Abstract
The changes in intra-atrial blood coagulability of acute phase after development of atrial fibrillation (AF) have not been elucidated in human. In the present study, blood coagulability were examined in the intra-atrial and peripheral regions during the acute phase after development of rapid atrial pacing (RAP) in experimentally created model dog similar to AF, using Total Thrombus-formation Analysis System (T-TAS) that is capable of comprehensively evaluating thrombogenicity in the bloodstream in the microvascular channel. According to the results, both the coagulating function-evaluating time to +10 kPa (T10) and occlusion time (OT) of the AR chip (chip for thrombus analysis mixed with coagulation and platelet) were significantly shortened in the atrial blood as early as 30 min after pacing (T10, 150.5 ± 40.5 s; OT, 212.4 ± 44.3 s) compared to the pre-pacing levels (T10, 194.5 ± 47.5 s; OT, 259.9 ± 49.5 s) (P<0.05). The OT of PL chip (chip for platelet thrombus analysis) was significantly shortened 30 min after pacing (231.8 ± 57.6 s), compared to the pre-pacing level (289.5 ± 96.0 s) (P<0.05). Meanwhile, none of T10 and OT of AR and PL chips showed any significant changes in the peripheral blood. The study demonstrated increase of blood coagulability 30 min after development of RAP. While no significant changes were observed in the peripheral blood in the present study, the outcome suggested that the anti-thrombus treatments are better to be started early after AF even if coagulability of the peripheral blood shows no change.
Keywords: atrial fibrillation, dog, rapid atrial pacing, thrombogenicity, total thrombus-formation analysis system
Introduction
Atrial fibrillation (AF) is one of the arrhythmias often observed in humans and dogs [23, 30, 41]. While several AF classifications exist, the Guidelines for Pharmacotherapy of Atrial Fibrillation for humans (JCS 2013) adopt the “incipient, paroxysmal, sustained and persistent” AF classification because it is simple and easy to use for clinicians [20]. According to the classification, paroxysmal AF is restored to normal sinus rhythm within 7 days (mostly 48 h) regardless of whether medication or no-medication therapy is implemented [5, 20, 22], and corresponds to the early phase of AF, considering its long chronic process. Among patients with paroxysmal AF, 5.6–33.1% shift to sustained AF after 7 days [32, 39].
It is known that thrombus is formed in the heart, especially in the atrium of humans with AF [5, 6, 14, 20, 22,23,24,25,26,27, 30, 32, 34, 36, 37, 39, 42]. In human medicine, AF accounts for the largest proportion (45%) among the causes of cardiogenic cerebral embolism [14], and has been the focus of considerable attention among the thromboembolism-causing diseases. Therefore, thrombus formation or predisposition to thrombus formation should be detected at an early stage. When cerebral infarction is caused by cardiogenic thrombus, the prognosis is very poor with a 1-year survival rate of about 50% [24]. According to the findings currently available, no significant difference exists in the cumulative occurrence rate of cerebral infarction between paroxysmal and sustained AF [17]. Even if AF continues for a short time period, the risk of thrombus formation should be considered at all times. However, human and veterinary medicine have not yet revealed at which time do the blood coagulability change in the atrial or peripheral regions after triggered AF [4, 11, 20, 29, 31, 36, 37].
The risk of developing AF-associated cerebral infarction can be evaluated using CHADS2 (C: congestive heart failure, H: hypertension, A: age of ≥75 years, D: diabetes mellitus, S2: stroke/TIA) or CHADS2-VASc (V: vascular disease, A: age of 65–74 years, Sc: sex category) scores of the American Heart Association/American College of Cardiology and European Society of Cardiology. Although not all high-risk patients develop thromboembolism, anticoagulant therapy has been provided for patients depending on their risk score [5, 10, 22]. Thrombus formation was visualized on transesophageal echocardiography [1, 13, 25, 30, 33]. However, this examination was not considered to be applicable to all patients because of its invasiveness. Therefore, the development of any alternative examination index other than clinical findings was desirable for patients who require anticoagulant therapy.
Very recently, the Total Thrombus-formation Analysis System (T-TAS) has been developed for medical use, which can measure the platelet function and coagulation factor function under a condition resembling that of a living person [18, 19, 44]. This system can numerically and visually evaluate, in detail, the thrombus formation in the bloodstream. And T-TAS has also been reported to be clinically applicable in dogs [21].
In addition to the conventional blood coagulation tests including prothrombin time (PT), active partial thromboplastin time (APTT), and tests for measuring fibrinogen (Fibn), D-dimer, and antithrombin III (AT III) levels, the present study compared and evaluated the blood coagulability in the atrial and peripheral regions immediately after RAP in experimentally created model dog similar to AF, using the new blood-coagulation-evaluating equipment T-TAS, aiming to detect, at an early stage, the risk of thrombus formation.
Materials and Methods
Approvals
This study was conducted in accordance with the Ethical Code of Animal Experiment of Tokyo University of Agriculture and Technology (approval No. 27-39). All experimental animal procedures were performed in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals (2011).
Animals
Sixteen (8 male and 8 female) healthy beagles were included in the study. The animals were 1 to 5 years old, with body weight of 9.0 to 12.5 kg, at the start of the study. They were housed individually in a dog run maintained at a temperature of 21 ± 2°C and a relative humidity of 50 ± 20%, with a 12 h : 12 h light : dark cycle. All animals were received commercial chow twice daily, and water was provided ad libitum in stainless steel containers. Physical examination, routine lead II electrocardiogram (ECG) recording, complete blood count, and blood chemistry analysis were performed to evaluate healthy status in all dogs before the beginning of the experiment.
