Abstract
Inhalation of pathogenic bacteria transported by particulate matter (PM) presents an important potential threat to human health. Therefore, the pulmonary toxicity in mice caused by Staphylococcus aureus (S. aureus) and PM as individual matter and mixtures was studied. PM and S. aureus were instilled intratracheally into Kunming mice at doses of 0.2 mg/mouse and 5.08 × 106 CFU /mouse, respectively, as individual matter and in combination two times at 5-day intervals. After the exposure period, oxidative stress markers and nitric oxide (NO) in the lung, cellular infiltration, neurotrophins, chemokines, and cytokines in bronchoalveolar lavage fluid (BALF), and immunoglobulin (Ig) in sera were examined. Exposure to the combination of PM and S. aureus caused significant increases in malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and NO and significant decreases in total antioxidant capacity (T-AOC) and the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) in the lung. Meanwhile, the ratio of interleukin (IL)-4 to interferon (INF)-γ, the IL-4 level in BALF, and the IgE concentration in sera were significantly increased in the groups exposed to S. aureus or the combination of PM and S. aureus. Substance P and IL-8 in BALF were significantly increased in mice exposed to PM, S. aureus or their combination. In addition, PM, S. aureus, and their combination caused infiltration of leukocytes into the alveolar tissue spaces. The results suggested that exposure to the combination of PM and S. aureus induced a lung inflammatory response that was at least partly caused by oxidative stress and mediators from the activated eosinophils, neutrophils, alveolar macrophages, and epithelial cells.
Keywords: lung inflammation, mediator, oxidative stress, particulate matter, Staphylococcus aureus
Introduction
Particulate matter (PM) is one of the most widespread air pollutants. It can pass through the different parts of the lungs and ultimately get to the capillaries and circulating cells or components, e.g., erythrocytes. Particulates are then delivered by the circulation to other organs such as the liver, kidney, spleen, heart, and brain, where they may be accumulated [22]. Inhalation of enhanced levels of ambient PM has been causally related to increased mortality and morbidity in human populations, especially those with pre-existing pulmonary inflammation [4].
Pulmonary exposure to PM has been associated with the development of pulmonary inflammation which can lead to pulmonary illnesses and diseases [21]. Oxidative stress has been reported as a possible inducer of apoptosis in lungs from animals exposed to low concentrations of urban particulates [5, 24]. Particulates could cause toxicities in alveolar macrophages and bronchial epithelial cells to induce inflammation in the lung, the mechanism of which is mainly involved in producing inflammatory factors and reactive oxygen species (ROS) [3, 17]. Pulmonary responses to diesel exhaust particulate exposure are mediated through elevated production of ROS and nitric oxide (NO) by alveolar macrophages [33].
Air pollution is also a mixture of many pollutants such as particulates, microbial pollutants, and some harmful chemical molecules. Inhalation of pathogenic bacteria or circulating viruses which might be transported by PM presents an important potential threat to human health [10, 31]. Pathogenic bacteria contribute to other globally important diseases, such as pneumonia, which can be caused by bacteria such as Streptococcus and Pseudomonas. Staphylococcus aureus (S. aureus) is an important opportunistic pathogen and etiologically agent of many hospital- and community-acquired infections [9]. S. aureus isolates secrete identifiable enterotoxins such as S. aureus enterotoxin A and B (SEA, SEB). SEA and SEB can induce upper airway and lung diseases related to eosinophil activation and recruitment. The pro-inflammatory effect of SEB on human nasal epithelial cells in vitro, which results in augmented granulocyte migration and survival, has been demonstrated [11].
The mechanisms involved in PM- or S. aureus-induced lung toxicology are possibly related to leukocyte infiltration, pro-inflammatory cytokines, oxidative stress [3, 5, 17, 24], NO level in the lung [33], eosinophil activation and recruitment, and granulocyte migration and survival [11]. Relatively little is known about murine lung toxicology caused by mixtures of PM and S. aureus.
In the present study, the acute pulmonary toxicity in mice exposed to S. aureus and PM as individual matter and mixtures was studied, and the association between the immune-related factors and lung inflammation were examined. In addition, the molecular mechanism for lung inflammation was also discussed based on the results.
Material and Methods
Animals
Male Kunming mice (5 weeks of age) weighing 18–22 g were obtained from the Experimental Animal Care Center of Dalian Medical University (Liaoning, China). All animals use was approved by the Animal Experimental Biosafety Committee, Dalian University of Technology (approval no. DUT2013576A) and complied with the Institutional Guidelines for the Care and Use of Laboratory Animals. These mice were acclimatized to the laboratory for one week and maintained in a humidity (50 ± 10%)- and temperature (22 ± 2°C)-controlled room on a 12 h light, 12 h dark cycle. The animals were given access to food and water ad libitum. All the mice were weighed before the exposure experiment.
Preparation of particulates and bacteria
Particulates in the experiment were composite artificial dust (CABR-AK- FHRGC-72235) obtained from the China Academy of Building Research. The components of the artificial dust were 72 ± 1% loess dust, 23 ± 1% black carbon, and 5 ± 1% short velveteen. The dust size distribution was as follows: 0–5 µm, 34 ± 2%; 5–10 µm, 27 ± 2%; 10–20 µm, 27 ± 2%; 20–40 µm, 11 ± 2%; and 40–80 µm, 1 ± 0.3%. The samples used in our experiment were heated at 360°C for 30 min in an electric heater to remove toxic materials (microbiological materials, sulfate, nitrate, etc.) adhering to them.
