Abstract
To investigate the effects of environmental enrichment on laboratory monkeys, we studied behavioral and physiological differences following changes in housing conditions. Ten male and female juvenile cynomolgus monkeys were first housed in pairs for 8 weeks after quarantine/acclimatization (singly housed) and subsequently housed alone for the next 8 weeks. Monkeys were subjected to evaluations of body weight gain, stereotypic or affiliative behaviors, cortisol, 4-ethylphenyl sulfate (4EPS) and catecholamine concentrations in biological samples, and blood chemistry tests under both housing conditions. Under paired housing, the stereotypic behavioral score decreased in both sexes, and the affiliative behavioral score increased in males and showed an increasing trend in females. Under single housing, the stereotypic score increased in both sexes, and the affiliative score decreased in males. Paired housing decreased serum calcium and urine cortisol concentrations in both sexes and decreased plasma cortisol in males and plasma 4EPS concentrations in females. The stereotypic score was positively correlated with serum calcium, plasma and urine cortisol, and plasma 4EPS concentration and negatively correlated with the affiliative score. The feces painting score, affiliative score, and plasma cortisol and serum calcium concentrations showed sex differences, suggesting differences in responsiveness to environmental changes between males and females. In conclusion, paired housing improved behavioral abnormalities in juvenile cynomolgus monkeys, suggesting that it may be an effective environmental enrichment paradigm. Calcium, cortisol, and 4EPS concentrations in biological samples may be useful indices for evaluating the effects of environmental enrichment.
Keywords: behavior, biomarkers, monkeys, social housing, stress
Introduction
In the current scientific environment, experimental animals are indispensable for the progression of biomedical science. However, the welfare and well-being of laboratory animals and compliance with the 3Rs (Replacement, Refinement and Reduction) [37] needs to be improved [27]. Among experimental animals, monkeys are the most genetically and physiologically similar to humans and are used in many biomedical studies [19]. Furthermore, given that they are highly socialized animals with high intelligence, sufficient improvement in their well-being is warranted in laboratory settings.
The Guide for the Care and Use of Laboratory Animals 8th Edition (ILAR guide) states that “the primary aim of environmental enrichment is to enhance animal well-being by providing animals with sensory and motor stimulation, through structures and resources that facilitate the expression of species-typical behaviors and promote psychological well-being through physical exercise, manipulative activities, and cognitive challenges according to species-specific characteristics” [27]. Use of environmental enrichment is recommended for improving the well-being of experimental animals [27, 36, 43]. Environmental enrichment includes improving the space (behavior-conscious space, perch tree), social (physical, visual, and social interactions among animals or with humans), exercise (exercise opportunities for animals with high athletic ability), and sensory aspects (nest material, bent tree, ingenuity of extension of foraging time) of housing, among others [7]. In particular, it is important to enrich the social environment for highly social animals such as nonhuman primates [36]. For rhesus and cynomolgus monkeys, single housing induces behavioral abnormalities such as autism-like behaviors (stereotypic behaviors) and depression-like behaviors (sitting in a hunched posture, head lower than shoulders), while group housing decreases such abnormal behaviors [2, 24, 39]. In addition, housing conditions reportedly affect immune system activities such as natural killer cell activity and interferon production [40]. Even in rodents, single housing induces anxiety behaviors and impairs associative learning and memory compared with group housing [10, 44]. These behavioral and physiological abnormalities are undesirable for achieving biomedical research objectives and for improving animal welfare, indicating the importance of providing social environmental enrichment to animals. However, social housing is not always used in research, especially for animals such as monkeys and dogs, because the effects of the structure of rearing cages, limitations on experimental protocols, and the effects of environmental enrichment on experimental results have not been clarified [1]. Therefore, many studies have been conducted recently to investigate the influence of environmental enrichment on the psychology, behavior, and physiology of laboratory animals. However, further research is required on the effects of the type and usage of environmental enrichment on various animal species [7, 40, 43].
Evaluation methods such as behavioral assessment and measurement of biochemical parameters are necessary to thoroughly investigate the effect of environmental enrichment. Behavioral assessment is frequently used as an index for environmental stress [29]. Environmental stress such as single housing leads to abnormal behaviors in animals [2, 3, 39]. In particular, a number of reports have shown that laboratory monkeys kept in a closed environment exhibit abnormal behaviors such as stereotyped behaviors or self-injurious behaviors [2, 12, 24]. Cortisol and catecholamines are well-known biomarkers for evaluating stress. Cortisol is released into the peripheral blood via the hypothalamic-pituitary-adrenocortical (HPA) axis following stress exposure and increases resistance to the stressor. In laboratory monkeys, cortisol concentrations in blood, urine, feces, and hair increase in response to environmental stress [9, 12, 24]. Catecholamines are neurotransmitters such as dopamine, noradrenaline, and adrenaline that are released under stress conditions and play important roles in the stress response [21, 29].
Recently, many reports have demonstrated that stress induces changes in the gut microbiota and increases the permeability of the gut epithelium [22, 49], which can lead to increases in the concentration of uremic toxins such as p-cresol, 4-ethylphenyl sulfate (4EPS), and kynurenine in the blood stream [17, 31]. Furthermore, these changes are suspected to induce blood-brain barrier dysfunction, intracerebral inflammation, abnormal behaviors, and psychiatric disorders [22, 26]. Hsiao et al. [17] reported that the 4EPS concentration in blood was associated with autism symptoms in an autism mouse model (MIA mice). Therefore, 4EPS can be a useful biomarker for environmental stress; however, the association between 4EPS levels and stress has not been investigated in monkeys.
Blood chemistry tests are routinely performed in biomedical studies, such as toxicity studies, to determine the physiological state and health of test animals. Given that there is an abundance of background information on a number of animal species and that blood chemistry tests are easy to perform, it would be useful to determine an effective index for evaluating environmental enrichment.
Based on the above background, we investigated the behavioral and physiological effects of environmental enrichment on laboratory monkeys by studying the behavioral differences associated with changes in housing conditions (single vs. paired housing) in juvenile cynomolgus monkeys. In addition, we investigated the usefulness of cortisol, catecholamines, and 4EPS and the usefulness of blood chemical parameters as biomarkers for evaluating the effect of environmental enrichment in juvenile cynomolgus monkeys.
Materials and Methods
Ethical declaration
This study was approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc., Tsukuba Research Center, which is accredited by AAALAC International (approval protocol number: C-T 16305).
Subjects and housing
The subjects were 20 healthy juvenile cynomolgus monkeys (Macaca fascicularis, 10 males and 10 females) ranging in age from 2 to 3 years and were transported from the breeding facility of Shin Nippon Biomedical Laboratories, Ltd. (Kagoshima, Japan) to Tsukuba Research Center, Astellas Pharma Inc.