Methods for creating the model dog similar to AF
Under general anesthesia, a pacing lead was implanted in the animals in accordance with the guidelines provided by Fukushima [9] and Gaspo et al. [12]. In the present study, for stimulation of the atrium by RAP (2 mV, 390 bpm), artificial tachycardia was immediately triggered and maintained in the dog, and hemodynamics resembling the spontaneously developed similar to AF were obtained [9, 12] (Fig. 1). Briefly, the animals were pre-anesthetized with 30 µg/kg atropine sulfate (subcutaneous injection, atropine sulfate, Mitsubishi Tanabe Pharma Co., Osaka, Japan), 0.2 mg/kg of butorphanol tartrate (intravenous injection, Betorufaru, Meiji Seika Pharma Co., Ltd., Tokyo, Japan), and 0.2 mg/kg of midazolam (intravenous injection, Dormicum, Astellas Pharma, Tokyo, Japan). For induction and maintenance of anesthesia, 6 mg/kg propofol (intravenous injection, propofol, Fresenius Kabi, Tokyo, Japan) and isoflurane (inhalation administration, Isoflu, DS Pharma Animal Health, Osaka, Japan) were administered, respectively. The anesthetized animals were held in the right-side recumbent position, and a thoracotomy was performed at the left fourth intercostal region. A pacemaker electrode lead terminal, dipole electrode lead (TY216-033, Unique Medical Co., Ltd., Tokyo, Japan), was fixed on the left atrium by 5-0 polypropylene sutures (Proline, Johnson and Johnson Co., Ltd., Tokyo, Japan). The intercostal region was closed in accordance with the routine procedures, and the operation was completed after introducing the connecting terminal of the pacing lead with the main body of the pacemaker from the back region to outside the body. Animals were housed to rest for 10 days after the implantation surgery, considering the effect of the surgery. During the resting period, 0.2 mg/kg meloxicam (subcutaneous injection, injection for 3 days, Metacam 0.5%, Nippon Boehringer Ingelheim Co., Ltd., Tokyo, Japan) and 5 mg/kg enrofloxacin (subcutaneous injection, injection for 10 days, Baytril, Bayer Pharmaceutical, Osaka, Japan) were administered for analgesia and infection prevention, respectively. After the resting period, in 8 of 16 dogs (4 male and 4 female), the pacing lead was connected to an external pacemaker, and RAP (2 mV, 390 bpm) was started (Pacing group). On the other hand, pacing was not performed for the remaining 8 dogs (Control group).
Fig. 1.
Electrocardiogram during rapid atrial pacing (2 mV, 390 bpm). In the electrocardiogram, it was confirmed that the disappearance of P wave, absolute irregularity of R-R. The model can reproduce the state similar to AF by rapid atrial pacing.
Blood sampling
The model dogs were underwent general anesthetized (in accordance with the protocol similar to that used for creating the AF model), and blood samples were collected at 5:00 PM to 8:00 PM. The animals were held in the right-side recumbent position, and the skin was incised at the cervical region to expose the jugular vein. The jugular vein was held with a surgical support thread and slightly incised. A nutritional catheter (Atom nutritional catheter 5 Fr, Atom Medical International Inc., Tokyo, Japan) was inserted in the jugular vein from the incised region. The catheter was led to the right atrium while confirming the tip under X-ray illumination, and “Atrial blood sample” was collected. “Peripheral blood sample” was collected from the lateral saphenous vein by using a 20-G winged needle. Blood samples were collected before and 30 min after the start of RAP. In addition, blood sampling is to be carried out while performing the RAP.
T-TAS
Two different kinds of exclusive chips, the AR chip (chip for thrombus analysis mixed with coagulation and platelet) and the PL chip (chip for platelet thrombus analysis), were used in the T-TAS. The AR chip was coated inside with collagen and thromboplastin. By flowing whole blood into the AR chip, thrombus formation was accelerated by the interaction between platelets and the blood coagulating system. Platelet-containing fibrin-rich thrombus was formed, and the pressure inside the chip increased owing to the occlusion of the flow channel. The PL chips were coated only with collagen. By flowing hirudin-treated whole blood into the PL chip, platelets adhered inside the chip and agglutinated (Fig. 2). The pressure inside the chip increased owing to the occlusion of the flow channel by the formation of platelet-specific thrombus. Coagulation statuses were continuously monitored by determining increases in the pressure inside the chip [17, 18, 43]. Thrombus formation-associated increases in the internal pressures of the AR and PL chips were measured after putting whole atrial and peripheral blood samples into the micro flow channels of the chips. Clogging start time (T10; the time required to reach internal pressure of +10 kPa from the baseline) and occlusion time (OT; the time required to reach +80 kPa from the baseline in the AR chip and +60 kPa from the baseline in the PL chip, respectively) were measured, and the area under the flow pressure curve (AUC: area under the flow pressure curve) of the pressure-increasing curve was calculated (Fig. 3).
Fig. 2.
Schematic diagram of T-TAS (PL chip). The PL chip was coated with collagen (a). By flowing hirudin-treated whole blood into the PL chip, platelets adhered inside the chip and agglutinated. The pressure inside the chip increased owing to the occlusion of the flow channel by the formation of platelet-specific thrombus (b).
Fig. 3.
