Abstract
Introduction:
Our primary goal was to examine demographic and clinicopathologic differences across an ethnoracially diverse autopsy-confirmed cohort of Alzheimer’s disease cases.
Methods:
A retrospective study was conducted in the Florida Autopsied Multi-Ethnic cohort on 1625 Alzheimer’s disease cases, including decedents who self-reported as Hispanic/Latino (n = 67), black/African American (n = 19), and white/European American (n = 1539).
Results:
Hispanic decedents had a higher frequency of family history of cognitive impairment (58%), an earlier age at onset (median age of 70 years), longer disease duration (median of 12 years), and lower MMSE proximal to death (median of 4 points) compared with the other ethnoracial groups. Black decedents had a lower Braak tangle stage (stage V) and higher frequency of coexisting hippocampal sclerosis (21%); however, only hippocampal sclerosis differences survived adjustment for sex, age at onset, and disease duration. Neither Thal amyloid phase nor coexisting Lewy body disease differed across ethnoracial groups.
Discussion:
Despite a smaller sample size, Hispanics demonstrated longer disease duration with Alzheimer’s disease, but not greater lifespan. Neuropathologic differences across ethnoracial groups supported differences in tau pathology distribution and coexisting hippocampal sclerosis, which may impact biomarker studies.
Keywords: Alzheimer disease, African American, Hispanic, Survival, Brain, Autopsy, Ethnoracial
1. Background
In the next few decades, the United States population will become proportionally older and more ethnoracially diverse, contributing to a projected increase in the prevalence of dementia. By 2030, approximately one in five Americans will be over the age of 65 years and, by 2060, Hispanic Americans and black/African Americans are projected to constitute 29% and 14% of the population, respectively [1]. The prevalence of dementia is estimated to more than double by 2050 [2]. Despite these trends, our understanding of dementia across ethnoracial groups remains limited and represents an important topic of investigation [3–5].
Race/ethnicity is often used as a proxy for interrelated psychosociocultural characteristics that may be associated with dementia risk [5]. Compared with the risk for older white individuals in the United States, the current risk for developing Alzheimer’s disease (AD) and other dementias among older black individuals is twice as high and among older Hispanic individuals is one and one-half times higher [2]. Hispanic Americans constitute a heterogeneous population based on their country of origin, race, ethnicity, immigration patterns, and consequently their rates of dementia also vary [4]. For instance, the estimated overall dementia prevalence among Mexican Americans is 4.8% for those ≥60 years old and 31% for those ≥85 years old [6]; among Puerto Rican veterans, those estimates are 13% for those ≥65 years old [7]; among a predominantly Dominican sample of Caribbean Hispanics, the prevalence estimates are 7.5% for adults 65 to 74 years old, 28% for adults 75 to 84 years old, and 63% for adults ≥85 years old [8]; and among Cuban Americans, the estimates are 13% for women ≥65 years old. Moreover, the estimated annual incidence of dementia among Hispanic Caribbean adults (3.6%) is higher than the estimate for Mexican American adults (0.8%) [4]. In sum, the epidemiologic data depict variable rates of dementia prevalence and incidence among Hispanic Americans, with higher rates in Caribbean Hispanics than Mexican Americans [4].
The higher incidence rate of AD among black and Hispanic individuals to age 90 years was not observed to change after adjusting for common demographics or cardiovascular conditions [3]. This may be of particular importance given that cardiovascular and sociodemographic risk factors for AD and other dementias are more prevalent in Hispanic individuals and black individuals than in white individuals [3,9]. Interestingly, Hispanic individuals have been found to survive longer with AD [10] and have lower mortality risk estimates from any cause [11] relative to black individuals and white individuals. This phenomenon, known as the “Hispanic mortality paradox” [11,12], has received limited neurobiologic investigation due to the scarcity of autopsy-confirmed study cohorts containing minorities. Autopsy-based neuropathologic diagnoses are not only fundamental to AD research, but are also important in historically underserved populations where clinical and social factors may track differently than among traditionally studied populations. Moreover, this information may also apply to or be compared with other underserved populations worldwide [5].
Among the sociodemographic factors important to AD research, both educational and occupational attainments have been used as proxies for cognitive reserve [13]. Education, in particular, is believed to be neuroprotective, as it appears to alleviate the impact of pathology on the clinical manifestation of dementia before death [14]. In addition, older age, family history of AD, and APOE ε4 are the greatest risk factors for late-onset AD [15–17]. Specifically, APOE ε4 is the strongest genetic risk factor for more common late-onset forms of AD [15]. Hispanic individuals have been found to have a lower frequency of APOE ε4 relative to black individuals and white individuals [6,16,18]. Also, compared with white individuals, Hispanic individuals and black individuals have been found to have an increased frequency of AD regardless of APOE status [16,19], and similar cumulative risk of AD to age 90 years in APOE ε4 carriers [20].
The extent to which neuropathologic and genetic factors contribute to disparities in neurocognitive deficits among ethnoracial groups remains poorly understood. Thus, we investigated clinical, genetic, and neuropathologic differences in AD across three ethnoracial groups from the FLorida Autopsied Multi-Ethnic (FLAME) study. Our primary goal was to assess demographics, APOE genotype, clinical progression, and neuropathologic differences or similarities in the context of autopsy-confirmed AD. Our secondary goal was to determine demographic and frequency differences across a wide range of neurodegenerative diseases in the overall FLAME cohort.