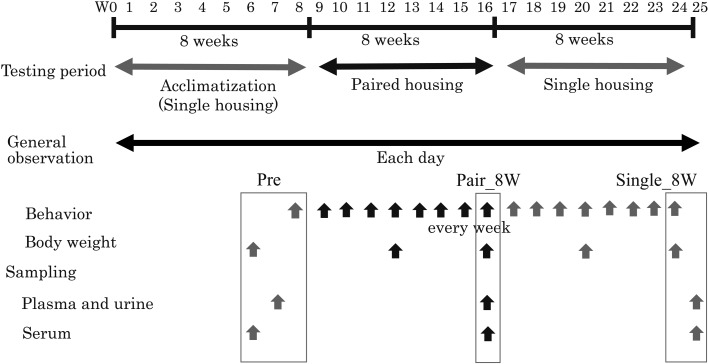
After transportation, monkeys were reared in individual cages for 8 weeks for quarantine/acclimatization (Acc). Subsequently, they were reared under paired housing for 8 weeks (Pair) and then under single housing for 8 weeks (Single). Each rearing period (8 weeks) was defined as the period during which changes in behaviors and biochemical parameters were evaluated [6]. All animals were reared in pairs of the same sex for 2 days (7 h per day) by removing a stainless steel plate between their cages in Acc. As a result, pairs with good compatibility without fighting, etc., were selected as test animals [36]. The experimental design is showed in Fig. 1.
Fig. 1.
Experimental design. Acclimatization in individual cages (single housing) for 8 weeks, Acc; paired housing for 8 weeks, Pair; single housing for 8 weeks, Single.
Animals were reared in compliance with Astellas Global Policy for the Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals [27]. Monkeys were housed in stainless steel cages with a perch (cage size: 650 × 680 × 1,253 mm; Try-Tech Co., Ltd., Osaka, Japan). The cages were double cages that could be converted from single to paired cages by removing a stainless steel plate between the individual cages. During Pair, animals were housed in pairs for about 7 h from 09:00 to 16:00 every day for 8 weeks. During the other hours, the animals were kept isolated for individual feed intake measurements and general condition assessments such as feces properties [3]. Animals were reared under a 12:12 h light-dark cycle (lights on from 07:00 to 19:00) in a controlled temperature (25 ± 1°C) and humidity (55 ± 5%) environment. Filtered 100% fresh air was used with approximately 15 changes per hour. Monkeys were fed standard laboratory food (approximately 100 g / animal / day, PS-A, Oriental Yeast, Tokyo, Japan) from 16:00 to the next morning (09:00), and tap water filtered through a 30-µm filter was available ad libitum. As part of the environmental enrichment paradigm, music from a local FM radio station was played from 13:00 to 15:00, and monkeys had access to plastic toys (Dura-Chews, Certified, Bio-Serv, Flemington, NJ, USA).
General observation
At around 09:00 each day, the general condition of each animal was observed, and individual feed intake was scored (none, 0; small, 1; half or more, 2; all, 3) by a full-time caretaker. Animals were weighed once a month. The percentage body weight gain was calculated according to the following calculation formula:
Percentage body weight gain = (current measured body weight − previous measured body weight) / previous measured body weight × 100
Behavioral assessment
Behaviors of individual monkeys were observed each week from the 6th, 7th, or 8th week of Acc (Pre) from 11:00 to 17:00 throughout the entire test period. Behavioral analysis was based on a previous report [3, 4, 24]. Behavioral assessment was conducted by three trained observers who evaluated the behaviors consistently. Each behavioral assessment lasted 5 min and was conducted by an observer who was unfamiliar to the animal. The observer stood approximately 1 m away from the animal’s cage and recorded the frequency and duration of unique behaviors (Table 1). Behavioral observations were classified and scored according to the criteria in Table 2. In addition, feces painting behavior was recorded as a daily general condition observation and scored (none, 0; present, 1). The feces painting behavior score was combined with the stereotypic behavioral score to generate the abnormal behavioral score.
Table 1. Ethogram of behaviors (Lutz et al., 2003, Cannon et al., 2016, Baker et al., 2017) [3, 4, 24].
| Behavior | Definition | |
|---|---|---|
| Stereotypic behaviors | ||
| Bob | Rapid and repetitive up and down motion of the body on flexed limbs; animal does not leave the cage surface. | |
| Bounce | Repetitively using one’s hind legs or all four limbs to push oneself off the cage surface. | |
| Flip | Repeated forward or backward somersaults, may utilize the cage sides or celing. | |
| Floating limb | An arm or leg rises into the air and may or may not contact the body. | |
| Hair pluck | Removal of hair from one’s own body by pulling with teeth or hands, often seen with a quick jerking motion. | |
| Pace | Repetitive locomotion following the same path; for example, walking back and forth on the ground, around the enclosure. | |
| Periorbital contact (saluting, eye poke) | Animal holding hand, digit, and/or object against/near one’s eyebrow or eye. | |
| Lick | Prolonged or excessive contact of the tongue with a surface or object for no apparent reason. | |
| Rock | Any repetitive motion of the body from a stationary position. Animal remains sitting or standing, while the upper torso sways back and forth. | |
| Self-oral | Sucking a part of one’s own body. | |
| Other abnormal behaviors | ||
| Self-injurious behavior | Activities such as self-biting, head banging, injurious hair plucking, and self-injury with which can result in tissue trauma. | |
| Feces paint | Smearing and/or rubbing feces on a surface. | |
| Affiliative behaviors | ||
| With peers | Groom, rest in contact, social play, cling with peer animals, etc. | |
| With human | Present at the front of cage, extend a limb toward the observer. | |
Table 2. Scoring procedure for behaviors observed.
| Score | Observation |
|---|---|
| 0 | Repetitive abnormal behaviors or affiliative behaviors were not observed. |
| 1 | Repetitive abnormal behaviors or affiliative behaviors were moderately observed (two times or more / min) |
| 2 | Repetitive abnormal behaviors or affiliative behaviors were frequently observed (five times or 20 s / min or more). After observation, the behavior disappeared if the observer approached the cage closely. |
| 3 | Repetitive abnormal behaviors or affiliative behaviors were frequently observed (five times or 20 s / min or more). After observation, the behavior continued even if the observer approached the cage closely. |
Blood chemistry analyses
Blood chemistry analyses were conducted in Pre, Pair_8W (8th week of Pair), and Single_8W (8 or 9th week of Single).
For blood chemistry analyses, a blood sample was collected from the cephalic vein into plastic tubes using a winged needle from 09:30 to 11:30 in Pre, Pair_8W, and Single_8W. Blood collection was performed under manual restraint without anesthesia in front of the animal’s home cage. The blood sample was allowed to clot and was centrifuged at 1,700 × g for 15 min at 4°C to separate the serum. Samples were analyzed using an automatic analyzer (LABOSPECT 006, Hitachi High-Technologies Corporation, Tokyo, Japan). The analyzed parameters were total protein, albumin, total bilirubin, total cholesterol, triglyceride, alkaline phosphatase, aspartate transaminase, alanine transaminase, glucose, urea nitrogen, creatinine, inorganic phosphorus, calcium, sodium, potassium, and chloride.
Plasma and urine cortisol, catecholamine, and 4EPS analyses
Cortisol, catecholamine, and 4EPS analyses were conducted in Pre, Pair_8W, and Single_8W. Plasma samples were obtained by centrifuging (1,700 × g, 15 min, 4°C) a blood sample collected from the cephalic vein into plastic tubes containing EDTA-2K 10% solution using a winged needle. Blood collection was performed under manual restraint without anesthesia in front of the animal’s home cage. Excreted urine was collected in a stainless steel urine collection tray with a stainless steel mesh under the cage for about 4 h (from approximately 09:30 to 13:30). Urine samples in the trays were collected in ice-cold plastic bottles. After measuring the collected urine volume using a measuring cylinder, a maximum of 10 ml of urine was collected in a plastic tube for centrifugation. The supernatant obtained by centrifugation (450 × g, 5 min, 4°C) was used as the urine sample for measurement of urine parameters.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses of cortisol and 4EPS in plasma and urine samples were performed on a UHPLC system, which consisted of LC30AD pumps and an SIL-30AC autosampler (Shimadzu, Kyoto, Japan) coupled to a Triple Quad 5500 triple-quadrupole mass spectrometer (AB Sciex, Framingham, MA, USA). Plasma and urine catecholamine concentrations were analyzed using the Automatic Catecholamine Analyzer HLC-725CAII (Tosoh, Tokyo, Japan) [16] in the Tsukuba laboratory of KOTOBIKEN Medical Laboratories Inc. (Ibaraki, Japan).