Figure of pressure rise curve (one example of PL with atrial blood as a sample). In the PL chip of the T-TAS, clogging start time (T10, the time required to reach internal pressure +10 kPa from the baseline) and occlusion time (OT, the time required to reach +60 kPa from the baseline of the PL chip) were measured, and the area under the flow pressure curve (AUC) of the pressure increase was calculated. In this figure, T10 is expressed as 148 s and OT as 202 s. Compared with Pre, it can be seen that OT is shortened to 30 min.
Conventional blood coagulation tests
Each of 0.45 ml the atrial and peripheral blood samples were placed in centrifugal tubes containing 0.05 ml of 3.2% citric acid and centrifuged to obtain plasmas. The atrial blood plasma in Pacing group was tested for prothrombin time (PT), active partial thromboplastin time (APTT), and fibrinogen (Fibn), D-dimer, and antithrombin III (AT III) levels, whereas the peripheral blood plasma in Pacing and Control group, and the atrial blood plasma in Control group were tested for PT, APTT, and Fibn level. A blood coagulation analyzer (Wako Coag 2V: M&T Corporation, Hiroshima, Japan) was used to determine PT, APTT, and Fibn level. D-dimer and AT III levels were analyzed using latex turbidimetric immunoassay (Fujifilm Monolith Co., Ltd., Tokyo, Japan) and a synthetic substrate method (Fujifilm Monolith Co., Ltd.), respectively. These test systems used in the present study are an inspection device developed for human use, but they have been proved to be clinically usable also in dogs.
Statistical analysis
Measurement values were expressed as mean ± SD. To confirm the normal distribution of the measurement values at pre-pacing and 30 min-pacing, normal probability plots were obtained. For measurement values within the Pacing and Control groups, the normality of the distribution was tested using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Measurement values with normal distributions were analyzed using paired t-test. Meanwhile, those without normal distributions were analyzed using the Wilcoxon signed-rank test. To compare the pre-pacing values between Pacing groups and Control group, t-test or Mann-Whitney U-test was performed. P-values <0.05 were considered statistically significant. All statistical processing was performed by statistical software (BellCurve for Excel, Social Survey Research Information Co., Ltd., Tokyo, Japan).
Results
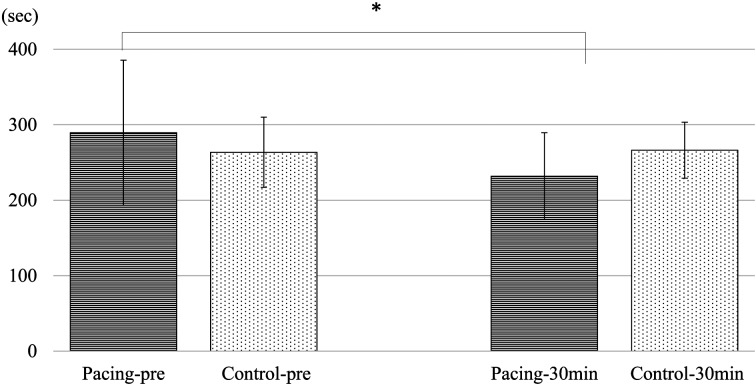
T-TAS: In the PL chip of the atrial blood of the Pacing group, OT significantly shortened to 231.8 ± 57.6 s at 30 min after pacing, compared to 289.5 ± 96.0 s before pacing (P<0.05) (Fig. 2). Meanwhile, T10 showed a shortening tendency, though not significantly, at 136.5 ± 29.8 s, compared to 143.0 ± 43.1 s before pacing. AUC significantly increased to 426.0 ± 42.4 at 30 min after pacing, compared to 386.8 ± 72.2 before pacing (P<0.05). In the AR chip of the atrial blood, T10 significantly shortened to 150.5 ± 40.5 s at 30 min after pacing, compared to 194.5 ± 47.5 s before pacing (P<0.05). OT similarly shortened to 212.4 ± 44.3 s at 30 min after pacing, compared to 259.9 ± 49.5 s before pacing (P<0.05). AUC significantly increased to 2,164.3 ± 53.0 at 30 min after pacing, compared to 2,100.8 ± 60.3 before pacing (P<0.05). In the PL and AR chips of the peripheral blood, no significant changes were observed in any of the test items.
Meanwhile, no significant changes were observed in T10, OT, and AUC of the AR and PL chips of the atrial and peripheral blood of the Control group. Further, no significant difference was observed in the pre- pacing measurement values between the Pacing and Control groups (Figs. 4 and 5) (Tables 1 and 2)
Fig. 4.
Occlusion time of the PL chip for the atrial blood in the Pacing and Control groups. Occlusion time of the PL chip for the atrial blood in the Pacing group significantly shortened at 30 min after rapid atrial pacing compared to the pre-rapid atrial pacing level (*compared with the pre-rapid atrial pacing level, a significant difference in P<0.05). No significant changes were observed in the Control group. Pacing group (Pacing): Group with rapid atrial pacing of 390 bpm, 2 mV, n=8. Control group (Control): Group with non-rapid atrial pacing, n=8. Pre: pre-rapid atrial pacing, 30 min: 30 min after rapid atrial pacing. Measurement values are expressed as mean ± SD.
Fig. 5.
Occlusion time of the AR chip for atrial blood in the Pacing and Control groups. Occlusion time of the AR chip for the atrial blood in the Pacing group significantly shortened at 30 min after stimulation compared to the pre-rapid atrial pacing level (*compared with the pre-rapid atrial pacing level, a significant difference in P<0.05). No significant changes were observed in the Control group. Pacing group (Pacing): Group with rapid atrial pacing of 390 bpm, 2 mV, n=8. Control group (Control): Group with non-rapid atrial pacing, n=8. Pre: pre-rapid atrial pacing, 30 min: 30 min after rapid atrial pacing. Pre: pre-rapid atrial pacing, 30 min: 30 min after rapid atrial pacing. Measurement values are expressed as mean ± SD.