2. Methods
2.1. Study samples
The FLAME cohort is derived from the State of Florida brain bank housed at the Mayo Clinic Florida. Participating Memory Disorder Clinics in the State of Florida’s Alzheimer’s Disease Initiative offer to register individuals for autopsy regardless of sex, race, or ethnicity. Other referrals may include educational talks to the community by Memory Disorder Center staff and family members of the brain bank participants. The major requirement is that a documented neurologic or psychiatric workup for cognitive disorders be available. Participating centers include West Florida Regional Medical Center, Tallahassee Memorial, Mayo Clinic Jacksonville, University of Florida, Orlando Health Center for Aging, Florida Hospital Orlando, East Central Florida, Morton Plant, University of South Florida, St. Mary’s Medical Center, Florida Atlantic University, Sarasota Memorial, Lee Memorial, Broward Health North, University of Miami, and Mount Sinai Medical Center (http://elderaffairs.state.fl.us/doea/alz/clinicmap.pdf ). All individuals in this study have come to autopsy and are thus referred to as decedents. The FLAME cohort consists of individuals self-identifying as Hispanic/Latino, black/African, and non-Hispanic white/European; hereafter referred to as Hispanic decedents, black decedents, and white decedents, respectively. The overall FLAME cohort consists of a total of n = 2809 autopsied individuals whose brain tissue was received on or before August 2015, with a wide range of neurodegenerative diseases (Supplementary Table 1). The overall cohort was queried for autopsy-confirmed AD cases regardless of clinical diagnosis. After exclusion of non-AD cases (n = 1166) and AD cases with known mutations (n = 18), we identified n = 1625/2809 (875 females and 750 males) individuals neuropathologically diagnosed as AD. All brains were acquired with informed consent, and procedures were conducted according to the approved Institutional Review Board protocol (IRB# 16–003061).
2.2. Clinicopathologic procedures
All cases underwent standard neuroanatomic sampling by a single neuropathologist (DWD), using optimized procedures developed by Terry et al. [21]. Briefly, the fixed hemibrain (typically left hemisphere) is weighed and doubled to obtain brain weight in grams. At the time of brain cutting, the infratentorial structures (brainstem and cerebellum) are first removed at the level of the midbrain and mammillary body. The cerebellar vermis is sampled with subsequent 1 cm thick transverse sections made through the midbrain, pons, medulla, and spinal cord when available. Cortical sections (frontal, temporal, parietal, motor, and visual) are next sampled perpendicular to the gyrus to ensure uniform laminar structure of the cortical ribbon. The supratentorial tissue is then cut at approximately 1 cm thick sections. To optimize sampling of the nucleus basalis of Meynert, the Dickson sampling scheme uses an oblique coronal plane defined by the anterior commissure, infundibulum, and uncus. Coronal sections are then slabbed in both the anterior and posterior extent. Subcortical regions are subsequently sampled, including amygdala (with basal ganglia), ventral/dorsal striatum, hippocampus (anterior and posterior), and thalamus (with subthalamic nucleus). After sampling, tissue cassettes are placed in 10% formalin solution before embedding.
AD neuropathologic change was assessed using thioflavin-S microscopy, including Braak tangle stage [22] and Thal amyloid phase [23], as previously described [24]. TAR DNA binding protein 43 (TDP-43) immunohistochemistry was performed using MC2085 (1:2500), which is a rabbit antibody that recognizes amino acids 220–227 in the 25-kDa C-terminal fragment [25]. Hippocampal sclerosis of a TDP-43 etiology was assigned to cases with hippocampal neuronal loss disproportionate to the severity of neurofibrillary tangle pathology [26]. Coexisting Lewy body disease was assessed using the rabbit antibody NACP (1:3000), which recognizes amino acids 98–115 with a cysteine residue at its C-terminus [27]. Lewy body disease subtypes were classified based on neuroanatomical distribution [28]. Genetic screening of APOE was available for n = 1208/1625 (74%) of the AD cohort.
Demographic and clinical data were abstracted from available clinical history notes for the AD cohort, including self-reported sex and ethnoracial status that was available for all individuals. Years of education was available for n = 952/1625(59%) of all decedents. Job-level score was categorized based on an individual’s highest occupation according to the United States Department of Labor occupation, as previously described [29] (see Supplementary Table 2, http://www.govtusa.com/dot/ ). Job-level score was available for n = 897/1625 (55%) of all decedents. Family history was based on self-reported presence or absence of apparent or diagnosed cognitive problems in any of the patient’s family members and was available for n = 1500/1625 (92%) of all decedents. Age at symptom onset consisted of the initial patient’s and/or caregiver’s complaint of cognitive dysfunction and was available for n = 1152/1625 (71%) of all decedents. Disease duration represented the time interval between age at symptom onset and death. Test date and score were recorded for every Mini-Mental State Examination (MMSE) [30]. At least one MMSE was performed in n = 724/1625 (45%) of all decedents, and a final MMSE performed within three years of death was available for n = 309/1625 (19%). Rate of cognitive decline was evaluated using three or more MMSE test dates relative to the date of death, and calculated as points lost per year [24]; this was available for n = 297/1625 (18%). Individuals who reported that English was not their first language were given the option at each MMSE test date to take the English or Spanish version of the test. Specific data availability by ethnoracial group has been summarized in Supplementary Table 3. Country of origin was reviewed for Hispanic decedents (available in 52/67 [78%]) and black decedents (available in all 19) who were neuropathologically diagnosed with AD. Among Hispanic decedents whose information was available, most were of Caribbean origin (46/52 [88%]), primarily from Cuba (34/52 [65%]) and Puerto Rico (9/52 [17%]), whereas the minority were of Mexican, Central or South American origin (6/52 [12%]). All black decedents identified as having been born in the United States with the exception of one individual born in Guyana and another born in Jamaica. Primary language was not available for either of these cases; however, all other black decedents identified English as their primary language. Supplementary Table 4 contains breakdown of self-reported language and country of origin for Hispanic decedents.