S. aureus obtained from the China General Microbiological Culture Collection Center was cultured in a growth medium comprised of nutrient broth overnight in a thermostat at 37°C. After S. aureus was cultured for two generations, it was washed twice in sterilized normal saline and prepared at a concentration of 5.08 × 107 CFU/ml for intratracheal instillation into mice.
Study protocol
The study protocol was based on the exposure patterns in cases in which people are always exposed to PM together with highly pathogenic bacteria. The exposure doses of PM and S. aureus were chosen on the basis of previous information [10] and our experimental conditions. The details for procedures concerning exposure conditions and concentrations were in accordance with established protocols [31]. In brief, male Kunming mice were divided into four groups (n=12, each group) on the basis of the treatment with PM and S. aureus: control (normal saline), G1 (PM, 0.2 mg/mouse), G2 (S. aureus, 5.08 × 106 CFU/mouse), and G3 (PM, 0.2 mg/mouse + S. aureus, 5.08 × 106 CFU/mouse). Each mouse in the control group was instilled intratracheally with 0.1 ml of normal saline two times at 5-day intervals. Each mice mouse in the G1 group was intratracheally instilled with 0.1 mg PM two times at 5-day intervals. Each mice mouse in the G2 group was intratracheally instilled with 2.54 × 106 CFU S. aureus two times at 5-day intervals. Each mice mouse in the G3 group was simultaneously injected with 0.1 mg PM and 2.54 × 106 CFU S. aureus two times at 5-day intervals.
Bronchoalveolar lavage fluid (BALF) and tissues collection
After the 10 days of exposure, the mice were sacrificed under pentobarbital anesthesia. Five mice were randomly chosen from each group for blood extraction from the right ventricle, then the blood was centrifuged to obtain the sera for immunoglobulin (Ig) analysis; meanwhile, BALF from each of these mice was collected by cannulating the trachea and lavaging the lung four times with 1 ml of sterile saline as described previously [6]. The recovered fluids, about 3.5 ml, were pooled. Collected BALF was centrifuged, and the supernatant was removed and stored in an ultra-low temperature freezer (−80°C) for cytokines, chemokines, and neurotrophins analysis, while the resulting BALF cell pellet was diluted. Aliquots were analyzed by hemocytometer and trypan blue dye exclusion for viable cell count, and differential inflammatory cells were counted by optical microscopy after Wright- Giemsa staining.
The lungs of the other seven mice in each group of 12 animals were collected for detection of oxidative stress markers and histological analysis.
Measurement of lung oxidative stress markers
Malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG), NO, and total antioxidant capacity (T-AOC) were measured by using commercially available kits according to the manufacturer’s protocol (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The lungs of the mice were washed in normal saline, 10% lung homogenates were prepared in 1.15% w/v of potassium chloride. Then, the homogenates were centrifuged at 7,000 g for 10 min at 4°C, and their supernatants were used for determination of oxidative stress markers.
Degree of lipid peroxidation in lung tissue homogenates of mice was determined according to thiobarbituric acid reactive substances formation with maximal absorbance at 532 nm by following the protocol of the kit. The concentration of MDA in the mouse lung was calculated by comparing the absorbance to that produced by the control standard 1, 1, 3, 3 - tetraethoxypropane and expressed as nmol/mg prot.
CAT activity was measured using the ammonium molybdate spectrophotometric method, which is based on the fact that ammonium molybdate can rapidly terminate the hydrogen peroxide (H2O2) degradation reaction catalyzed by CAT and react with the residual H2O2 to generate a yellow complex, which can be detected by the absorbance at 405 nm. CAT activity was expressed as U/mg prot.
SOD activity was assayed by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium in absorbance at 550 nm. SOD data for the lung was expressed as U/mg prot as compared with the standard.
Total glutathione (TGSH) and GSH were determined spectrophotometrically by monitoring the chromophoric product resulting from reaction of the 5, 50-dithiobis- (2-nitrobenzoic acid) with GSH in the presence of nicotinamide adenine dinucleotide phosphate (NADPH) and glutathione reductase at 412 nm. GSSG was calculated by subtracting the GSH content from the TGSH. The ratio of GSH to GSSG in lung homogenates was calculated from the GSH and GSSG contents.
NO levels were determined by a spectrophotometer at 550 nm. The final level of NO in lung homogenates was calculated and expressed as nmol/mg prot.
T-AOC was measured by the ferric reducing-antioxidant power assay method and detected at 520 nm with a spectrophotometer. T-AOC in lung homogenates was calculated and expressed as U/mg prot.
Assay of cytokines, chemokines, and substance P
The amounts of various biochemical mediators in each sample supernatant from BALF of mice were determined by using ELISA kits for IL-8, IL-4, interferon (INF)-γ, eotaxin, neurotrophin (NT)-3, and substance P (R&D systems, Minneapolis, MN, USA). ELISA plate absorbance was measured at 450 and 550 nm wavelengths using a microplate reader (Model 550, Bio-Rad, Hercules, CA, USA). Determinations were made in duplicates for every sample using standard curves accordance with the manufacturer’s instructions.
Histopathological observation
The lung specimens of mice were fixed with 10% formaldehyde and processed conventionally for embedding in paraffin, cut into 5-µm-thick sections, stained with hematoxylin and eosin (H&E) for conventional histopathological examination, and then observed under a light microscope.