Statistical analyses
Differences between two groups were assessed using the Mann-Whitney U test for unpaired nonparametric data, the Wilcoxon matched-pairs signed rank test for paired nonparametric data, and the unpaired t test for parametric data. Differences among three or more groups were assessed using repeated measures one-way analysis of variance (ANOVA) with Dunn’s multiple comparison test as a post hoc test for nonparametric data and Tukey’s multiple comparison test as a post hoc test for parametric data. Fisher’s exact test was used to analyze the proportion of animals showing stereotypic and affiliative behaviors. Spearman’s rank correlation test was used to analyze the degree of the association between the relative behavioral score and calcium, cortisol, and 4EPS concentrations. The pearson r correlation test was used to measure the degree of the relationship between calcium and cortisol and 4EPS concentrations.
Statistical analyses were performed using the GraphPad Prism 7 software (GraphPad Software, La Jolla, CA, USA). Data in the figures and tables are indicated as the mean ± SEM calculated using Excel 2016 (Microsoft, Redmond, WA, USA). All tests were two tailed and considered significant at P<0.05. Significant differences are indicated in the figures as follows: *P<0.05; **P<0.01; ***P<0.001; #P<0.05; ##P<0.01; ###P<0.001; †P<0.05. Near-significant differences that showed a trend (0.05<P<0.1) are also indicated in the Figures.
Results
General observation, feed intake, and body weight
On general observation, hair loss was observed in 3 males in Acc, 2 males in Pair, 3 males in Single, 4 females in Acc and Pair, and 3 females in Single. No other marked changes in general observation were noted. The average percentage body weight gain was significantly higher in the latter 4 weeks of Pair (Pair_5W to 8W) than in the first 4 weeks of Single (Single_1W to 4W) and the latter 4 weeks of Single (Single_5W to 8W) in both males and females (Fig. 2). There was no difference in average feed intake scores between Pair and Single.
Fig. 2.
Body weight gain during Pair and Single (mean ± SEM). *P<0.05 for the first 4 weeks of Single (Single_first) vs. the latter 4 weeks of Pair (Pair_latter; repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). **P<0.01 for the latter 4 weeks of Single (Single _latter) vs. Pair_latter (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). ***P<0.001 for Pair_latter and Single_first vs. Pre to Pair_4W; Single_first and Single_latter vs. Pair_latter; and Single_latter vs. Single_first (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test).
Behavioral assessment
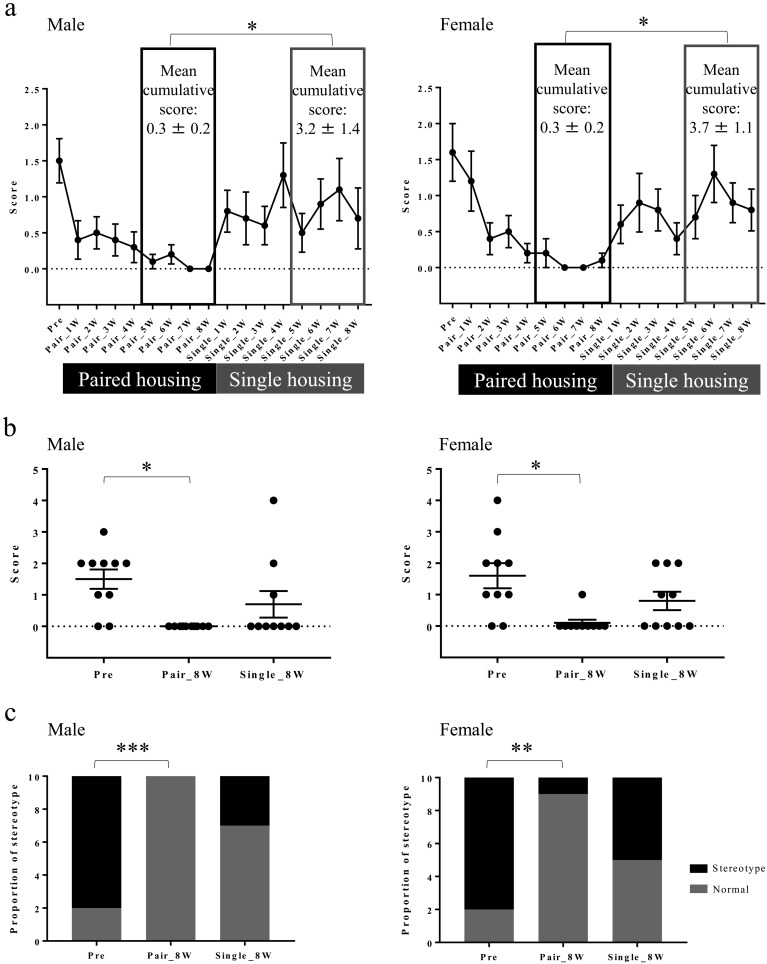
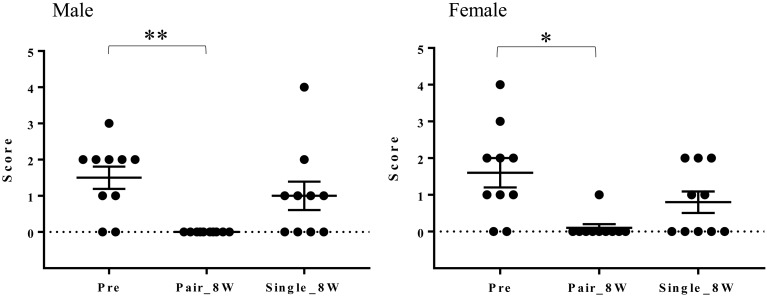
The mean cumulative stereotypic behavioral score was lower in the latter 4 weeks of Pair than in the latter 4 weeks of Single in both sexes (Fig. 3a). The stereotypic behavioral score (ST score) decreased in Pair_8W compared with Pre in both sexes (Fig. 3b). The proportion of animals that displayed stereotypic behaviors decreased in Pair_8W compared with Pre in both sexes (Fig. 3c). As shown in Table 3, the mean cumulative amount number of animals that displayed stereotypic behaviors was lowest in the latter 4 weeks of Pair in both sexes. The mean cumulative number of monkeys showing stereotypic behaviors was highest in the first 4 weeks of Single in males and in the latter 4 weeks of Single in females. The most common stereotypic behavior was pacing in the latter 4 weeks of Single (Table 3).
Fig. 3.