2.3. Statistical analyses
All analyses were conducted using SAS 9.4 Version (Statistical Analysis Software, Cary, NC) using a P value of, 0.05 for significance. Associations of categorical variables were assessed using Fisher’s Exact Test. Continuous variables were summarized with medians and interquartile ranges and compared between ethnoracial groups using the Kruskal-Wallis test. Ethnoracial differences in brain weight, Braak tangle stage, coexisting hippocampal sclerosis of a TDP-43 etiology, and final MMSE score were further examined using regression analyses. The final MMSE score was adjusted for education, sex, age at onset, and disease duration. The brain weight, Braak tangle stage, and hippocampal sclerosis regression analyses were adjusted for sex, age at onset, and disease duration. Linear regression was used for analysis of MMSE and brain weight, proportional odds regression for Braak stage, and logistic regression for HpScl.
3. Results
A summary of the demographic, clinical, and neuropathologic findings in the overall FLAME cohort stratified by self-reported ethnoracial status is found in Supplementary Table 1. Of the total n = 2809 overall autopsied cohort, there were n = 118 (4%) Hispanic decedents (ages 39–101), n = 36 (1%) black decedents (ages 36–97), and n = 2655 (95%) white decedents (ages 36–104). Findings in the AD cohort stratified by ethnoracial status are summarized in Table 1. Of the total n = 1625 AD cases, there were n = 67 (4%) Hispanic decedents (ages 58–93), n = 19 (1%) black decedents (ages 60–97), and n = 1539 (95%) white decedents (ages 53–102). We did not observe sex differences across ethnoracial groups (P = .865) in this autopsy-confirmed AD cohort. Differences in years of education were marginally significant (P =.055), with Hispanic decedents (median = 13 years) and black decedents (14 years) observed to have the lowest years of education compared with white decedents (14 years). Highest job level score attained did not differ across ethnoracial groups (P =.898). The frequency of APOE ε4 carriers did not differ among the groups (P = .118). Family history of cognitive problems differed (P = .003), with Hispanic decedents observed to have a higher frequency (58%) of family members with cognitive problems or dementia compared with white decedents (37%) and black decedents (29%).
Table 1.
Clinicopathologic differences identified among Alzheimer’s disease cases stratified by ethnoracial group
| Characteristics | Hispanic decedents (n = 67) | Black decedents (n = 19) | White decedents (n = 1539) | P value |
|---|---|---|---|---|
| Demographic differences | ||||
| Females | 36/67 (54%) | 9/19 (47%) | 830/1539 (54%) | .865† |
| Education, years | 13 (12, 16) | 14 (12, 16) | 14 (12, 16) | .055 |
| Job level score | 4 (3, 6) | 3 (3,6) | 4 (3, 6) | .898 |
| Apolipoprotein E ε4 | 32/62 (52%) | 10/14 (71%) | 727/1132 (64%) | .118† |
| Family history | 38/66 (58%) | 5/17 (29%) | 522/1417 (37%) | .003† |
| Clinical differences | ||||
| Age onset, years. | 70 (62, 75) | 71 (68, 75) | 72 (66, 78) | .047 |
| Disease duration, years | 12 (8, 14) | 8 (6, 10) | 9 (6, 12) | <.001 |
| Cognitive decline, pts/year | −1.8 (−4.1, −0.50) | −3.6 (−4.8, 0.50) | −1.6 (−3.8, −0.20) | .715 |
| MMSE final score, points* | 4.0 (2.0, 7.0) | 10 (5.0, 16) | 14 (7.0, 19) | <.001 |
| Neuropathologic differences | ||||
| Age at death, years | 82 (76, 86) | 78 (73, 82) | 82 (76, 87) | .217 |
| Brain weight, g. | 960 (880, 1080) | 940 (860, 1200) | 1040 (940, 1140) | .002 |
| Thal amyloid phase | 5 (5, 5) | 5 (5, 5) | 5 (5, 5) | .450 |
| Braak tangle stage | VI (V, VI) | V (V, VI) | VI (V, VI) | .015 |
| Lewy body disease | 17/67 (25%) | 4/19 (21%) | 373/1539 (24%) | .976† |
| Hippocampal sclerosis | 8/67 (12%) | 4/19 (21%) | 110/1539 (7%) | .032† |
NOTE: Ethnoracial group is self-reported.
NOTE: Data availability summarized in Supplementary Table 3.
Abbreviation: MMSE, Mini-Mental State Examination.
Last MMSE score was tested within three years of death (time from test date to death did not differ).
Unless noted, data are presented as median (25th, 75th).
Significance tested using Kruskal-Wallis One-Way Analysis of Variance on Ranks
Fisher Exact Test where indicated.