Statistical analysis
Statistical analyses of means were carried out with SPSS software (Ver13.0, SPSS, Inc., Chicago, IL, USA). Data were expressed as means ± SD. One-way analysis of variance (ANOVA) was conducted to compare the differences of means among multigroup data. Duncan’s test was used for multiple comparisons. Differences among groups were considered statistically significant at the level of P<0.05 or P<0.01.
Results
Animal body weight and lung organ mass
After the 10 days of exposure, the lung organ coefficient in treated mice was increased, and body weight, net increase in body weight, and lung weight were decreased compared with the control, but no significant differences were found among them (Table 1).
Table 1. Body weight and lung organ weight in male mice exposed to PM, S. aureus, or their combination.
| Index | Control | G1 | G2 | G3 |
|---|---|---|---|---|
| Body weight/g | 31.95 ± 3.25 | 29.19 ± 3.31 | 30.27 ± 4.49 | 28.74 ± 4.01 |
| Weight change/g | 13.45 ± 1.12 | 10.57 ± 2.50 | 11.8 ± 3.21 | 9.68 ± 3.77 |
| Lung weight/g | 0.32 ± 0.03 | 0.30 ± 0.02 | 0.30 ± 0.03 | 0.31 ± 0.04 |
| Lung organ coefficient | 1.01 ± 0.09 | 1.07 ± 0.21 | 1.13 ± 0.36 | 1.12 ± 0.27 |
Data are presented as the mean ± SD (n=5 in each group).
Changes in numbers of BALF cells
To examine the effect of exposure to PM, S. aureus, and their combination on lung inflammation in mice, we determined the numbers of BALF cells after exposure to PM, S. aureus, or their combination for 10 days (Table 2). PM exposure resulted in no significant increase in various types of inflammatory cells in BALF. The numbers of BALF cells, macrophages, neutrophils, lymphocytes, and eosinophils in group 2 and 3 mice were significantly increased compared with those in the control mice. In addition, the numbers of cells, macrophage cells, and eosinophils in BALF in the group exposed to the combination of PM and S. aureus were higher than those in the group exposed to PM; neutrophils and lymphocytes were higher than those in the groups exposed to PM or S. aureus alone.
Table 2. Effect of exposure to PM, S. aureus, or their combination on the numbers of inflammatory cells in BALF cells of mice.
| Group | BALF cells (×104) | Macrophages | Neutrophils | Lymphocytes | Eosinophils |
|---|---|---|---|---|---|
| Control | 2.42 ± 0.33 | 2.26 ± 0.22 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 |
| 1 | 2.44 ± 0.28 | 2.31 ± 0.18 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 |
| 2 | 2.69 ± 0.11* | 2.48 ± 0.13* | 0.03 ± 0.02* | 0.05 ± 0.02* | 0.07 ± 0.03* |
| 3 | 2.95 ± 0.41*# | 2.63 ± 0.17*# | 0.06 ± 0.02*#$ | 0.12 ± 0.04*#$ | 0.09 ± 0.02*# |
Data are reported as the mean ± SD (n=5 in each group). *P<0.05 compared with control; #P<0.05 compared with G1; $P<0.05 compared with G2.
Oxidative stress markers in the lung
Oxidative stress levels in the lungs of mice exposed to PM, S. aureus, or their combination are shown in Table 3. MDA, SOD, and CAT levels in G3 group mice were significantly increased compared with the control (P<0.05 or P<0.01); moreover, CAT activities in G2 mice were higher than those in the control (P<0.05), and SOD and CAT activities in G3 mice were higher than those in G1 mice. However, T-AOC and the ratio of GSH to GSSH in all treated groups were significantly decreased compared with the control, and these levels in G3 mice were lower than those in G1 mice.
Table 3. Effect on MDA, T-AOC, CAT, SOD, and GSH/GSSG in mice exposed to PM, S. aureus, or their combination.
| Group | MDA (nmol/mg prot) | T-AOC (U/mg prot) | CAT (U/mg prot) | SOD (U/mg prot) | Ratio of GSH to GSSG |
|---|---|---|---|---|---|
| Control | 4.65 ± 0.71 | 2.46 ± 0.57 | 21.51 ± 3.66 | 225.09 ± 21.07 | 2.49 ± 0.18 |
| 1 | 5.15 ± 1.09 | 2.26 ± 0.34 | 20.45 ± 4.79 | 209.75 ± 29.2 | 2.28 ± 0.35 |
| 2 | 5.59 ± 1.29 | 1.92 ± 0.29 | 33.01 ± 8.08* | 275.99 ± 62.58 | 1.85 ± 0.55 |
| 3 | 5.89 ± 1.65* | 1.56 ± 0.35*# | 41.76 ± 7.29**## | 334.24 ± 24.05**## | 1.51 ± 0.21**# |
Data are presented as the mean ± SD (n=5 in each group); *P<0.05 compared with control; **P<0.01 compared with control; #P<0.05 compared with G1; ##P<0.01 compared with G1.