Table 1. Changes with blood coagulation test items in the Pacing and Control groups.
| Pre | 30 min | |||
|---|---|---|---|---|
| Pacing group | Atrial blood | PT (sec) | 7.5 ± 0.5 | 7.7 ± 0.5 |
| APTT (sec) | 17.5 ± 1.9 | 18.0 ± 5.3 | ||
| Fibn (mg/dl) | 250.2 ± 36.5 | 283.0 ± 66.0 | ||
| D-dimer (μg/ml) | 1.46 ± 1.0 | 1.78 ± 1.5 | ||
| AT III (%) | 152.4 ± 39.3 | 169.5 ± 28.8 | ||
| Peripheral blood | PT (sec) | 7.4 ± 0.3 | 7.4 ± 0.4 | |
| APTT (sec) | 18.3 ± 3.5 | 16.9 ± 4.1 | ||
| Fibn (mg/dl) | 267.6 ± 68.4 | 256.3 ± 51.4 | ||
| Control group | Atrial blood | PT (sec) | 7.9 ± 0.3 | 7.8 ± 0.2 |
| APTT (sec) | 21.2 ± 2.4 | 20.4 ± 3.8 | ||
| Fibn (mg/dl) | 210.4 ± 35.2 | 217.8 ± 25.2 | ||
| Peripheral blood | PT (sec) | 7.8 ± 0.3 | 7.9 ± 0.4 | |
| APTT (sec) | 22.5 ± 5.5 | 20.7 ± 5.2 | ||
| Fibn (mg/dl) | 223.0 ± 42.2 | 205.6 ± 26.5 | ||
There was no significant difference from before to after rapid atrial pacing in any of the measurement variables in the Pacing and Control groups. No significant differences were observed in the pre-stimulation levels between the Pacing and Control groups. Measurement values are expressed as mean ± SD. Pacing group: Group with rapid atrial pacing of 390 bpm, 2 mV, n=8. Control group: Group with non-rapid atrial pacing, n=8. Pre, pre- rapid atrial pacing; 30 min, 30 min after rapid atrial pacing; PT, prothrombin time (reference value, 7.4–8.8 s); APTT, active partial thromboplastin time (reference value, 12.0–28.8 s); fibn, fibrinogen (reference value, 150–350 mg/dl); D-dimer (reference value, <2.0 µg/dl); AT III, antithrombin III (reference value, 102–156%).
Table 2. T-TAS measurements for atrial blood samples in the Pacing and Control groups.
| Pre | 30 min | |||
|---|---|---|---|---|
| Pacing group | AR | T10 (sec) | 194.5 ± 47.5 | 150.5 ± 40.5* |
| OT (sec) | 259.9 ± 49.5 | 212.4 ± 44.3* | ||
| AUC | 2,100.8 ± 60.3 | 2,164.3 ± 53.0* | ||
| PL | T10 (sec) | 143.0 ± 43.1 | 136.5 ± 29.8 | |
| OT (sec) | 289.5 ± 96.0 | 231.8 ± 57.6* | ||
| AUC | 386.8 ± 72.2 | 426.0 ± 42.4* | ||
| Control group | AR | T10 (sec) | 184.8 ± 35.2 | 187.3 ± 34.2 |
| OT (sec) | 263.6 ± 46.1 | 268.3 ± 46.3 | ||
| AUC | 2,106.9 ± 47.9 | 2,108.5 ± 56.8 | ||
| PL | T10 (sec) | 121.6 ± 21.6 | 128.2 ± 31.8 | |
| OT (sec) | 263.5 ± 46.6 | 266.3 ± 37.1 | ||
| AUC | 437.4 ± 29.9 | 441.0 ± 38.8 | ||
OT and AUC of the PL chip for the atrial blood significantly decreased at 30 min after stimulation compared to the pre-stimulation level. T10, OT and AUC of the AR chip for the atrial blood significantly decreased at 30 min after stimulation compared to the pre-stimulation level. No significant changes were observed in T10, OT, and AUC of the AR and PL chips of the Control group. No significant differences were observed in the pre-stimulation levels between the Pacing and Control groups. (*Compared with the pre-stimulation level, a significant difference in P<0.05). Measurement values are expressed as mean ± SD. Pacing group: Group with rapid atrial pacing of 390 bpm, 2 mV, n=8. Control group: Group with non-rapid atrial pacing, n=8. Pre, pre-papid atrial pacing; 30 min, 30 min after-papid atrial pacing; AR,chip for thrombus analysis mixed with coagulation and platelet; PL, chip for platelet thrombus analysis; T10, the time required to reach +10 kPa of the internal pressure; OT, the time required to reach +80 kPa in the AR chip and +60 kPa in the PL chip; AUC, the area under the flow pressure curve.
Conventional blood coagulation tests: No significant changes were observed from the pre-pacing to 30 min-pacing values in the Pacing group, regarding PT, APTT, and Fibn, D-dimer, and AT III levels of atrial or peripheral blood, as they were all within the normal range. Similar observations were obtained in the Control group. Further, no significant difference was observed in the pre-pacing measurements between the Pacing and Control groups (Table 3).