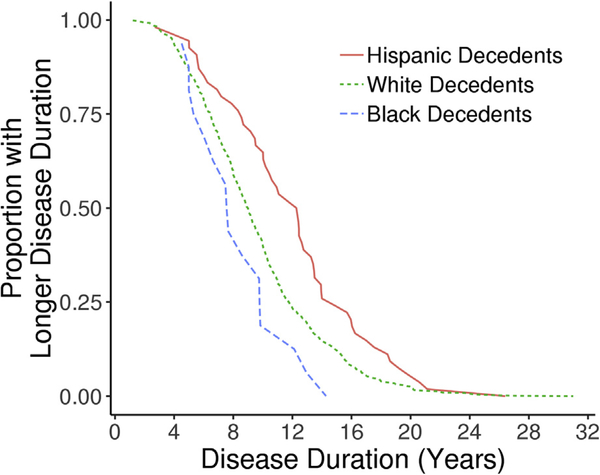
Age at symptom onset differed across ethnoracial groups (P =.047), with Hispanic decedents (70 years) and black decedents (71 years) observed to report cognitive complaints at an earlier age compared with white decedents (72 years). Disease duration differed across groups (P = .0004), with Hispanic decedents (12 years) observed to survive longer from onset to death compared with white decedents (9 years), who in turn survived longer than black decedents (8 years). Fig. 1 graphically displays the cumulative probability of longer disease duration at each time point. Extrapolation from a disease duration of 10 years demonstrates that only 22% of black decedents and 37% of white decedents had survived, whereas 67% of Hispanic decedents survived. Although longitudinal cognitive decline (i.e., points lost on the MMSE per year) did not differ across ethnoracial groups (P = .715), the MMSE score proximal to death differed across groups (P ≤.0001). Lower MMSE scores were observed in Hispanic decedents (4 points) followed by black decedents (10 points) relative to white decedents (14 points). A multiple linear regression model was built to examine whether ethnoracial differences in the MMSE final score remained after adjusting for education, age at onset, sex, and disease duration (Supplementary Table 5). As expected based on a lack of groupwise difference, the MMSE final score did not differ between black and white decedents (estimate = −2.5, 95% confidence interval [CI] =−7.1– 2.1, P = .290); however, the MMSE final score remained lower in Hispanic decedents compared with white decedents by 7 points (estimate = −7.1, 95% CI = −11 to −3.4, P =.0002).
Fig. 1.
Across the autopsied Alzheimer’s disease cases, the distributions of disease durations were examined by ethnoracial group. The x-axis reflects disease duration from age at onset of cognitive symptoms to the age at death. For each disease duration time on the x-axis, the y-axis was calculated as the proportion of disease durations that were greater than that time. For example, it shows that only 16% of black decedents and 40% of white decedents had a disease duration of 10 years or greater, whereas 66% of Hispanic decedents had a disease duration of 10 years or greater.
Age at death did not differ across groups (P = .217). Brain weight differed (P =.002), with lower values observed in black decedents (940 g) and Hispanic decedents (960 g) compared with white decedents (1040 g). Thal amyloid phase did not differ across groups (P =.450). Braak tangle stage differed (P = .015), with black decedents observed to have a lower Braak tangle stage (stage V) relative to the Hispanic decedents (stage VI) and white decedents (stage VI). Coexisting Lewy body disease did not differ across groups (P =.976). Coexisting hippocampal sclerosis of a TDP-43 etiology differed (P = .032), with black decedents found to have the highest frequency (21%) followed by Hispanic decedents (12%), with the lowest frequency observed in white decedents (7%).
The three neuropathologic variables found to significantly differ across ethnoracial groups (brain weight, Braak tangle stage, and coexisting hippocampal sclerosis) were further evaluated using regression modeling (Table 2). When compared with white decedents, Hispanic decedents were found to have a 61 gram lower brain weight (estimate = −61, CI = −98 to −24, P = .001); however, black decedents were not found to differ (estimate = −50, CI = −120–18, P = .15). The lower brain weight observed in Hispanic decedents did not survive adjustment of sex, age at onset, or disease duration (estimate = −25, CI = −57–6.3, P = .12). When compared with white decedents, Hispanic decedents were two times as likely to have a higher Braak tangle stage (odds ratio [OR] = 2.0, 95% CI = 1.2–3.4, P = .005); however, black decedents were not found to differ (OR = 0.7, CI = 0.3–1.7, P = .400). The higher Braak tangle stage observed in Hispanic decedents did not survive adjustment of sex, age at onset, or disease duration (OR = 1.3, CI = 0.7–2.3, P = .400). When compared with white decedents, black decedents were 3.5 times as likely to have coexisting hippocampal sclerosis of a TDP-43 etiology (OR = 3.5, CI = 1.0–9.7, P =.054), but Hispanic decedents were not found to differ (OR = 1.8, CI = 0.8–3.6, P = .180). The likelihood of observing coexisting hippocampal sclerosis in black decedents was even higher after adjusting for sex, age at onset, and disease duration (OR = 5.4, CI = 1.4–17, P = .016).
Table 2.
Multiple linear regression modeling of significant neuropathologic variables
| Brain weight | Braak tangle stage | Coexisting HpScl-TDP | ||||
|---|---|---|---|---|---|---|
| Regression estimate (95% CI) | P value | OR* (95% CI) | P value | OR* (95% CI) | P value | |
| Unadjusted | ||||||
| Ethnoracial Group* | ||||||
| Hispanic decedents | −61 (−98, −24) | .001 | 2.0 (1.2, 3.4) | .005 | 1.8 (0.8, 3.6) | 180 |
| Black decedents | −50 (−120, 18) | .150 | 0.7 (0.3, 1.7) | .400 | 3.5 (1.0, 9.7) | .054 |
| Adjusted for age of onset, sex, and disease duration | ||||||
| Ethnoracial group* | ||||||
| Hispanic decedents | −25 (−57, 6.3) | .120 | 1.3 (0.7, 2.3) | .400 | 1.1 (0.4, 2.4) | .900 |
| Black decedents | −47 (−100, 9.0) | .099 | 0.7 (0.3, 1.6) | .340 | 5.4 (1.4, 17) | .016 |
| Covariates | ||||||
| Age of onset | 15 (7.3, 24) | .0003 | 0.5 (0.4, 0.6) | <.0001 | 2.8 (2.0, 3.8) | <.0001 |
| Sex | −140 (−150, −130) | <.0001 | 1.9 (1.5, 2.5) | <.0001 | 0.8 (0.5, 1.2) | .3 |
| Disease duration | −9.4 (−11, −7.7) | <.0001 | 1.1 (1.1, 1.1) | <.0001 | 1.3 (1.2, 1.3) | <.0001 |
Abbreviations: OR, odds ratio; 95% CI, confidence interval; HpScl, Hippocampal Sclerosis of a TDP-43 etiology.