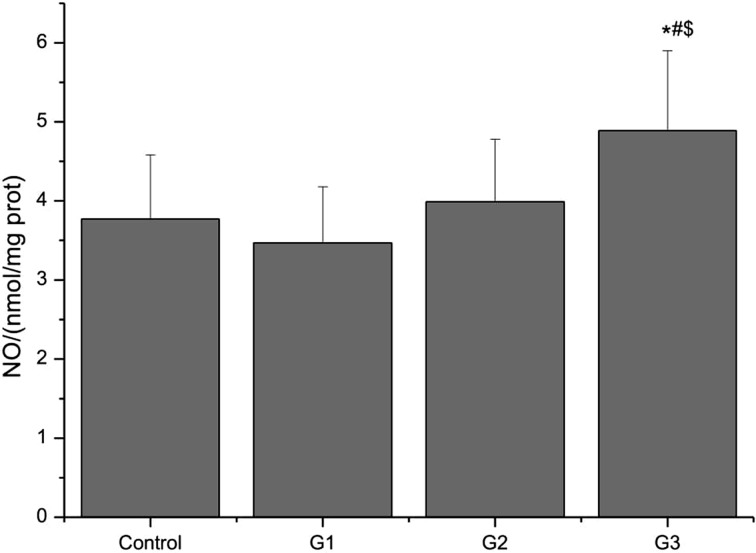
NO level in the lung
NO levels in the lungs of mice exposed to PM, S. aureus, or their combination are shown in Fig. 1. The NO level in G3 mice was significantly increased compared with that in control, G1, or G2 mice (P<0.05), while exposure to PM or S. aureus alone did not lead to a significant change in NO level (P>0.05).
Fig. 1.
Effect on NO levels in mice exposed to PM, S. aureus, or their combination. Data are presented as the mean ± SD. n=5 in each group. *P<0.05 versus control; #P<0.05 versus G1; $P<0.05 versus G2.
Chemokines and cytokines levels in BALF
Cytokine levels in BALF of mice exposed to PM, S. aureus, or their combination are shown in Fig. 2. The IL-8 levels in BALF of all treated groups of mice were significantly increased compared with the control (P<0.01), and the ratio of IL-4 to INF-γ and the IL-4 levels in the G2 and G3 groups were higher than those of the control (P<0.05 or P<0.01), but there were no significant differences in INF-γ and eotaxin levels between the treated groups and control (P>0.05).
Fig. 2.
Effect on chemokine and cytokines levels in mice exposed to PM, S. aureus, or their combination. (A) IL-4, (B) INF-γ, (C) IL-4/INF-γ, (D) IL-8, (E) Eotaxin. Data are presented as the mean ± SD (n=5). *P<0.05 versus control; **P<0.01 versus control.
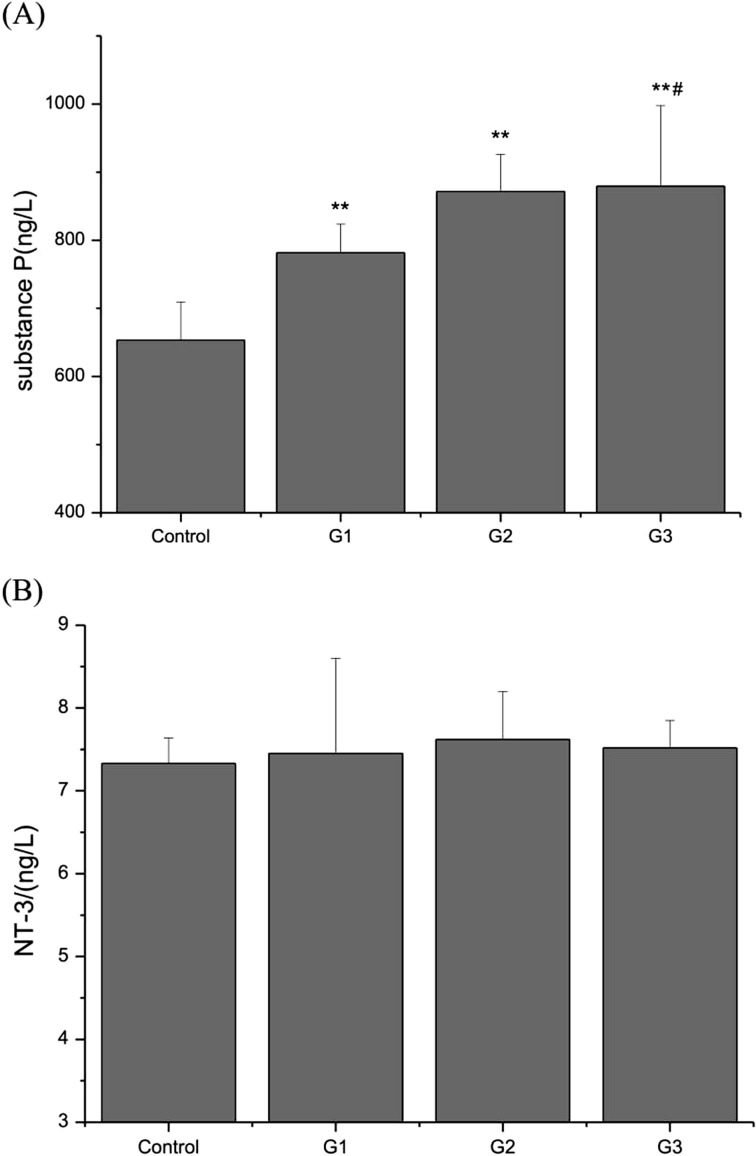
NT-3 and substance P levels in BALF
To investigate the effect of exposure to PM, S. aureus, or their combination on neurogenic inflammation, we measured the contents of NT-3 and substance P. NT-3 and substance P in BALF in all treated groups of mice were increased compared with the control, and substance P was significantly increased (P<0.01) (Fig. 3A); moreover, the substance P level in G3 mice was higher than that in G1 mice. However, the NT-3 level in treated mice was slight high compared with the control (P>0.05) (Fig. 3B).