Table 3. T-TAS measurements of peripheral blood samples in the Pacing and Control groups.
| Pre | 30 min | |||
|---|---|---|---|---|
| Pacing group | AR | T10 (sec) | 174.7 ± 56.7 | 162.2 ± 49.6 |
| OT (sec) | 253.3 ± 102.0 | 233.5 ± 83.4 | ||
| AUC | 2,108.1 ± 117.9 | 2,134.0 ± 87.0 | ||
| PL | T10 (sec) | 122.1 ± 23.5 | 128.6 ± 44.1 | |
| OT (sec) | 239.2 ± 78.4 | 229.6 ± 77.2 | ||
| AUC | 424.7 ± 43.2 | 429.1 ± 57.7 | ||
| Control group | AR | T10 (sec) | 177.5 ± 60.4 | 179.3 ± 38.5 |
| OT (sec) | 255.4 ± 45.7 | 254.5 ± 59.1 | ||
| AUC | 2,099.5 ± 51.7 | 2,106.2 ± 44.2 | ||
| PL | T10 (sec) | 119.9 ± 21.1 | 127.6 ± 28.5 | |
| OT (sec) | 260.5 ± 53.1 | 257.3 ± 60.9 | ||
| AUC | 426.2 ± 35.5 | 425.2 ± 51.8 | ||
There was no significant difference from before to after rapid atrial pacing in any of the measurement variables in the Pacing and Control groups. No significant differences were observed in the pre-stimulation levels between the Pacing and Control groups. Measurement values are expressed as mean ± SD. Pacing group: Group with rapid atrial pacing of 390 bpm, 2 mV, n=8. Control group: Group with non-rapid atrial pacing, n=8. Pre, pre-papid atrial pacing; 30 min, 30 min after-papid atrial pacing; AR,chip for thrombus analysis mixed with coagulation and platelet; PL, chip for platelet thrombus analysis; T10, the time required to reach +10 kPa of the internal pressure; OT, the time required to reach +80 kPa in the AR chip and +60 kPa in the PL chip; AUC, the area under the flow pressure curve.
Discussion
The Virchow triad consisting of (1) change in blood flow, (2) change in blood properties, and (3) change in blood components presents 3 thrombosis-causing factors [2, 26, 43]. Of the 3 factors, hemostasis and change in blood coagulability are considered to be mainly involved in induced AF in human medicine [40]. Thrombus formed by AF in humans used to be called red thrombus, rich in fibrin and erythrocytes, or venous thrombus [27], but it is now considered to be a mixed thrombus that additionally contains platelets [34]. Nevertheless, our experiment suggested that both platelets and the coagulation system interactively contribute to RAP-caused thrombus formation, since OT of both AR and PL chips of the atrial blood was shortened at 30 min after AF development.
PT and APTT are used in screening tests to examine deficiency or adequacy of coagulation factors in the extrinsic and intrinsic coagulation pathway, respectively [3, 28]. And Fibn, a precursor of fibrin, has a strong netlike membrane structure, with the function of reinforcing a platelet-containing thrombus. In the present study, no significant changes were observed in PT, APTT and Fibn of the atrial blood in either group.
The increase in D-dimer level in human patients with AF is considered useful in confirming thrombus formation [7, 15, 38]. AT III is a physiological serine protease inhibitor that suppresses coagulation reaction in the blood [8, 16]. As a result, coagulation-suppressing function decreases and predisposition to thrombosis develops, as generally known. In the present study, no significant changes were observed in either D-dimer or AT III possibly because complete thrombus formation did not occur for a short 30-min period, though blood coagulability changed through activation of the intra-atrial coagulation factor.
Measurement of the abovementioned coagulation system factors may be useful in evaluating the extent of reaction occurring partly in the entire coagulation cascade [3, 7, 8, 15, 16, 28, 38]. However, such evaluation is done only by piecing together the in vitro reactions. Hemostasis and bleeding under actual biological reactions are controlled by many common factors, a complex reaction consisting of thrombus formation (platelet activity and coagulation factor activity), and the fibrinolytic system. This is a conventional coagulation system test, although it has been considered difficult to use it to monitor thrombus formation. In recent years, coagulation systems using whole blood, such as ROTEM, PFA-100, and T-TAS, have attracted attention [3, 18, 19, 35, 44]. However, ROTEM and PFA-100 are measurement systems used under conditions in which there is no blood flow, which greatly contributes to thrombus formation. On the other hand, in T-TAS, collagen and tissue factors in the flow path activate part of the specimen via the flow of the coagulation-treated whole blood into the microchannel, which is a simulated blood vessel, while changing the flow rate, and this triggers. It is an instrument that can induce physiological thrombus formation that matches that which occurs in vivo. Therefore, by performing T-TAS examination, it is now considered possible to evaluate thrombogenicity, which was not previously evaluable [18, 19, 44]. In the future, it will be important to perform comparisons of T-TAS with other measurement systems.
According to the reports on the timing of thrombus formation in humans, thrombus was formed in 5–14% of the patients with AF, continuing for 2 or more days, and 14% of patients with acute AF [29, 37], and therefore, platelets and coagulation factors may become active within 12 h after the induced AF [36]. However, T-TAS used in the present study demonstrated that the platelet function and coagulability increased in the atrium as quickly as 30 min after RAP development. In addition, since there was no significant difference in T10, OT, and AUC of the AR and PL chips of the atrial blood of the Control group, it was revealed that the thrombogenicity depended on pacing. Nevertheless, formation of thrombus in the atrium was not confirmed at a short 30-min period, and therefore, the 30-min process may not have been long enough for thrombus formation.