NOTE. White decedents were inputted as the referent for the ethnoracial group.
All odds ratio estimates calculated by profile likelihood.
4. Discussion
In a large AD series from the FLAME cohort, we report intriguing ethnoracial differences in demographics, clinical progression, and neuropathologic severity. Of particular interest, we found that, despite the observation of more common risk factors for AD in Hispanic decedents, their length of survival from age at onset of cognitive problems to death (i.e., disease duration) was significantly longer. Moreover, after controlling for education, sex, age at onset, and disease duration, the final MMSE scores remained lower in Hispanic decedents. Braak tangle stage and the presence of coexisting hippocampal sclerosis differed across ethnoracial groups, but not Thal amyloid phase or presence of coexisting Lewy body disease.
Neuropathologic studies investigating differences in ethnoracially diverse autopsy cohorts have been limited. Given that ethnoracial minorities are less likely to consent to autopsy for historical and sociopolitical reasons [5,31], knowledge of AD neuropathologic changes comes primarily from studies on white decedents. Two autopsybased studies comparing the presence of amyloid-β plaques and neurofibrillary tangle pathology did not find differences between black decedents and white decedents [32,33]. This is in contrast to a study investigating autopsied individuals from the National Alzheimer’s Coordinating Center that found a greater proportion of black decedents with a higher Braak tangle stage compared with white decedents [34]. In the present study, we did not observe a difference in Braak tangle stage between black decedents and white decedents, nor did we observe that Hispanic decedents had a higher Braak tangle stage than white decedents after adjusting for sex, age at onset of earliest cognitive symptoms, and disease duration. Similarly, a study investigating an ethnoracially diverse cohort with a wide range of AD neuropathologic change did not find differences when they stratified by clinical severity [35]. As a proxy for global effect of Alzheimer’s pathology, brain weight was investigated and found to not differ between black decedents and white decedents. This is consistent with a study showing no difference in brain weight among these ethnoracial groups [33]. We also found that Hispanic decedents with AD did not have a lower brain weight than white decedents after controlling for sex, age at onset, and disease duration. In addition, we investigated differences in coexisting/mixed pathology given the striking contribution to variance in dementia risk [36] and preponderance of additional pathologic changes observed in black decedents [9]. We did not observe a difference in frequency of coexisting Lewy body disease pathology, but did note a difference in the frequency of hippocampal sclerosis of a TDP-43 etiology. Although the presence of hippocampal sclerosis in black decedents has been previously observed [9], to our knowledge, this is the first report of a higher frequency in black decedents, even after accounting for sex, age at onset, and disease duration. Given our small sample size of Hispanic decedents and black decedents, we are cautious in our interpretation of neuropathologic differences.
Although we did not find ethnoracial differences in the age at death (i.e., length of life) within the autopsy-confirmed AD cases, we did find that Hispanic decedents developed symptoms earlier and experienced longer disease duration compared with white decedents. This is consistent with studies showing younger age at onset and/or longer survival time in Hispanic decedents with dementia [35,37], and slightly diverges from the “Hispanic mortality paradox” [11,12]. That is, Hispanic decedents in the United States (both immigrants and non-immigrants) live longer than black decedents and white decedents despite having at least as many risk factors [38]. The significant mortality advantage in Hispanic decedents appears to be moderated by age, with a stronger effect among older adults [11]. Some argue that this advantage may be the result of genetics [11,12] and sociocultural characteristics [39]. While the cause of this resilience remains unknown, our results provide new evidence of noticeably longer disease duration in Hispanic decedents with autopsy-confirmed AD compared with white decedents. We observed shorter disease duration in black decedents, which is in contrast to the longer survival that has been reported using data from National Alzheimer’s Coordinating Center [35]. In addition, one methodologic difference that could explain the discrepant findings is that we calculated age at onset of cognitive symptoms to death instead of time from first clinical visit to death. Regardless of methodology, differences in disease duration have important implications in terms of likely caregiver, social, and financial burden [4], and warrants further research to determine factors contributing to differences in disease course relevant to AD.
Differences in the length of education approached significance, suggesting that Hispanic decedents and black decedents had fewer years of education than white decedents is consistent with the 2015 United States Census data, showing that white decedents are more likely to have 16 or more years of education compared with Hispanic decedents and black decedents [40]. Lower education has also been associated with less cognitive reserve [13,41], lower socioeconomic status [42], and more cardiovascular risk factors [43], all of which have been found to contribute to the increased dementia risk in late life [2]. Both education and occupational attainment are often used as proxies, given their putative role in cognitive reserve, with occupation providing a more life-long metric [13,29]. Interestingly, we did not find ethnoracial differences in job level score within the AD cases from the FLAME cohort. This lack of a difference requires replication in future autopsy-confirmed studies given the growing evidence of mentally stimulating occupations in potentially reducing AD risk [13,44]. Likewise, more work is needed to look at the interaction between education and occupation to decipher their impact on AD risk, especially in the context of an ethnoracially diverse cohort.
