Nguyen VT, Le T, Barnes AM, Grindle S, Dunny GM: Development and use of an efficient system for random mariner transposon mutagenesis to identify novel genetic determinants of biofilm formation in the core Enterococcus faecalis genome. Appl Environ Microbiol 2008, 74:3377–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nallapareddy SR, Qin X, Weinstock GM, Hook M, Murray BE: Enterococcus faecalis adhesin, ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect Immun 2000, 68:5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nallapareddy SR, Singh KV, Sillanpaa J, Zhao M, Murray BE: Relative contributions of Ebp Pili and the collagen adhesin ace to host extracellular matrix protein adherence and experimental urinary tract infection by Enterococcus faecalis OG1RF. Infect Immun 2011, 79:2901–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nallapareddy SR, Singh KV, Sillanpaa J, Garsin DA, Hook M, Erlandsen SL, Murray BE: Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest 2006, 116:2799–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. *.Abt MC, Buffie CG, Susac B, Becattini S, Carter RA, Leiner I, Keith JW, Artis D, Osborne LC, Pamer EG: TLR-7 activation enhances IL-22-mediated colonization resistance against vancomycin-resistant Enterococcus. Sci Transl Med 2016, 8:327ra325. Using a mouse model, this study revealed the potential to restore colonization resistance against VRE to an antibiotic-treated GIT ecosystem by stimulating innate antiviral immune signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG: Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature 2008, 455:804–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG: Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis 2010, 201:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ubeda C, Bucci V, Caballero S, Djukovic A, Toussaint NC, Equinda M, Lipuma L, Ling L, Gobourne A, No D, et al. : Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect Immun 2013, 81:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. *.Caballero S, Kim S, Carter RA, Leiner IM, Susac B, Miller L, Kim GJ, Ling L, Pamer EG: Cooperating Commensals Restore Colonization Resistance to Vancomycin-Resistant Enterococcus faecium. Cell Host Microbe 2017, 21:592–602 e594. This study defined a specific microbial consortium with the ability to restore colonization resistance and clear VRE from the intestines of mice. [DOI] [PMC free article] [PubMed] [Google Scholar]