As far as we know, no reports that compared the changes in blood coagulability between intra-atrial and peripheral regions immediately after induced RAP have been published. In the present study, dramatic changes in blood coagulability were observed only in the atrium, while no significant changes were observed in any test items for the peripheral region, possibly because blood stagnation may be less and endothelial disorder may be less caused by physical fibrillating stimulus in the peripheral region. These results suggested that coagulability may have increased in the atrium, even if no coagulative abnormality is observed in the peripheral blood of humans with AF.
The AR chip and PL chip used in this experiment it is said that the platelet count are not affected [18, 19], but in the present study, the detailed examination of these values is not done, so it is necessary to be careful about the interpretation. Also, we have not examined hematocrit in atrial blood. It is said that a difference in hematocrit causes artificial changes in PT and APTT due to changing ratio of anticoagulant in the citrated plasma, and changing hematocrit modulates platelet function due to rheological change in the whole blood. Therefore, the interpretation of the results of atrial blood is considered necessary to be careful enough. In the future, evaluation of platelet count, hematocrit value, atrial motility and histopathological examination may be needed to prove these occurrences.
The animals used were healthy and young at the time of the study. RAP of these animals may be defined as incipient, according to the Guidelines for Pharmacotherapy of Atrial Fibrillation (JCS 2013). Therefore, atrial pathological change in the atrium, which was the basic condition of RAP, should have been mild. Accordingly, the change in atrial blood coagulability of the patients with spontaneous AF may possibly be more dramatic compared with the observation of the present study. And in the present study, we created a hemodynamics similar to AF by RAP at the site where the pacemaker was installed. However, in clinical AF, because the microreentry occurs in various site of the atrium, dogs in this AF model are different from the clinical AF. This may have some influence on the results of this present study. Meanwhile, as pacing was conducted for the left atrium in the present study, the blood was collected from the right atrium, in order to remove the effect of cell damage that might cause thrombus formation and also for the sake of ease in blood collection.
Conclusion
The present experiment demonstrated, using T-TAS, that the platelet function and coagulability increased in the atrial blood at 30 min after induced RAP. The present experiment produced the first report on an analysis of the changes in the coagulability of the atrial blood during the acute phase of RAP in dogs. The observation of the early appearance of thrombotic predisposition suggested that anti-thrombus treatments are better to be started quickly in clinical practice once AF is detected, which may help present a new therapeutic direction.
Conflict of Interest
This study was supported by the MEXT/JSPS KAKENHI (grant no. 26450425).
Acknowledgments
We would like to thank Kazuya Hosokawa and Tomoko Ohnishi-Wada, Fujimori Kogyo Co., Ltd., for their cooperation for the instruction and operation of the T-TAS and adjustment of the apparatus.
References
- 1.Asinger R.W., Koehler J., Pearce L.A., Zabalgoitia M., Blackshear J.L., Fenster P.E., Strauss R., Hess D., Pennock G.D., Rothbart R.M., Halperin J.L.1999. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: II. Dense spontaneous echocardiographic contrast (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study). J. Am. Soc. Echocardiogr. 12: 1088–1096. doi: 10.1016/S0894-7317(99)70106-9 [DOI] [PubMed] [Google Scholar]
- 2.Bagot C.N., Arya R.2008. Virchow and his triad: a question of attribution. Br. J. Haematol. 143: 180–190. doi: 10.1111/j.1365-2141.2008.07323.x [DOI] [PubMed] [Google Scholar]
- 3.Brooks M.B., Catalfamo J.L.2013. Current diagnostic trends in coagulation disorders among dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 43: 1349–1372, vii. doi: 10.1016/j.cvsm.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 4.Brownlie S.E., Cobb M.A.1999. Observations on the development of congestive heart failure in Irish wolfhounds with dilated cardiomyopathy. J. Small Anim. Pract. 40: 371–377. doi: 10.1111/j.1748-5827.1999.tb03102.x [DOI] [PubMed] [Google Scholar]
- 5.Camm A.J., Lip G.Y., De Caterina R., Savelieva I., Atar D., Hohnloser S.H., Hindricks G., Kirchhof P., ESC Committee for Practice Guidelines (CPG)2012. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 33: 2719–2747. doi: 10.1093/eurheartj/ehs253 [DOI] [PubMed] [Google Scholar]
- 6.Choudhury A., Lip G.Y.2003–2004. Atrial fibrillation and the hypercoagulable state: from basic science to clinical practice. Pathophysiol. Haemost. Thromb. 33: 282–289. doi: 10.1159/000083815 [DOI] [PubMed] [Google Scholar]