Fig. 3.
Effect on NT-3 and substance P contents in BALF of mice exposed to PM, S. aureus, or their combination. (A) substance P, (B) NT-3. Data are presented as the mean ± SD (n=5). **P<0.01 versus control; #P<0.05 versus G1.
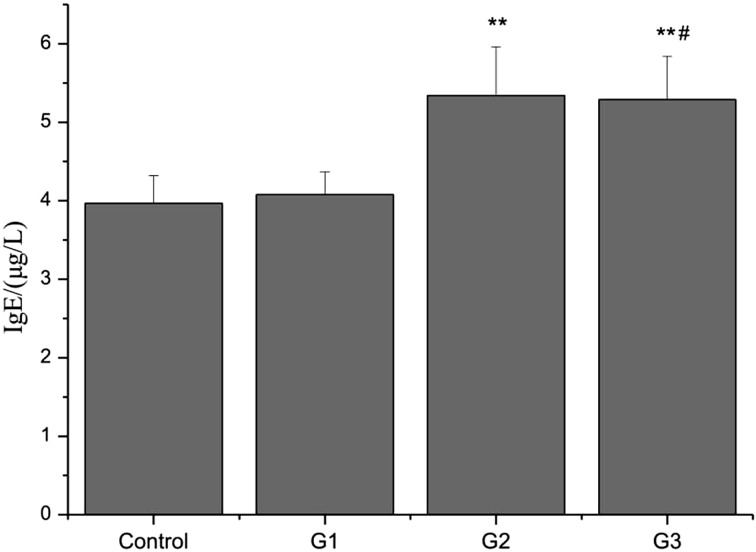
Ig production in sera
To validate the predominance of Th2 cytokine expression in mice exposed to PM, S. aureus, or their combination, the levels of Ig isotype in sera of the mice were measured by using an isotype-specific ELISA. Exposure to S. aureus or the combination of PM and S. aureus led to a considerable increase in IgE concentration in serum compared with the control group. Moreover, the IgE concentration in serum of the mice exposed to the combination of PM and S. aureus was higher than that in the mice exposed to PM (Fig. 4).
Fig. 4.
IgE production in the sera of the mice exposed to PM, S. aureus, or their combination. Data are presented as mean ± SD (n=5). **P<0.01 versus control; #P<0.05 versus G1.
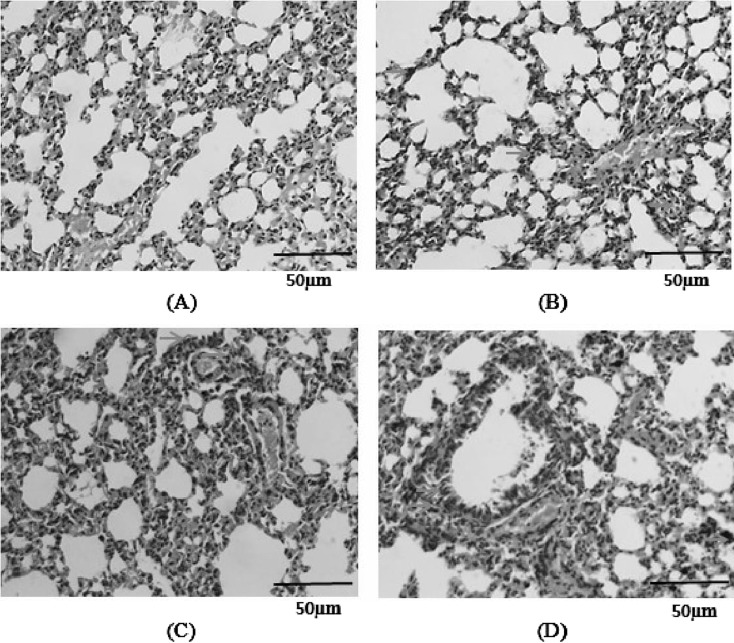
Pathologic changes in the lung
No pathological alterations were found in the lungs of the control group (Fig. 5A). PM or S. aureus alone caused slight infiltration of leukocytes into the submucosa of the airways with slight goblet cell proliferation in the bronchial epithelium and invasion of leukocytes into the alveolar area (Figs. 5B and C). However, the combination of PM and S. aureus caused more prominent infiltration of leukocytes into the alveolar tissue spaces and the connective tissue in the airway compared with PM or S. aureus alone (Fig. 5D).
Fig. 5.
Histopathological analysis of the lung in mice exposed to PM, S. aureus, or their combination. Hematoxylin and Eosin (H&E) staining is shown (×400). (A) Control, (B) G1, (C) G2, (D) G3. Yellow arrows indicate leukocytes, red arrows indicate goblet cells, and blue arrows indicate particulates.
Discussion
PM has been shown to adhere to many kinds of microorganisms [10, 15], some of which are highly pathogenic. It is one of the important air pollutants, and it often adheres to microorganisms in air. Therefore, the acute pulmonary toxicity in mice exposed to S. aureus, PM, or their combination was investigated in this study.
The present study demonstrated that S. aureus or a combination of PM and S. aureus induces mice lung inflammation in mice which is evidenced by oxidative stress in the lung, inflammation factors in BALF, Ig in sera, and pathological examinations. Furthermore, all these effects were almost always more remarkable in the group exposed to the combination of PM and S. aureus than in the group exposed to PM.