Stereotypic behavioral changes in Pre, Pair, and Single. (a) Stereotypic behavioral scores in the Pre, paired housing, and single housing periods at each observation and mean cumulative scores for the latter 4 weeks of Pair and Single (mean ± SEM). *P<0.05 for the latter 4 weeks of Pair vs. the latter 4 weeks of Single (Wilcoxon matched-pairs signed rank test). (b) Stereotypic behavioral scores in Pre, Pair_8W, and Single_8W (mean ± SEM). *P<0.05 for Pair_8W vs. Pre (repeated measures one-way ANOVA and Friedman test with Dunn’s multiple comparisons post hoc test). (c) Proportion of monkeys that displayed stereotypic behaviors in Pre, Pair_8W, and Single_8W. **P<0.01 for Pair_8W vs. Pre in females (Fisher’s exact test). ***P<0.001 for Pair_8W vs. Pre in males (Fisher’s exact test).
Table 3. Mean cumulative numbers of monkeys that displayed stereotypic behaviors under paired and single housing.
| Sex | Stereotypic behaviors | Paired housing | Single housing | ||
|---|---|---|---|---|---|
| First 4 weeks | Latter 4 weeks | First 4 weeks | Latter 4 weeks | ||
| Male | Pace | 0.3 ± 0.3 | 0.3 ± 0.3 | 1.8 ± 0.3 | 2.5 ± 0.3 |
| Rock | 0.8 ± 0.3 | 0.5 ± 0.3 | 1.0 ± 0.0 | 1.0 ± 0.0 | |
| Self-oral | 1.5 ± 0.3 | 0 | 1.3 ± 0.6 | 1.5 ± 0.3 | |
| Bob | 0.3 ± 0.3 | 0 | 0.5 ± 0.3 | 0 | |
| Periorbital contact | 0 | 0 | 0 | 0 | |
| Bounce | 0 | 0 | 0 | 0 | |
| Lick | 0.3 ± 0.3 | 0 | 0.8 ± 0.5 | 0 | |
| Other | 0 | 0 | 0 | 0 | |
| Amount | 3.0 ± 0.6 | 0.8 ± 0.5aa, bb | 5.3 ± 0.9aa | 5 ± 0.6bb | |
| Female | Pace | 2.0 ± 0.8 | 0.3 ± 0.3 | 3.3 ± 0.3 | 4.3 ± 0.3 |
| Rock | 0 | 0 | 0 | 0 | |
| Self-oral | 0.5 ± 0.3 | 0 | 0 | 0.5 ± 0.5 | |
| Bob | 0.8 ± 0.3 | 0.3 ± 0.3 | 0 | 0.8 ± 0.8 | |
| Periorbital contact | 0 | 0 | 0 | 0 | |
| Bounce | 0.5 ± 0.3 | 0 | 0 | 0.5 ± 0.3 | |
| Lick | 0.3 ± 0.3 | 0 | 0 | 0 | |
| Other | 0 | 0 | 1.3 ± 0.5 | 0 | |
| Amount | 4.0 ± 1.1 | 0.5 ± 0.25a,bb | 4.5 ± 0.6a | 6.0 ± 1.1bb | |
Values indicate the mean ± SEM cumulative numbers of monkeys that displayed the stereotypic behaviors. Significant differences were detected between values labelled with the same superscript letters; a, bP<0.05; aa, bbP<0.01. Comparisons were performed with one-way ANOVA with Tukey’s multiple comparison post hoc test.
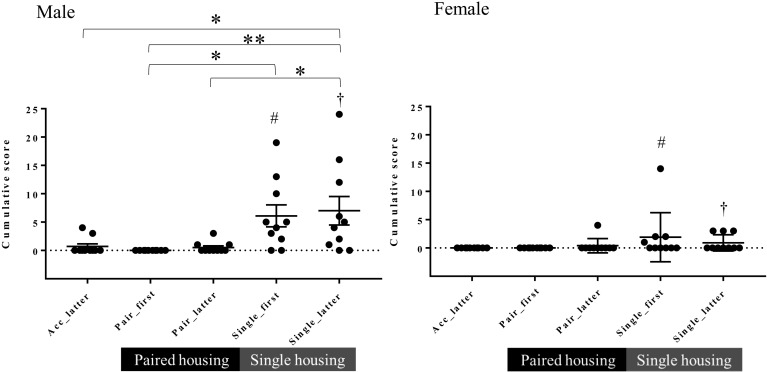
In males, the mean cumulative feces painting behavioral score (FP score) was increased in the first 4 weeks of Single compared with the latter 4 weeks of Pair (Fig. 4). Males showed higher cumulative FP scores than females in the first and latter 4 weeks of Single (Fig. 4).
Fig. 4.
Cumulative feces painting behavioral scores in Pre, Pair, and Single (mean ± SEM). *P<0.05 for latter 4 weeks of Single (Single_latter) vs. the latter 4 weeks of acclimatization (Acc_latter); the first 4 weeks of Single (Single_first) vs. the first 4 weeks of Pair (Pair_first); and Single_latter vs. the latter 4 weeks of Pair (Pair_latter; repeated measures one-way ANOVA and Friedman test with Dunn’s multiple comparisons post hoc test). **P<0.01 for Single_latter vs. Pair_first (repeated measures one-way ANOVA and Friedman test with Dunn’s multiple comparisons post hoc test). #P<0.05 for males vs. females (Mann-Whitney U test). †P<0.05 for males vs. females (Mann-Whitney U test).
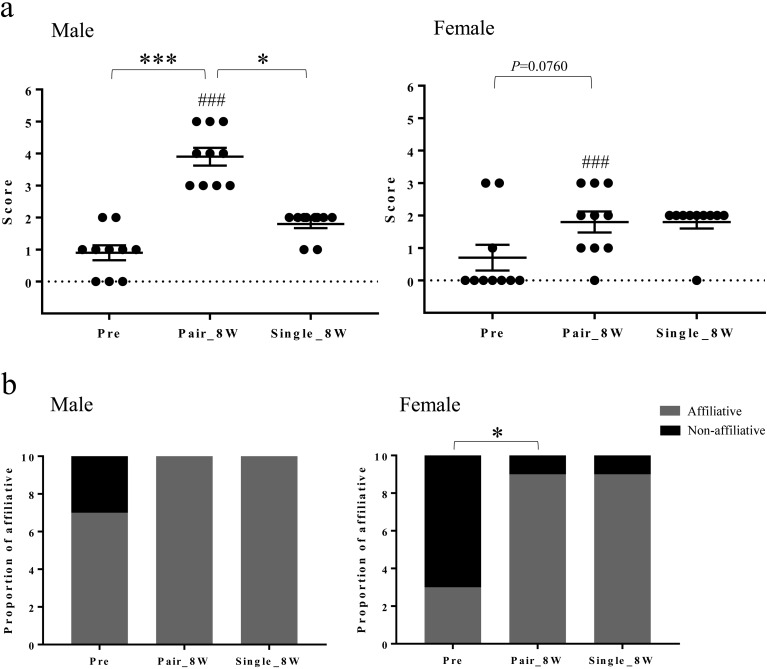
The mean abnormal behavioral score (AB score: ST score + FP score) was lower in Pair_8W than in Pre for both sexes (Fig. 5). The mean affiliative behavioral score (AF score) in Pair_8W increased in males and showed an increasing trend in females compared with Pre (Fig. 6a). The AF score in Single_8W was lower than that in Pair_8W in males (Fig. 6a). The proportion of animals that displayed affiliative behaviors increased in Pair_8W compared with Pre in females (Fig. 6b) but did not change in the transition from Pair_8W to Single_8W. The AF score in males was higher than that in females in Pair_8W (Fig. 6a). The affiliative behavior observed in Pre, Pair_8W, and Single_8W was classified as affiliative behavior with humans (either “present at the front of cage” or “extend a limb toward the observer”).