- 7.Danese E., Montagnana M., Cervellin G., Lippi G.2014. Hypercoagulability, D-dimer and atrial fibrillation: an overview of biological and clinical evidence. Ann. Med. 46: 364–371. doi: 10.3109/07853890.2014.912835 [DOI] [PubMed] [Google Scholar]
- 8.Fukuda Y., Kuroiwa Y., Okumiya K., Kuroiwa N., Ohshige T., Tabuchi H., Sanada J., Takaoka S., Minami Y., Kataoka H., Furukawa S., Miyahara K., Nakamura K., Hashimoto S.1980. Hypercoagulability in patients with mitral stenosis -- from the viewpoint of the behavior of plasma antithrombin III and alpha2-plasmin inhibitor. Jpn. Circ. J. 44: 867–874. doi: 10.1253/jcj.44.867 [DOI] [PubMed] [Google Scholar]
- 9.Fukushima R., Tanaka R., Matsumoto H., Machida N., Hirose H., Yamane Y., Koyama H.2010. Effects of enfonidipine hydrochloride in dogs with experimental supraventricular tachyarrhythmia. J. Vet. Med. Sci. 72: 833–838. doi: 10.1292/jvms.09-0358 [DOI] [PubMed] [Google Scholar]
- 10.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J.2001. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285: 2864–2870. doi: 10.1001/jama.285.22.2864 [DOI] [PubMed] [Google Scholar]
- 11.Gallagher M.M., Hennessy B.J., Edvardsson N., Hart C.M., Shannon M.S., Obel O.A., Al-Saady N.M., Camm A.J.2002. Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J. Am. Coll. Cardiol. 40: 926–933. doi: 10.1016/S0735-1097(02)02052-1 [DOI] [PubMed] [Google Scholar]
- 12.Gaspo R., Bosch R.F., Talajic M., Nattel S.1997. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation 96: 4027–4035. doi: 10.1161/01.CIR.96.11.4027 [DOI] [PubMed] [Google Scholar]
- 13.Goldman M.E., Pearce L.A., Hart R.G., Zabalgoitia M., Asinger R.W., Safford R., Halperin J.L.1999. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study). J. Am. Soc. Echocardiogr. 12: 1080–1087. doi: 10.1016/S0894-7317(99)70105-7 [DOI] [PubMed] [Google Scholar]
- 14.Greer D.M., Homma S., Furie K.L.2011. Cardiac diseases. pp. 814–827. In: Stroke 5th ed: Pathophysiology, Diagnosis, and Management (Mohr, J.P., Wolf, P.A., Grotta, J.C., Moskowitz, M.A., Mayberg, M. and Kummer, R.V., eds.), Elsevier Saunders, Philadelphia. [Google Scholar]
- 15.Habara S., Dote K., Kato M., Sasaki S., Goto K., Takemoto H., Hasegawa D., Matsuda O.2007. Prediction of left atrial appendage thrombi in non-valvular atrial fibrillation. Eur. Heart J. 28: 2217–2222. doi: 10.1093/eurheartj/ehm356 [DOI] [PubMed] [Google Scholar]
- 16.Hoefer J., Ulmer H., Kilo J., Margreiter R., Grimm M., Mair P., Ruttmann E., Innsbruck Liver-in-Heart-Failure Program2017. Antithrombin III is associated with acute liver failure in patients with end-stage heart failure undergoing mechanical circulatory support. J. Thorac. Cardiovasc. Surg. 153: 1374–1382. doi: 10.1016/j.jtcvs.2017.01.053 [DOI] [PubMed] [Google Scholar]
- 17.Hohnloser S.H., Pajitnev D., Pogue J., Healey J.S., Pfeffer M.A., Yusuf S., Connolly S.J., ACTIVE W Investigators2007. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J. Am. Coll. Cardiol. 50: 2156–2161. doi: 10.1016/j.jacc.2007.07.076 [DOI] [PubMed] [Google Scholar]
- 18.Hosokawa K., Ohnishi T., Fukasawa M., Kondo T., Sameshima H., Koide T., Tanaka K.A., Maruyama I.2012. A microchip flow-chamber system for quantitative assessment of the platelet thrombus formation process. Microvasc. Res. 83: 154–161. doi: 10.1016/j.mvr.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 19.Hosokawa K., Ohnishi T., Kondo T., Fukasawa M., Koide T., Maruyama I., Tanaka K.A.2011. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J. Thromb. Haemost. 9: 2029–2037. doi: 10.1111/j.1538-7836.2011.04464.x [DOI] [PubMed] [Google Scholar]
- 20.Inoue H., Atarashi H., Kamakura S., Koretsune Y., Kumagai K., Okumura K., Sugi K., Yamashita T., Yasaka M., Satomi K., Kodama I., Ogawa S., Ohe T., Tsutsui H., JCS Joint Working Group2014. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013). Circ. J. 78: 1997–2021. doi: 10.1253/circj.CJ-66-0092 [DOI] [PubMed] [Google Scholar]
- 21.Iwanaga T., Yamada S., Fukushima R., Nagasato T., Maruyama I., Miura N.2017. Novel thrombogenicity examination of whole blood: Total Thrombus-formation analysis System in dogs. J. Vet. Intern. Med. 31: 1301. [Google Scholar]
- 22.January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C., Jr, Conti J.B., Ellinor P.T., Ezekowitz M.D., Field M.E., Murray K.T., Sacco R.L., Stevenson W.G., Tchou P.J., Tracy C.M., Yancy C.W., ACC/AHA Task Force Members2014. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 130: 2071–2104. doi: 10.1161/CIR.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 23.Kannel W.B., Abbott R.D., Savage D.D., McNamara P.M.1982. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N. Engl. J. Med. 306: 1018–1022. doi: 10.1056/NEJM198204293061703 [DOI] [PubMed] [Google Scholar]