We did not observe a difference in longitudinal cognitive decline (i.e., points lost per year) on the MMSE across ethnoracial groups. However, when the final MMSE test scores were examined, Hispanic decedents and black decedents were observed to have lower MMSE scores compared with white decedents. These results are consistent with studies indicating that older white decedents, with or without clinically diagnosed AD, generally outperform older Hispanic decedents and/or black decedents on the MMSE [45–50]. Notably, ethnoracial disadvantages in cognitive aging are reflected in generally poorer outcomes among older Hispanic decedents and black decedents [51]. Adjusting for years of education has been shown to either ameliorate or eliminate ethnoracial differences [44,45,47,52]. However, the MMSE final score remained significantly lower for Hispanic decedents compared with the other ethnoracial groups even after controlling for education, sex, age at onset, and disease duration. This is in line with previous research showing persistent ethnoracial differences after controlling for education [5]. The differences seen on the MMSE final score could be due to other unaccounted for variables (e.g., item bias, reading level, acculturation, greater prevalence of neuropsychiatric disturbances in dementia, income) shown to contribute to ethnoracial differences in neuropsychological test performance in aging, dementia, and population studies [41,45,53].
Although the observed numerically lower frequency of APOE ε4 among Hispanic decedents relative to black decedents and white decedents did not reach statistical significance, other studies have demonstrated lower APOE ε4 frequency in Hispanic individuals affected by AD [6,16,18,19]. Despite the lower frequency, APOE ε4 represents a significant risk factor for dementia in several groups of Hispanic adults [6,54] and black adults [55,56]. Hispanic decedents were observed to more frequently have a family history of cognitive problems than white decedents and black decedents, respectively. A community-based study found a greater prevalence of dementia among black decedents compared with white decedents, despite a substantially greater family history of dementia among white decedents, suggesting that dementia is more likely to go undetected among black decedents than white decedents [54].
This study has several limitations related to data collection and sample characteristics. Specifically, clinical progression was studied both cross-sectionally and retrospectively using antemortem information made available at the time of brain donation. Given that cause of death and ethnic differences in social relationships, health-related behaviors, and family networks were either rarely available or lacking, survival bias was not investigated. We used self-reported ethnoracial status to classify decedents, but within each cohort considerable genetic and cultural heterogeneity exists [57]. Given that geographical differences in immigration patterns may differ across states with diverse populations (e.g. Florida, California, New York, and Illinois), replication from other geographic areas of the United States will be necessary. This heterogeneity is a particular concern for studies of Hispanic Americans, and future prospective ethnoracial studies would benefit from a more granular ascertainment of study sample data with respect to self-identified ethnicity, genetic markers, country or region of origin, socioeducational factors, and other variables that will help characterize homogenous subgroups. Although there were no sex differences across the ethnoracial groups in the overall or AD cohort, self-selection bias (i.e., individual’s control over whether to participate for the deeded autopsy program) warrants further examination – especially as it applies to cultural diversity. Participation in the deeded autopsy program through the Alzheimer’s Disease Initiative is not restricted by sex, race, or ethnicity; however, greater efforts are needed to enhance participation to minimize selection bias. Given the aforementioned limitations, study results were interpreted with caution and should be considered preliminary.
The results of this autopsy-based AD study provide evidence of substantial ethnoracial differences in age at onset of cognitive impairment, disease duration, and end-stage cognitive decline. Neuropathologic differences across ethnoracial groups were suggestive of differences in the topographic distribution of tau pathology and coexisting hippocampal sclerosis of a TDP-43 etiology. The focus of the present study was on differences in neurodegenerative pathology; however, future work should investigate cerebrovascular disparity given known ethnoracial differences in cardiovascular risk [3,43]. This may be especially important given recent evidence suggesting cerebral amyloid angiopathy may not differ between black decedents and white decedents [58]. However, our data supports the need for consideration of clinical variability when assessing neuropathologic outcomes. This has important implications for therapeutic trials when assessing efficacy of interventions, especially when neuroimaging biomarkers measures are used as surrogates for neuropathologic severity as one of the endpoints. A culturally sensitive approach regarding deeded autopsy participation by ethnoracial minorities is needed [5,59]. Moreover, psychosocial and cultural factors impacting cognitive test performance should be considered, as these may vary depending on acculturation, geographic and socioeconomic factors [41,44,53]. We hope these preliminary findings motivate future mechanistic, biomarker, and clinical studies, with the ultimate goal of understanding and improving dementia treatments for historically underserved populations.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: We reviewed available literature using PubMed for scientific articles examining ethnoracial differences in Alzheimer’s disease (AD). While growing evidence indicates that ethnoracial minorities are found to be at a higher risk of AD or other dementias compared with non-Hispanic white Americans, our understanding of neuropathologic differences remains relatively understudied. Thus, we conducted a retrospective study to determine clinicopathologic differences across an autopsy-confirmed AD cohort of Hispanic, black, and white decedents.
Interpretation: Consistent with previous findings, substantial ethnoracial differences in age of onset of cognitive impairment, disease duration, and endstage cognitive decline were found. Notably, neuropathologic differences across ethnoracial groups supported differences in tau pathology distribution and coexisting hippocampal sclerosis of a TDP-43 etiology. Overall, results underscore the need for consideration of clinical variability when assessing neuropathologic outcomes.
Future directions: These preliminary findings could guide future mechanistic, biomarker, and clinical studies, with the goal of understanding and improving dementia treatments in underserved populations.