Fig. 5.
Abnormal behavior scores in Pre, Pair_8W, and Single_8W (mean ± SEM). *P<0.05 for Pair_8W vs. Pre in females (repeated measures one-way ANOVA and Friedman test with Dunn’s multiple comparisons post hoc test). **P<0.01 for Pair_8W vs. Pre in males (repeated measures one-way ANOVA and Friedman test with Dunn’s multiple comparisons post hoc test).
Fig. 6.
Affiliative behavioral changes in Pre, Pair, and Single. (a) Affiliative behavioral scores of Pre, Pair_8W, and Single_8W (mean ± SEM). *P<0.05 for Single_8W vs. Pair_8W (repeated measures one-way ANOVA and Friedman test with Dunn’s multiple comparisons post hoc test). ***P<0.001 for Pair_8W vs. Pre (repeated measures one-way ANOVA and Friedman test with Dunn’s multiple comparisons post hoc test). ###P<0.001 for male vs. females (Mann-Whitney U test). (b) Proportion of monkeys that displayed affiliative behaviors in Pre, Pair_8W, and Single_8W. *P<0.05 for Pair_8W vs. Pre in females (Fisher’s exact test).
Blood chemistry
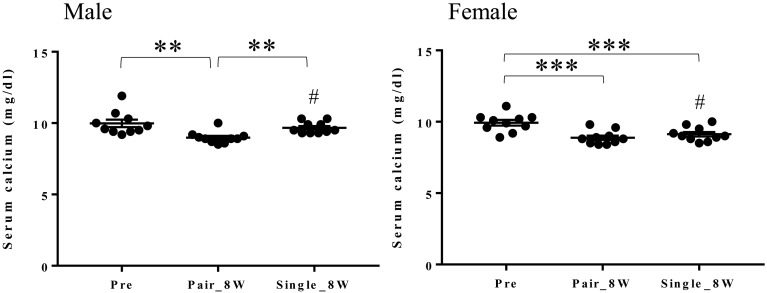
Serum calcium concentrations decreased after the transition from Pre to Pair_8W and increased after the transition from Pair_8W to Single_8W in males (Fig. 7). In females, serum calcium concentrations were significantly lower in Pair_8W and Single_8W compared with Pre (Fig. 7). Serum calcium concentrations were higher in males than in females in Single_8W (Fig. 7).
Fig. 7.
Serum calcium concentration in Pre, Pair_8W, and Single_8W (mean ± SEM). **P<0.01 for Pair_8W vs. Pre and Single_8W vs. Pair_8W (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). ***P<0.001 for Pair_8W and Single_8W vs. Pre (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). #P<0.05 for males vs. females (unpaired t test).
No other marked changes were noted in blood chemistry parameters due to changes in housing conditions.
Cortisol, 4EPS, and catecholamine concentrations in plasma and urine
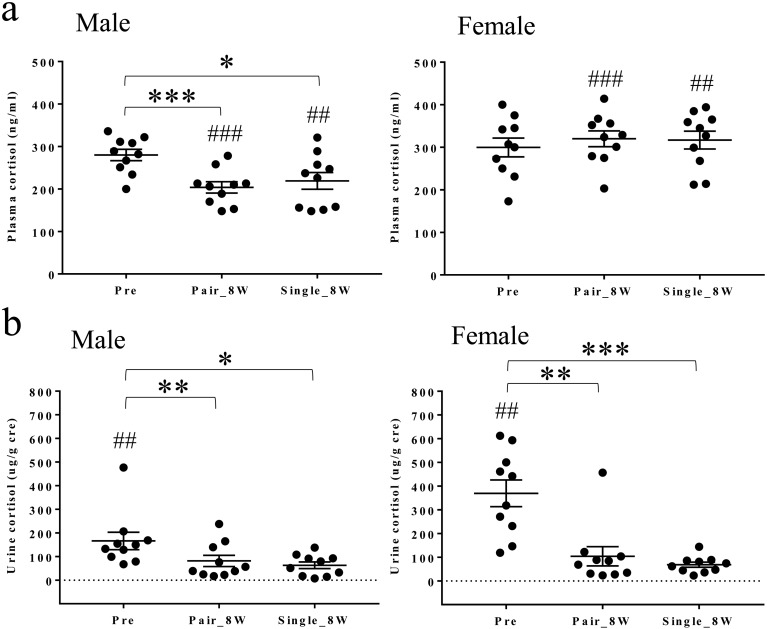
Plasma cortisol concentrations decreased in Pair_8W and Single_8W compared with Pre in males (Fig. 8a). There were no changes in females. Plasma cortisol concentrations were higher in females than in males in Pair_8W and Single_8W (Fig. 8a). Urine cortisol concentrations decreased in Pair_8W and Single_8W compared with Pre in males and females (Fig. 8b). Urine cortisol concentrations were higher in females than males in Pre (Fig. 8b). Plasma and urine cortisol concentrations increased in 6 males and 5 females and in 2 males and 5 females, respectively, in Single_8W compared with Pair_8W.
Fig. 8.
Plasma and urine cortisol concentrations in Pre, Pair_8W, and Single_8W. (a) Plasma cortisol concentrations in Pre, Pair_8W, and Single_8W (mean ± SEM). *P<0.05 for Single_8W vs. Pre (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). ***P<0.001 for Pair_8W vs. Pre (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). ##P<0.01 for males vs. females (unpaired t test). ###P<0.001 for males vs. females (unpaired t test). (b) Urine cortisol concentrations in Pre, Pair_8W, and Single_8W (mean ± SEM). *P<0.05 for Single_8W vs. Pre (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). **P<0.01 for Pair_8W vs. Pre (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). ***P<0.001 for Single_8W vs. Pre (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). ##P<0.01 for males vs. females (unpaired t test).
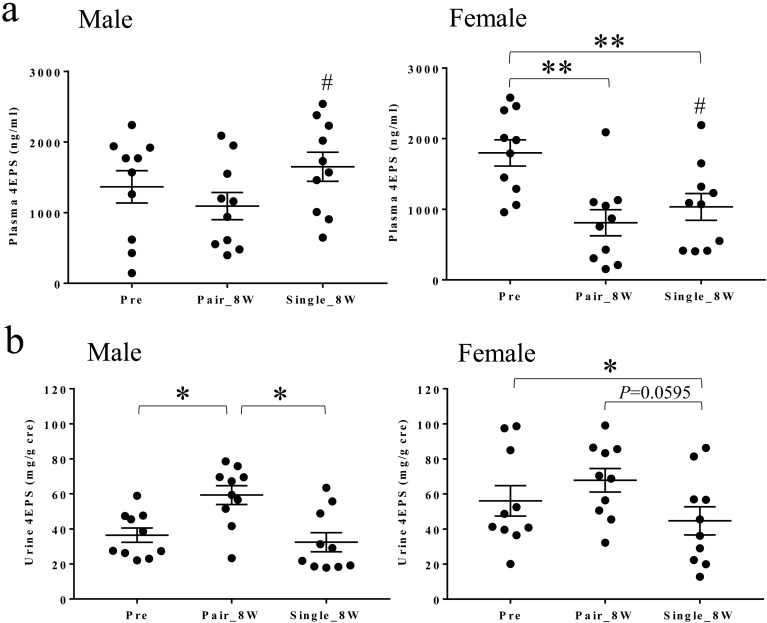
Plasma 4EPS concentrations decreased in Pair_8W and Single_8W compared with Pre in females (Fig. 9a). Plasma 4EPS concentrations were higher in males than in females in Single_8W (Fig. 9a). Urine 4EPS concentrations increased after the transition from Pre to Pair_8W in males (Fig. 9b) and decreased from Pair_8W to Single_8W in males and females (Fig. 9b). Plasma and urine 4EPS concentrations increased in 8 males and 7 females and in 1 male and 1 female, respectively, in Single_8W compared with Pair_8W.