- 24.Kubo M., Kiyohara Y., Ninomiya T., Tanizaki Y., Yonemoto K., Doi Y., Hata J., Oishi Y., Shikata K., Iida M.2006. Decreasing incidence of lacunar vs other types of cerebral infarction in a Japanese population. Neurology 66: 1539–1544. doi: 10.1212/01.wnl.0000216132.95207.b4 [DOI] [PubMed] [Google Scholar]
- 25.Li Y.H., Lai L.P., Shyu K.G., Hwang J.J., Kuan P., Lien W.P.1994. Clinical implications of left atrial appendage flow patterns in nonrheumatic atrial fibrillation. Chest 105: 748–752. doi: 10.1378/chest.105.3.748 [DOI] [PubMed] [Google Scholar]
- 26.Lip G.Y., Gibbs C.R.1999. Does heart failure confer a hypercoagulable state? Virchow’s triad revisited. J. Am. Coll. Cardiol. 33: 1424–1426. [DOI] [PubMed] [Google Scholar]
- 27.Marsh J.D., Keyrouz S.G.2010. Stroke prevention and treatment. J. Am. Coll. Cardiol. 56: 683–691. doi: 10.1016/j.jacc.2009.12.072 [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K., Takahashi Y., Yoshitake S., Noguchi T.1996. [A hematologic coagulation monitoring system for bedside use]. Masui 45: 1525–1528. (in Japanese) [PubMed] [Google Scholar]
- 29.Menke J., Lüthje L., Kastrup A., Larsen J.2010. Thromboembolism in atrial fibrillation. Am. J. Cardiol. 105: 502–510. doi: 10.1016/j.amjcard.2009.10.018 [DOI] [PubMed] [Google Scholar]
- 30.Mügge A., Kühn H., Nikutta P., Grote J., Lopez J.A., Daniel W.G.1994. Assessment of left atrial appendage function by biplane transesophageal echocardiography in patients with nonrheumatic atrial fibrillation: identification of a subgroup of patients at increased embolic risk. J. Am. Coll. Cardiol. 23: 599–607. doi: 10.1016/0735-1097(94)90743-9 [DOI] [PubMed] [Google Scholar]
- 31.Usechak P.J., Bright J.M., Day T.K.2012. Thrombotic complications associated with atrial fibrillation in three dogs. J. Vet. Cardiol. 14: 453–458. doi: 10.1016/j.jvc.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 32.Petersen P., Godtfredsen J.1986. Embolic complications in paroxysmal atrial fibrillation. Stroke 17: 622–626. doi: 10.1161/01.STR.17.4.622 [DOI] [PubMed] [Google Scholar]
- 33.Pop G.A., Meeder H.J., Roelandt J.R., van Oudenaarden W., Bulens C., Verweij W., Gijsbers C., van Domburg R., Koudstaal P.J.1994. Transthoracic echo/Doppler in the identification of patients with chronic non-valvular atrial fibrillation at risk for thromboembolic events. Eur. Heart J. 15: 1545–1551. doi: 10.1093/oxfordjournals.eurheartj.a060428 [DOI] [PubMed] [Google Scholar]
- 34.Sato Y., Ishibashi-Ueda H., Iwakiri T., Ikeda Y., Matsuyama T., Hatakeyama K., Asada Y.2012. Thrombus components in cardioembolic and atherothrombotic strokes. Thromb. Res. 130: 278–280. doi: 10.1016/j.thromres.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 35.Schött U., Johansson P.I.2013. II. Bringing flow into haemostasis diagnostics. Br. J. Anaesth. 111: 864–867. doi: 10.1093/bja/aet289 [DOI] [PubMed] [Google Scholar]
- 36.Sohara H., Amitani S., Kurose M., Miyahara K.1997. Atrial fibrillation activates platelets and coagulation in a time-dependent manner: a study in patients with paroxysmal atrial fibrillation. J. Am. Coll. Cardiol. 29: 106–112. doi: 10.1016/S0735-1097(96)00427-5 [DOI] [PubMed] [Google Scholar]
- 37.Stoddard M.F., Dawkins P.R., Prince C.R., Ammash N.M.1995. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J. Am. Coll. Cardiol. 25: 452–459. doi: 10.1016/0735-1097(94)00396-8 [DOI] [PubMed] [Google Scholar]
- 38.Stokol T.2003. Plasma D-dimer for the diagnosis of thromboembolic disorders in dogs. Vet. Clin. North Am. Small Anim. Pract. 33: 1419–1435. doi: 10.1016/S0195-5616(03)00096-2 [DOI] [PubMed] [Google Scholar]
- 39.Suttorp M.J., Kingma J.H., Koomen E.M., van ’t Hof A., Tijssen J.G., Lie K.I.1993. Recurrence of paroxysmal atrial fibrillation or flutter after successful cardioversion in patients with normal left ventricular function. Am. J. Cardiol. 71: 710–713. doi: 10.1016/0002-9149(93)91015-A [DOI] [PubMed] [Google Scholar]
- 40.Tondo C., Scherlag B.J., Otomo K., Antz M., Patterson E., Arruda M., Jackman W.M., Lazzara R.1997. Critical atrial site for ablation of pacing-induced atrial fibrillation in the normal dog heart. J. Cardiovasc. Electrophysiol. 8: 1255–1265. doi: 10.1111/j.1540-8167.1997.tb01016.x [DOI] [PubMed] [Google Scholar]
- 41.Trappe H.J., Brandts B., Weismueller P.2003. Arrhythmias in the intensive care patient. Curr. Opin. Crit. Care 9: 345–355. doi: 10.1097/00075198-200310000-00003 [DOI] [PubMed] [Google Scholar]
- 42.Watson T., Shantsila E., Lip G.Y.2009. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet 373: 155–166. doi: 10.1016/S0140-6736(09)60040-4 [DOI] [PubMed] [Google Scholar]
- 43.Wolberg A.S., Aleman M.M., Leiderman K., Machlus K.R.2012. Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth. Analg. 114: 275–285. doi: 10.1213/ANE.0b013e31823a088c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaguchi Y., Moriki T., Igari A., Matsubara Y., Ohnishi T., Hosokawa K., Murata M.2013. Studies of a microchip flow-chamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb. Res. 132: 263–270. doi: 10.1016/j.thromres.2013.05.026 [DOI] [PubMed] [Google Scholar]