Oxidative stress refers to an increase in intracellular contents of ROS that cause damage to lipids, proteins, and DNA [27]. It plays a crucial role in the deleterious and inflammatory responses in airways diseases such as asthma and chronic obstructive pulmonary disease [18]. Our previous studies showed that ROS are generated following exposure to volatile organic compounds (VOCs) and play a pivotal part in lung damage [29, 30]. It has been suggested that the oxidative stress was one way for ambient particles to affect lung health [5]. Its mechanism of action mainly includes attacking the pulmonary antioxidant system and elevating the production of ROS in the lung, which may represent the initiation of inflammation and eventually activate apoptosis to induce pulmonary disease [24]. Oxidative stress arises from an oxidant/antioxidant imbalance in favor of oxidants. In the present study, the combination of PM and S. aureus led to significant increases in lipid peroxidation (MDA) and antioxidase (SOD and CAT) and significant decreases in T-AOC and the ratio of GSH/GSSG. The results showed that lungs of mice exposed to the combination of PM and S. aureus showed severe oxidative stress. In other words, the combination of PM and S. aureus induces generation of ROS, and excess ROS accumulation may lead to modified antioxidant defense mechanisms. Oxidative stress then causes damage to lipids, and ROS may represent the initiation of inflammation and eventually activate apoptosis to induce lung inflammation [16, 29, 30].
In the airway, nitric oxide synthase (NOS) may form NO and S-nitrosoglutathione (GSNO). NO may further generate nitrogen dioxide (NO2), which may exhaust GSH and result in airway inflammation [29]. ROS can also interact with NO to form other reactive nitrogen species (RNS), which include nitrosonium cation, nitroxyl anion, and peroxynitrite. RNS may damage DNA, lipids, proteins, and carbohydrates in environmental lung diseases. In the present study, NO levels in the mice exposed to the combination of PM and S. aureus were significantly increased, while the levels in the mice with single exposure alone were not significantly changed, indicating that NO plays an important role in the injurious and inflammatory responses in the airways of mice exposed to a combination of PM and S. aureus.
IL-8, a member of the proinflammatory protein family, is a chemokine produced by macrophages and other cell types like epithelial cells and airway smooth muscle cells [28]. It has been suggested to be an important biomarker for the evaluation of early inflammatory responses to chemical irritants due to its non-cell-specific nature [25]. The IL-8 release in BALF of mice exposed to S. aureus, PM, or their combination was significantly higher than that of the control in the present study, and the oxidative stress levels changed significantly following exposure to the combination of PM and S. aureus, suggesting that S. aureus, PM, or their combination may cause an inflammatory response and that the response caused by the combination of PM and S. aureus is at least partly caused by release of the ROS and mediators from the activated lymphocytes, neutrophils, macrophages, endothelial cells, epithelial cells, and fibroblasts [23]. Moreover, S. aureus or the combination of PM and S. aureus led to a significant increase in the numbers of BALF cells, macrophages, neutrophils, lymphocytes, and eosinophils in the present study.
Eosinophils are considered to play a critical role in the pathogenesis of airway inflammation through the release of inflammatory mediators such as IL-4, IFN-γ, and eotaxin [19, 30]. Conversely, the activation of eosinophils can be modulated by these eosinophil-derived cytokines as well as T cell-derived cytokines [2, 14]. We found that a Th2 cytokine (IL-4) was significantly higher in BALF of mice exposed to S. aureus or the combination of PM and S. aureus than that in the control, while INF-γ and eotaxin were not significantly changed in the present study. Th2 cytokines (such as IL-4) are the most potent factor that promote the differentiation of Th cells to Th2 effectors, but IL-4 also antagonizes the activity of Th1 cells [8]. The balance between Th1 and Th2 differentiation is critically related to the status of allergic disorders and airway inflammation [12, 13, 30]. Our results also demonstrated that the ratio of IL-4 to IFN-γ in BALF of mice exposed to S. aureus or the combination of PM and S. aureus was significantly increased compared with that in the control group. This finding suggests the possibility that exposure to S. aureus or the combination of PM and S. aureus may provoke the predominance of Th2 cytokine production due to the activated status of Th2 cells. Meanwhile, increased production of IgE in serum of mice exposed to S. aureus or the combination of PM and S. aureus correlated positively with the upregulated Th2 cytokine production. Several studies reported that the development of allergic airway inflammation and bronchial asthma was associated with elevated IgE levels, the predominance of Th2 cytokine production, and eosinophils related-inflammation of the airways [7, 26].
Substance P, an important mediator of neurogenic inflammation, is released from the terminals of specific sensory nerves and inflammatory cells like lymphocytes, macrophages, eosinophils, and dendritic cells and acts through the neurokinin-1 receptor [1]. It has proinflammatory effects in immune and epithelial cells and plays a part in inflammatory diseases of the airway. Many substances induce neuropeptide secretion from sensory nerves in the lung [20]. In the present study, substance P in BALF was significantly changed in G1, G2, and G3 (P<0.01), but NT-3 was not significantly changed. These results showed that substance P plays an important role in lung inflammation in mice exposed to PM, S. aureus, or their combination. In addition, substance P is known to augment the production of cytokines in macrophages [20]. It also induces NO production and an oxidative burst in macrophages, resulting in the production of reactive oxygen intermediates [32]. In our study, IL-8, IL-4, NO, typical oxidative stress biomarkers, and macrophages were significantly changed in mice exposed to the combination of PM and S. aureus. These results suggested that the combination of PM and S. aureus induced inflammatory cells to release substance P, and that substance P augmented the production of IL-8 and IL-4 also induced NO production and an oxidative burst in macrophages, resulting in the production of ROS intermediates.