Fig. 9.
Plasma and urine 4EPS concentrations in Pre, Pair_8W, and Single_8W. (a) Plasma 4EPS concentrations in Pre, Pair_8W, and Single_8W (mean ± SEM). **P<0.01 for Pair_8W and Single_8W vs. Pre (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test). #P<0.05 for males vs. females (unpaired t test). (b) Urine 4EPS concentrations in Pre, Pair_8W, and Single_8W (mean ± SEM). *P<0.05 for Pair_8W vs. Pre and Single_8W vs. Pair_8W in males and Single_8W vs. Pre in females (repeated measures one-way ANOVA with Tukey’s multiple comparisons post hoc test).
No marked changes were noted in catecholamine concentrations due to changes in housing conditions.
Correlations analyses
We conducted correlation analysis between behaviors and between behaviors and biochemical parameters using combined data from both sexes in Pre, Pair_8W, and Single_8W. The results of correlation analyses are shown in Table 4. There was a negative correlation between the ST score and the AF score. The serum calcium concentration showed a positive correlation with the ST score and AB score and a negative correlation with the AF score. Furthermore, the serum calcium concentration was correlated with urine cortisol and plasma 4EPS concentrations (r=0.3955 P=0.0018, and r=0.3708 P=0.0035, respectively). The plasma cortisol concentration showed a positive correlation with the ST score and a negative correlation with the AF score. The same correlations were observed for the urine cortisol concentration.
Table 4. Correlation between behavioral scores and calcium, cortisol, and 4EPS concentrations.
| Behavioral score | ||||
|---|---|---|---|---|
| Stereotypic | Abnormal | Affiliative | ||
| Behavior | Stereotypic | - | PC | NC |
| r=0.954, P<0.0001 | r=−0.5331, P<0.0001 | |||
| Calcium | Serum | PC | PC | NC |
| r=0.4046, P=0.0013 | r=0.436, P=0.0005 | r=−0.4193, P=0.0009 | ||
| Cortisol | Plasma | PC | ns | NC |
| r=0.2645, P=0.0412 | r=0.2062, P=0.1139 | r=−0.4399, P=0.0004 | ||
| Urine | PC | PC | NC | |
| r=0.4415, P=0.0004 | r=0.3918, P=0.0020 | r=−0.3971, P=0.0017 | ||
| 4EPS | Plasma | PC | PC | ns |
| r=0.2909, P=0.0241 | r=0.318, P=0.0133 | r=−0.03424, P=0.7951 | ||
| Urine | TNC | TNC | ns | |
| r=−0.2291, P=0.0783 | r=−0.2428, P=0.0616 | r=0.06666, P=0.6128 | ||
Statistical significance was analyzed using Spearman's rank correlation test. Correlation analysis was performed using combined data from both sexes in Pre (6, 7, or 8W), Pair_8W, and Single_8W. PC, positive correlation; NC, negative correlation; TNC, trend toward a negative correlation (0.05<P<0.1); ns, no significance (no correlation).
In plasma, the 4EPS concentration was positively correlated with the ST score and AB score. In urine, the 4EPS concentration showed a trend towards a negative correlation with the ST score and the AB score.
Discussion
General inspection and body weight gain
The percentage body weight gain decreased with the transition from the latter 4 weeks of Pair to Single (Fig. 2). This is in agreement with previous findings that changes in housing conditions cause psychological stress in animals, as observed by the presentation of abnormal behaviors, sleep disorders, and weight loss [8, 20]. The common marmoset is also known to show weight loss of about 10% following a transition to single housing [20]. Given that feed intake scores did not change with changes in housing conditions, we speculate that the decrease in the percentage body weight gain in Single may be attributed to changes in behavioral quantity or energy metabolism due to increased glucocorticoid secretion under chronic psychological stress. Grayson et al. [14] reported that chronic stress stimulates the production and release of glucocorticoid hormones (i.e., cortisol in nonhuman primates). Glucocorticoids have numerous effects throughout the body, including mobilizing stored energy to promote survival during stress, but also negative side effects such as reducing cognitive function, causing immune dysfunction, osteoporosis, and increased risk for mood and anxiety disorders.
Behavioral assessment
Paired housing for seven hours a day reduced the ST scores of juvenile cynomolgus monkeys housed for 8 weeks under single housing conditions for quarantine and acclimatization. ST scores were drastically decreased in Pair_8W, and few monkeys expressed stereotypic behaviors (Fig. 3 and Table 3).
The mean cumulative ST score in the latter 4 weeks of Single was significantly higher than that in the same period in Pair in males and females (Fig. 3a). Furthermore, the mean cumulative number of animals that displayed stereotypic behaviors also increased with the change from Pair to Single (Table 3). Chronic environmental stress such as single housing has physical and psychological effects on monkeys and consequently causes behavioral abnormalities [24, 47]. In laboratory monkeys, stereotypic behaviors are often observed among abnormal behaviors [3, 35, 47]. Stereotypic behaviors are defined as “repetitive, ritualistic behaviors that appear functionless” [30], and they are relatively frequently observed in laboratory monkeys reared in a closed environment [2, 12, 24]. Stereotypies can range from whole-body movements (e.g., rocking, pacing, somersaulting) to self-directed actions involving the hands or feet (e.g., eye covering, sucking, hair pulling, self-grasping) [2, 4, 30]. In this study, stereotypic behaviors such as pacing, self-oral behaviors (finger sucking, tail sucking), repetitive licking (licking cage), rocking, and periorbital contact were observed. Similar to previous reports [24, 35, 47], among the stereotypic behaviors, pacing (repeated walking in circles, forwards and backwards, left and right in a cage) was the most frequently observed (Table 3). In contrast, there is a view that stereotypic behaviors have a role in relieving environmental stress [4, 30]. Therefore, the frequent observation of these behaviors in Pre and Single may represent self-defense behaviors against environmental stress.
The transition from Pair to Single increased feces painting behaviors towards the wall of the rearing cage in males (Fig. 4). This behavior is thought to be associated with a lack of social interaction or opportunity for alternative behaviors and with insufficient stimulation. In Single, it seemed to be attributed to chronic stress [13]. In contrast, affiliative behaviors increased under the pair housing condition in both males and females and decreased under the single housing condition in males (Fig. 6a). Backer et al. [2] reported that socially housed monkeys show more affiliative interactions than those that are singly housed. Interestingly, we found that paired housing improved monkeys’ affinity with humans. The amount of time monkeys spent present at the front of their cage and extending limbs toward the observer increased in Pair but declined in Single. Nishimoto et al. [28] reported that affinity training by giving raisins directly to cynomolgus monkeys improves affinity with caretakers and researchers and decreases self-injury and aggressive behaviors. Schapiro et al. [41] and Perlman et al. [33] showed that positive reinforcement training suppresses behavioral abnormalities and improves affiliative behaviors in monkeys. These findings suggest that the psychological state of animals affects their affinity with humans.