Acknowledgments
The authors thank the patients and their families for their generous brain donations to help further our knowledge of ethnoracial differences in AD and related dementias. The authors would like to acknowledge the endless hours of commitment and teamwork offered by Virginia Phillips, Monica Castanedes-Casey, and Jessica F. Tranovich.
This study was supported by the Florida Department of Health, Ed and Ethel Moore Alzheimer’s Disease Research Program (Murray; 6AZ01 and 8AZ06) and the National Institute on Aging (NIA) of the National Institutes of Health (NIH) (under Award Number R01-AG054449 [Murray], P50-AG016574 [Graff-Radford], and P50-AG047266 [Ertekin-Taner]). This work was in part supported by the Alzheimer’s Association 2018-AARFD-592421 (Santos), Florida Department of Health, Ed and Ethel Moore Alzheimer’s Disease Research Program grants 7AZ17 (Ertekin-Taner), 7AZ07 (Carrasquillo), and NIH/NIA R03AG055677 (Carrasquillo). Additional funding for brain banking came from the State of Florida, Department of Elder Affairs, Alzheimer’s Disease Initiative.
Footnotes
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jalz.2018.12.013.
References
- [1].Colby SL, Ortman JM. Projections of the size and composition of the US population 2014 to 2060. Washington, DC: Current Population Reports; 2014. p. 25–1143. [Google Scholar]
- [2].Association As. 2017 Alzheimer’s disease facts and figures. Alzheimer’s Demen 2017;13:325–73. [Google Scholar]
- [3].Tang M-X, Cross P, Andrews H, Jacobs DM, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001;56:49–56. [DOI] [PubMed] [Google Scholar]
- [4].Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement 2017;13:72–83. [DOI] [PubMed] [Google Scholar]
- [5].Ighodaro ET, Nelson PT, Kukull WA, Schmitt FA, Abner EL, Caban-Holt A, et al. Challenges and considerations related to studying dementia in Blacks/African Americans. J Alzheimer’s Dis 2017;60:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: The influence of Type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 2003;51:169–77. [DOI] [PubMed] [Google Scholar]
- [7].Carrion-Baralt JR, Suarez-Perez E, del Rio R, Moore K, Silverman JM. Prevalence of dementia in Puerto Rican veterans is higher than in mainland U.S. veterans. J Am Geriatr Soc 2010; 58:798–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gurland B, Wilder D, Lantigua R, Mayeux R, Stern Y, Chen J, et al. Differences in Rates of Dementia Between Ethno-Racial Groups In: Martin LG, Soldo BJ, eds. Racial and Ethnic Differences in the Health of Older Americans. Washington (DC): National Academies Press; 1997. [PubMed] [Google Scholar]
- [9].Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 2015; 85:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: A multiethnic, population-based study of incident cases. Neurology 2008;71:1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ruiz JM, Steffen P, Smith TB. Hispanic mortality paradox: A systematic review and meta-analysis of the longitudinal literature. Am J Public Health 2013;103:e52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abraĺdo-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: a test of the “salmon bias” and healthy migrant hypotheses. Am J Public Health 1999;89:1543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stern Y Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 2012;11:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brayne C, Ince PG, Keage HAD, McKeith IG, Matthews FE, Polvikoski T, et al. Education, the brain and dementia: Neuroprotection or compensation?EClipSE Collaborative Members. Brain 2010; 133:2210–6. [DOI] [PubMed] [Google Scholar]
- [15].Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet 2007;39:17. [DOI] [PubMed] [Google Scholar]
- [16].Farrer LA, Cupples L, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein e genotype and alzheimer disease: A meta-analysis. JAMA 1997;278:1349–56. [PubMed] [Google Scholar]
- [17].Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med 2017;177:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PS, Pericak-Vance M, Joo S, et al. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993;43:1467. [DOI] [PubMed] [Google Scholar]
- [19].Campos M, Edland SD, Peavy GM. Exploratory study of apolipoprotein E epsilon4 genotype and risk of Alzheimer’s disease in Mexican Hispanics. J Am Geriatr Soc 2013;61:1038–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang M, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, et al. The apoe-ε4 allele and the risk of alzheimer disease among African Americans, Whites, and Hispanics. JAMA 1998;279:751–5. [DOI] [PubMed] [Google Scholar]
- [21].Terry RD, Hansen LA, DeTeresa R, Davies P, Tobias H, Katzman R. Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropathol Exp Neurol 1987;46:262–8. [DOI] [PubMed] [Google Scholar]
- [22].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–59. [DOI] [PubMed] [Google Scholar]
- [23].Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58:1791–800. [DOI] [PubMed] [Google Scholar]
- [24].Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 2015;138:1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A 2009;106:7607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol 2014;128:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 2008; 116:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–72. [DOI] [PubMed] [Google Scholar]
- [29].Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, et al. Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol 2012;72:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [31].Fillenbaum GG, Huber MS, Beekly D, Henderson VW, Mortimer J, Morris JC, et al. The consortium to establish a registry for Alzheimer’s Disease (CERAD). Part XIII. Obtaining autopsy in Alzheimer’s disease. Neurology 1996;46:142–5. [DOI] [PubMed] [Google Scholar]