Acknowledgments
This study was supported by the Joint Fund for Fostering Talents of National Natural Science Foundation of China and Henan province (U1504303), Foundation of Henan Provincial Youth Backbone Teachers (2016GGJS-119), and Open Foundation of Key Laboratory of Industrial Ecology and Environmental Engineering (MOE) (KLIEEE-14-03). All authors declare that there is no conflict of interest.
References
- 1.Bignami F., Rama P., Ferrari G.2016. Substance P and its Inhibition in Ocular Inflammation. Curr. Drug Targets 17: 1265–1274. doi: 10.2174/1389450116666151019100216 [DOI] [PubMed] [Google Scholar]
- 2.Cai Y., Zhou J., Webb D.C.2012. Estrogen stimulates Th2 cytokine production and regulates the compartmentalisation of eosinophils during allergen challenge in a mouse model of asthma. Int. Arch. Allergy Immunol. 158: 252–260. doi: 10.1159/000331437 [DOI] [PubMed] [Google Scholar]
- 3.Chen B.Y., Chan C.C., Lee C.T., Cheng T.J., Huang W.C., Jhou J.C., Han Y.Y., Chen C.C., Guo Y.L.2012. The association of ambient air pollution with airway inflammation in schoolchildren. Am. J. Epidemiol. 175: 764–774. doi: 10.1093/aje/kwr380 [DOI] [PubMed] [Google Scholar]
- 4.Clarke R., Murthy G.K., Wolfson J.M., Sioutas C., Koutrakis P., Catalano P., Paulauskis J., Godleski J.J.1998. Concentrated ambient air particles cause pulmonary inflammation in a dose-dependent manner in both normal and chronic bronchitic rats. Pathophysiology 5: 105. doi: 10.1016/S0928-4680(98)80693-7 [DOI] [Google Scholar]
- 5.Martin S., Fernandez-Alanis E., Delfosse V., Evelson P., Yakisich J.S., Saldiva P.H., Tasat D.R.2010. Low doses of urban air particles from Buenos Aires promote oxidative stress and apoptosis in mice lungs. Inhal. Toxicol. 22: 1064–1071. doi: 10.3109/08958378.2010.523030 [DOI] [PubMed] [Google Scholar]
- 6.Fujimaki H., Saneyoshi K., Shiraishi F., Imai T., Endo T.1997. Inhalation of diesel exhaust enhances antigen-specific IgE antibody production in mice. Toxicology 116: 227–233. doi: 10.1016/S0300-483X(96)03539-1 [DOI] [PubMed] [Google Scholar]
- 7.Elhaik Goldman S., Moshkovits I., Shemesh A., Filiba A., Tsirulsky Y., Vronov E., Shagan M., Apte R.N., Benharroch D.A., Karo-Atar D., Dagan R., Munitz A., Mizrachi Nebenzahl Y., Porgador A.2016. Natural Killer Receptor 1 Dampens the Development of Allergic Eosinophilic Airway Inflammation. PLoS One 11: e0160779. doi: 10.1371/journal.pone.0160779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guenova E., Watanabe R., Teague J.E., Desimone J.A., Jiang Y., Dowlatshahi M., Schlapbach C., Schaekel K., Rook A.H., Tawa M., Fisher D.C., Kupper T.S., Clark R.A.2013. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin. Cancer Res. 19: 3755–3763. doi: 10.1158/1078-0432.CCR-12-3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall J.W., Yang J., Guo H., Ji Y.2017. The Staphylococcus aureus AirSR Two-Component System Mediates Reactive Oxygen Species Resistance via Transcriptional Regulation of Staphyloxanthin Production. Infect. Immun. 85: e00838-16. doi: 10.1128/IAI.00838-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He M., Ichinose T., Yoshida S., Yamamoto S., Inoue K., Takano H., Yanagisawa R., Nishikawa M., Mori I., Sun G., Shibamoto T.2012. Asian sand dust enhances murine lung inflammation caused by Klebsiella pneumoniae. Toxicol. Appl. Pharmacol. 258: 237–247. doi: 10.1016/j.taap.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Huvenne W., Lanckacker E.A., Krysko O., Bracke K.R., Demoor T., Hellings P.W., Brusselle G.G., Joos G.F., Bachert C., Maes T.2011. Exacerbation of cigarette smoke-induced pulmonary inflammation by Staphylococcus aureus enterotoxin B in mice. Respir. Res. 12: 69. doi: 10.1186/1465-9921-12-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeon W.Y., Shin I.S., Shin H.K., Lee M.Y.2015. Samsoeum water extract attenuates allergic airway inflammation via modulation of Th1/Th2 cytokines and decrease of iNOS expression in asthmatic mice. BMC Complement. Altern. Med. 15: 47. doi: 10.1186/s12906-015-0561-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji X., Han M., Yun Y., Li G., Sang N.2015. Acute nitrogen dioxide (NO2) exposure enhances airway inflammation via modulating Th1/Th2 differentiation and activating JAK-STAT pathway. Chemosphere 120: 722–728. doi: 10.1016/j.chemosphere.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 14.Jung W.W., Kim E.M., Lee E.H., Yun H.J., Ju H.R., Jeong M.J., Hwang K.W., Sul D., Kang H.S.2007. Formaldehyde exposure induces airway inflammation by increasing eosinophil infiltrations through the regulation of reactive oxygen species production. Environ. Toxicol. Pharmacol. 24: 174–182. doi: 10.1016/j.etap.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi F., Kodanikuchi K., Kakikawa M., Maki T., Yamada M., Tobo Y., Hung C.S., Matsukt A., Iwasaka Y.2010. Direct samplings, separated culture, and identifications of kosa bioaerosols over Noto Peninsula, Suzu City (Japanese). Earozoru Kenkyu 25: 23–28. [Google Scholar]