Gender differences in behavioral changes were observed in affiliative behaviors and feces painting behaviors. Males showed a clear changes in both behaviors, whereas females tended to show moderate changes in their behaviors (Figs. 4 and 6a). Response to novel stimulation is reportedly higher in males than females [7, 24, 25]. Cohen et al. [6] suggested that this sex difference may be due to differences in cortisol/corticosterone concentrations. Our findings showed that the plasma cortisol concentration in females was higher than that in males in Pair_8W and Single_8W (Fig. 8), suggesting that gender differences in feces painting behaviors and affiliative behaviors may be associated with differences in susceptibility to stress related to the plasma cortisol concentration.
Our behavioral evaluations reconfirmed that paired housing (social housing) is extremely effective for reducing abnormal behaviors, which is a big problem in monkey rearing facilities [2]. This finding suggests that paired housing improves the psychological well-being of animals and the quality of experimental data [27, 30, 38]. In a drug discovery study using an animal model, however, it may be necessary to use time-limited (rather than full time) paired housing to enable the collection individual data such as food intake and fecal properties. The present study showed that paired housing in the daytime (7 h a day) and single housing at night may be an effective time-limited paired housing paradigm. Social housing sometimes causes injurious aggression if animals are not compatible partners. Reinhardt et al. [36] recommends placing a privacy panel for visual isolation in rearing cages to reduce potential conflicts between monkeys in paired housing. Hence, it is important to consider the compatibility, variables (such as weight, age, gender), and temperament of monkeys in social housing.
Blood chemistry
Serum calcium concentrations were decreased in Pair_8W compared with Pre in males and females and were increased in Single_8W compared with Pair_8W in males (Fig. 7). The control of calcium is very important because it is involved in many biological reactions. The serum calcium homeostasis is controlled by hormones such as parathyroid hormone (PTH) and calcitonin [23]. Serum calcium concentration has been reported to be associated with depression and schizophrenia and is a reported biomarker of psychiatric disorders [15, 32, 42]. Payne et al. [32] reported that serum calcium concentrations within the physiological standard range are correlated with neuropsychological performance. Cobb [5] showed that the serum calcium concentration increased in rats exposed to confinement and noise stress and suggested that the high calcium concentration was caused by PTH release in response to the stress. In the present study, we speculate that the decrease in serum calcium in Pair_8W compared with Pre or Single_8W was due to the PTH activity associated with a psychological stress state. Future studies should measure serum calcium and PTH concentrations to confirm this.
Cortisol, 4EPS, and catecholamine concentrations in plasma and urine
The plasma cortisol concentration in males and urine cortisol concentration in males and females decreased in Pair_8W compared with Pre (Fig. 8). Several studies have examined the relationship between behavioral abnormalities due to environmental stress and cortisol concentrations in monkeys [2, 4, 9]. Relocation of male rhesus monkeys has been reported to significantly increase plasma cortisol concentrations [8]. Suzuki et al. [46] also reported that psychological stress increases the plasma cortisol concentration in Japanese monkeys. We speculate that the decrease in cortisol concentration in Pair in this study may have been caused by the high stress state in Pre (transportation, rearing condition change, single housing for quarantine), which was reduced by social enrichment in Pair. This hypothesis is supported by the decrease in stereotypic behaviors and increase in affiliative behaviors in Pair.
The plasma cortisol concentration in males and urine cortisol concentrations in males and females decreased in Pair but did not increase in Single. Similar tendencies were observed in ST scores and AF scores in females. Sensory environmental enrichment (toys, music) or acclimatization to the rearing environment may have suppressed the increase in cortisol concentration in Single.
Since plasma and serum cortisol concentrations are affected by stress at the time of sampling, some reports suggest that these parameters do not accurately reflect the animal’s stress state [4, 29]. Therefore, cortisol concentrations in urine, feces, and hair, which are less affected by the sampling procedure, may be suitable for stress evaluation [30]. In this study, cortisol concentrations in plasma and urine showed similar changes (except the plasma concentration in females). Given that it takes several hours or more to raise cortisol levels in urine after exposure to stress [29, 30], we expect that any stress due to urine collection did not affect cortisol concentrations. Therefore, cortisol concentrations in plasma and urine likely reflect the animal’s stress state in this study. While the plasma cortisol concentration in males differed among housing conditions, those of females did not. A similar trend was observed in behavioral scores in females. As was suggested by Mallapur et al. [25], this discrepancy may reflect differences in responsiveness to stress between males and females [6, 24].
4EPS is produced from tyrosine by intestinal bacteria like p-cresol (4-methylphenol) [45]. It is a known uremic toxins like the tryptophan metabolites indolepyruvate and kynurenine [17, 18, 48]. In addition, some reports suggest a relationship between uremic toxins like 4EPS, indolepyruvate, and kynurenine and autism and depressive behaviors [17, 34]. Many studies indicate that blood levels of metabolites like 4EPS, indolepyruvate, and kynurenine are associated with changes in intestinal bacteria and leakage of the intestinal epithelium [11, 22]. An increase in such metabolites in circulating blood is suspected to cause behavioral abnormalities through invasion and inflammation in the brain [26]. Hsiao et al. [17] showed that the blood 4EPS concentration is associated with autism symptoms in MIA mice and that 4EPS administration to normal mice causes autism symptoms. In the present study, the plasma 4EPS level was decreased in females after the transition from Pre to Pair (Fig. 9a). In males, the pattern of changes in the 4EPS concentration was similar to that observed in females, but there was no significant difference between housing conditions. In contrast, the urine 4EPS concentration in males increased in Pair, decreased in Single, and showed a different trend from that of plasma 4EPS (Fig. 9b). It is unclear why 4EPS concentrations in plasma and urine showed different patterns. Renal clearance of uremic toxins such as p-cresyl sulfate and indoxyl sulfate is mediated by organic anion transporter 1 and 3 in the kidneys [48]. The increase in urinary 4EPS concentration in Pair may be due to activation of the renal excretion system, although further studies are required to elucidate the underlying mechanism.
Correlation analyses
A negative correlation was observed between ST scores and AF scores (Table 4). Exposure to environmental stress increased behavioral abnormalities such as stereotypic behaviors in monkeys. Simultaneously, affiliative behaviors towards observers and between animals decreased.
Serum calcium showed a positive correlation with the ST and AB scores and a negative correlation with the AF scores. We speculate that serum calcium concentrations increased due to environmental stress in association with PTH secretion and decreased following stress reduction. Serum calcium is a standard blood chemistry parameter and is therefore an easy-to-use index of stress. Plasma cortisol concentrations were positively correlated with ST scores but negatively correlated with AF scores. The relationship between urinary cortisol concentrations and behaviors showed similar trends to those observed for plasma cortisol concentrations. Cortisol concentrations in plasma and urine correlated well with behavioral changes due to environmental stress, suggesting that they may be useful biomarkers for evaluating environmental enrichment.