- [32].Sandberg G, Stewart W, Smialek J, Troncoso JC. The prevalence of the neuropathological lesions of Alzheimer’s disease is independent of race and gender. Neurobiol Aging 2001;22:169–75. [DOI] [PubMed] [Google Scholar]
- [33].Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and white individuals. Arch Neurol 2006;63:87–90. [DOI] [PubMed] [Google Scholar]
- [34].Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW. Neuropathologic differences by race from the National Alzheimer’s Coordinating Center. Alzheimers Dement 2016;12:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mehta KM, Yaffe K, Perez-Stable EJ, Stewart A, Barnes D, Kurland BF, et al. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology 2008;70:1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Power MC, Mormino E, Soldan A, James BD, Yu L, Armstrong NM, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol 2018;84:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Clark CM, DeCarli C, Mungas D, Chui HI, Higdon R, Nunez J, et al. Earlier onset of Alzheimer disease symptoms in latino individuals compared with anglo individuals. Arch Neurol 2005;62:774–8. [DOI] [PubMed] [Google Scholar]
- [38].Arias E, Eschbach K, Schauman WS, Backlund EL, Sorlie PD. The Hispanic mortality advantage and ethnic misclassification on US death certificates. Am J Public Health 2010;100:S171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Palloni A, Arias E. Paradox lost: Explaining the hispanic adult mortality advantage. Demography 2004;41:385–415. [DOI] [PubMed] [Google Scholar]
- [40].Ryan CL, Bauman K. Educational Attainment in the United States: 2015 Population Characteristics In: Commerce USDo. census.gov: U.S. Census Bureau; 2016. p. 1–12. [Google Scholar]
- [41].Mehta KM, Simonsick EM, Rooks R, Newman AB, Pope SK, Rubin SM, et al. Black and white differences in cognitive function test scores: What explains the difference? J Am Geriatr Soc 2004; 52:2120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McDowell I, Xi G, Lindsay J, Tierney M. Mapping the connections between education and dementia. J Clin Exp Neuropsychol 2007; 29:127–41. [DOI] [PubMed] [Google Scholar]
- [43].Steptoe A, Marmot M. Socioeconomic status and coronary heart disease: A psychobiological perspective. Popul Development Rev 2004;30:133–50. [Google Scholar]
- [44].Manly JJ, Jacobs DM, Sano M, Bell K, Merchant CA, Small SA, et al. Cognitive test performance among nondemented elderly African Americans and Whites. Neurology 1998;50:1238–45. [DOI] [PubMed] [Google Scholar]
- [45].Pedraza O, Clark JH, O’Bryant SE, Smith GE, Ivnik RJ, Graff-Radford NR, et al. Diagnostic validity of age and education corrections for the Mini-Mental State Examination in older African Americans. J Am Geriatr Soc 2012;60:328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].O’Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, et al. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2013;33:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Murden RA, McRae TD, Kaner S, Bucknam ME. Mini-Mental State exam scores vary with education in blacks and whites. J Am Geriatr Soc 1991;39:149–55. [DOI] [PubMed] [Google Scholar]
- [48].Brayne C, Calloway P. The association of education and socioeconomic status with the Mini Mental State Examination and the clinical diagnosis of dementia in elderly people. Age and ageing 1990; 19:91–6. [DOI] [PubMed] [Google Scholar]
- [49].Escobar JI, Burnam A, Karno M, Forsythe A, Landsverk J, Golding JM. Use of the Mini-Mental State Examination (MMSE) in a community population of mixed ethnicity. Cultural and linguistic artifacts. J nervous Ment Dis 1986;174:607–14. [DOI] [PubMed] [Google Scholar]
- [50].Welsh KA, Fillenbaum G, Wilkinson W, Heyman A, Mohs R, Stern Y, et al. Neuropsychological test performance in African-American* and white patients with Alzheimer’s disease. Neurology 1995; 45:2207–11. [DOI] [PubMed] [Google Scholar]
- [51].Weden MM, Miles JN, Friedman E, Escarce JJ, Peterson C, Langa KM, et al. The Hispanic paradox: Race/ethnicity and nativity, immigrant enclave residence and cognitive impairment among older US adults. J Am Geriatr Soc 2017;65:1085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Black SA, Espino DV, Mahurin R, Lichtenstein MJ, Hazuda HP, Fabrizio D, et al. The influence of noncognitive factors on the Mini-Mental State Examination in Older Mexican-Americans: Findings from the Hispanic EPESE. Established Population for the Epidemiologic Study of the Elderly. J Clin Epidemiol 1999; 52:1095–102. [DOI] [PubMed] [Google Scholar]
- [53].Manly JJ, Byrd DA, Touradji P, Stern Y. Acculturation, reading level, and neuropsychological test performance among African American elders. Appl Neuropsychol 2004;11:37–46. [DOI] [PubMed] [Google Scholar]
- [54].Demirovic J, Prineas R, Loewenstein D, Bean J, Duara R, Sevush S, et al. Prevalence of dementia in three ethnic groups: The South Florida program on aging and health. Ann Epidemiol 2003;13:472–8. [DOI] [PubMed] [Google Scholar]
- [55].Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, et al. Variants in the atp-binding cassette transporter (abca7), apolipoprotein e ε4, and the risk of late-onset Alzheimer disease in African Americans. JAMA 2013;309:1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Murrell JR, Price B, Lane KA, Baiyewu O, Gureje O, Ogunniyi A, et al. Association of apolipoprotein e genotype and alzheimer disease in African Americans. Arch Neurol 2006;63:431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bryc K, Durand EY, Macpherson JM, Reich D, Mountain JL. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am J Hum Genet 2015;96:37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kamara DM, Gangishetti U, Gearing M, Willis-Parker M, Zhao L, Hu WT, et al. Cerebral Amyloid Angiopathy: Similarity in African-Americans and Caucasians with Alzheimer’s disease. J Alzheimers Dis 2018;62:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res 2012;9:734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.