- 16.Lino-dos-Santos-Franco A., Correa-Costa M., Durão A.C., de Oliveira A.P., Breithaupt-Faloppa A.C., Bertoni J.A., Oliveira-Filho R.M., Câmara N.O., Marcourakis T., Tavares-de-Lima W.2011. Formaldehyde induces lung inflammation by an oxidant and antioxidant enzymes mediated mechanism in the lung tissue. Toxicol. Lett. 207: 278–285. doi: 10.1016/j.toxlet.2011.09.026 [DOI] [PubMed] [Google Scholar]
- 17.Luo B., Shi H., Wang L., Shi Y., Wang C., Yang J., Wan Y., Niu J.2014. Rat lung response to PM2.5 exposure under different cold stresses. Int. J. Environ. Res. Public Health 11: 12915–12926. doi: 10.3390/ijerph111212915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacNee W.2001. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 429: 195–207. doi: 10.1016/S0014-2999(01)01320-6 [DOI] [PubMed] [Google Scholar]
- 19.Nakagome K., Nagata M.2011. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx 38: 555–563. doi: 10.1016/j.anl.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 20.O’Connor T.M., O’Connell J., O’Brien D.I., Goode T., Bredin C.P., Shanahan F.2004. The role of substance P in inflammatory disease. J. Cell. Physiol. 201: 167–180. doi: 10.1002/jcp.20061 [DOI] [PubMed] [Google Scholar]
- 21.Parumasivam T., Ashhurst A.S., Nagalingam G., Britton W.J., Chan H.K.2017. Inhalation of Respirable Crystalline Rifapentine Particles Induces Pulmonary Inflammation. Mol. Pharm. 14: 328–335. doi: 10.1021/acs.molpharmaceut.6b00905 [DOI] [PubMed] [Google Scholar]
- 22.Peters A., Veronesi B., Calderón-Garcidueñas L., Gehr P., Chen L.C., Geiser M., Reed W., Rothen-Rutishauser B., Schürch S., Schulz H.2006. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part. Fibre Toxicol. 3: 13. doi: 10.1186/1743-8977-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman I., MacNee W.2000. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 16: 534–554. doi: 10.1034/j.1399-3003.2000.016003534.x [DOI] [PubMed] [Google Scholar]
- 24.Riva D.R., Magalhães C.B., Lopes A.A., Lanças T., Mauad T., Malm O., Valença S.S., Saldiva P.H., Faffe D.S., Zin W.A.2011. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal. Toxicol. 23: 257–267. doi: 10.3109/08958378.2011.566290 [DOI] [PubMed] [Google Scholar]
- 25.Roggen E.L., Soni N.K., Verheyen G.R.2006. Respiratory immunotoxicity: an in vitro assessment. Toxicol. In Vitro 20: 1249–1264. doi: 10.1016/j.tiv.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 26.Samitas K., Delimpoura V., Zervas E., Gaga M.2015. Anti-IgE treatment, airway inflammation and remodelling in severe allergic asthma: current knowledge and future perspectives. Eur. Respir. Rev. 24: 594–601. doi: 10.1183/16000617.00001715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schieber M., Chandel N.S.2014. ROS function in redox signaling and oxidative stress. Curr. Biol. 24: R453–R462. doi: 10.1016/j.cub.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh J.K., Simões B.M., Howell S.J., Farnie G., Clarke R.B.2013. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 15: 210–218. doi: 10.1186/bcr3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F., Li C., Liu W., Jin Y.2012. Effect of exposure to volatile organic compounds (VOCs) on airway inflammatory response in mice. J. Toxicol. Sci. 37: 739–748. doi: 10.2131/jts.37.739 [DOI] [PubMed] [Google Scholar]
- 30.Wang F., Li C., Liu W., Jin Y., Guo L.2014. Effects of subchronic exposure to low-dose volatile organic compounds on lung inflammation in mice. Environ. Toxicol. 29: 1089–1097. doi: 10.1002/tox.21844 [DOI] [PubMed] [Google Scholar]
- 31.Wang F., Liu F., Liu H.2016. Effect of exposure to staphylococcus aureus, particulate matter, and their combination on the neurobehavioral function of mice. Environ. Toxicol. Pharmacol. 47: 175–181. doi: 10.1016/j.etap.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 32.Yaraee R., Ebtekar M., Ahmadiani A., Sabahi F., Ghazanfari T.2007. The effect of substance P on nitric oxide production by HSV-1 infected macrophages. Int. Immunopharmacol. 7: 135–139. doi: 10.1016/j.intimp.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 33.Zhao H., Ma J.K., Barger M.W., Mercer R.R., Millecchia L., Schwegler-Berry D., Castranova V., Ma J.Y.2009. Reactive oxygen species- and nitric oxide-mediated lung inflammation and mitochondrial dysfunction in wild-type and iNOS-deficient mice exposed to diesel exhaust particles. J. Toxicol. Environ. Health A 72: 560–570. doi: 10.1080/15287390802706330 [DOI] [PubMed] [Google Scholar]