Plasma 4EPS concentrations showed a positive correlation with the ST and AB scores, while urine 4EPS concentrations were negatively correlated with the ST and AB scores. We found that plasma 4EPS levels were closely associated with abnormal behaviors, suggesting that plasma 4EPS may be one factor linked to the appearance of abnormal behaviors. Therefore, the plasma 4EPS concentration may be a useful indicator of psychological changes in monkeys. Given that 4EPS is a metabolite produced by intestinal bacteria, this parameter may be useful for investigating changes in microbiota related to environmental and behavioral changes.
In conclusion, our study of juvenile cynomolgus monkeys reared under paired housing and single housing conditions for 8 weeks each showed that paired housing is an effective environmental enrichment paradigm for improving behavior abnormalities. Moreover, in addition to behavioral evaluation, calcium, cortisol, and 4EPS concentrations in blood or urine may be useful biomarkers for evaluating the influence of environmental enrichment in juvenile cynomolgus monkeys.
Our findings demonstrate the benefits of social housing, especially given that behavioral abnormalities caused by environmental stress such as single housing can impair the well-being of animals and possibly lead to self-injurious behaviors or depression. However, further studies in adult subjects are needed to better understand the influence of housing condition changes on cynomolgus monkeys. We propose that, although there may be individual differences in the responses to the housing condition, it is important to use appropriate environmental enrichment paradigms for individual animal species to improve the accuracy of biomedical research data and well-being.
Acknowledgments
The authors would like to express their great appreciation to Azusa Yamada, Yuki Naganuma, Ippei Nishiura, Masahito Mizuno, Hiroyuki Satake, and Heijirou Ohnishi for the collection of experimental data and Dr. Takamitsu Tsukahara and Dr. Michiko Hashimoto for the provision of useful advice regarding the design of the experimental plan. We would like to thank Dr. Daisuke Kohari, Dr. Keiji Miyata, and Dr. Toshihiro Watanabe for their useful opinions in preparing the report. Finally, we deeply appreciate the efforts of KAC Co., Ltd., in conducting proper animal care.
References
- 1.Baker K.C., Weed J.L., Crockett C.M., Bloomsmith M.A.2007. Survey of environmental enhancement programs for laboratory primates. Am. J. Primatol. 69: 377–394. doi: 10.1002/ajp.20347 [DOI] [PubMed] [Google Scholar]
- 2.Baker K.C., Bloomsmith M.A., Oettinger B., Neu K., Griffis C., Schoof V., Maloney M.2012. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Appl. Anim. Behav. Sci. 137: 148–156. doi: 10.1016/j.applanim.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker K.C., Bloomsmith M.A., Coleman K., Crockett C.M., Wolein J., Lutz C.K., McCowan B., Pierre P., Weed J.2017. The behavioral management consortium A partnership for promoting consensus and best practices. pp. 14–16. In: Handbook of primate behavioral management (Schapiro, S.J. edt.), CRC press, NW. [Google Scholar]
- 4.Cannon T.H., Heistermann M., Hankison S.J., Hockings K.J., McLennan M.R.2016. Tailored Enrichment Strategies and Stereotypic Behavior in Captive Individually Housed Macaques (Macaca spp.). J. Appl. Anim. Welf. Sci. 19: 171–182. doi: 10.1080/10888705.2015.1126786 [DOI] [PubMed] [Google Scholar]
- 5.Cobb H. P.1985. The effects of stress on the blood calcium level in the male white rat (Rattus norvegicus). Master’s Theses. Paper 495. University of Richmond, UR Scholarship Repository.
- 6.Cohen H., Yehuda R.2011. Gender differences in animal models of posttraumatic stress disorder. Dis. Markers 30: 141–150. doi: 10.1155/2011/734372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman K., Bloomsmith M.A., Crockett C.M., Weed J.L., Schapiro S.J.2012. Behavioral management, enrichment, and psychological well-being of laboratory nonhuman primates. pp.156–164. In: Nonhuman primates in biomedical research Vol.1: Biology and management (Abee, C.R., Mansfield K., Tardif, S., Morris, T. eds.), 2nd ed., Academic press, UK. [Google Scholar]
- 8.Davenport M.D., Lutz C.K., Tiefenbacher S., Novak M.A., Meyer J.S.2008. A rhesus monkey model of self-injury: effects of relocation stress on behavior and neuroendocrine function. Biol. Psychiatry 63: 990–996. doi: 10.1016/j.biopsych.2007.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle L.A., Baker K.C., Cox L.D.2008. Physiological and behavioral effects of social introduction on adult male rhesus macaques. Am. J. Primatol. 70: 542–550. doi: 10.1002/ajp.20526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunphy-Doherty F., O’Mahony S.M., Peterson V.L., O’Sullivan O., Crispie F., Cotter P.D., Wigmore P., King M.V., Cryan J.F., Fone K.C.F.2018. Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav. Immun. 68: 261–273. doi: 10.1016/j.bbi.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 11.Fiorentino M., Sapone A., Senger S., Camhi S.S., Kadzielski S.M., Buie T.M., Kelly D.L., Cascella N., Fasano A.2016. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 7: 49. doi: 10.1186/s13229-016-0110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb D.H., Capitanio J.P., McCowan B.2013. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): animal’s history, current environment, and personality. Am. J. Primatol. 75: 995–1008. doi: 10.1002/ajp.22161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb D.H., O’Connor J.R., Coleman K.2014. Using porches to decrease feces painting in rhesus macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 53: 653–656. [PMC free article] [PubMed] [Google Scholar]
- 14.Grayson B.E., Hakala-Finch A.P., Kekulawala M., Laub H., Egan A.E., Ressler I.B., Woods S.C., Herman J.P., Seeley R.J., Benoit S.C., Ulrich-Lai Y.M.2014. Weight loss by calorie restriction versus bariatric surgery differentially regulates the hypothalamo-pituitary-adrenocortical axis in male rats. Stress 17: 484–493. doi: 10.3109/10253890.2014.967677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grützner T.M., Listunova L., Fabian G.A., Kramer B.A., Flach D., Weisbrod M., Roesch-Ely D., Sharma A.2018. Serum calcium levels and neuropsychological performance in depression and matched healthy controls: Reversal of correlation a marker of the aging cognitive clock? Psychoneuroendocrinology 91: 198–205. doi: 10.1016/j.psyneuen.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 16.Hirowatari Y., Ito Y., Kasai M., Takahashi H., Hayashi H.1999. Development of the automatic catecholamine analyzer HLC-725CAII. J. Tosoh Res. 43:3–12. [Google Scholar]
- 17.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., Patterson P.H., Mazmanian S.K.2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155: 1451–1463. doi: 10.1016/j.cell.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh Y., Ezawa A., Kikuchi K., Tsuruta Y., Niwa T.2013. Correlation between Serum Levels of Protein-Bound Uremic Toxins in Hemodialysis Patients Measured by LC/MS/MS. Mass Spectrom. (Tokyo) 2: S0017.(Tokyo). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnsen D.O., Johnson D.K., Whitney R.A., Jr2012. History of the use of nonhuman primates in biomedical research. pp.2–33. In: Nonhuman primates in biomedical research Vol.1: Biology and management (Abee, C.R., Mansfield K., Tardif, S., Morris, T. eds.). 2nd ed., Academic press, UK. [Google Scholar]
- 20.Kaplan E., Shelmidine N.2010. Factors influencing weight changes in callitrichids at the Bronx Zoo. Zoo Biol. 29: 551–566. doi: 10.1002/zoo.20290 [DOI] [PubMed] [Google Scholar]
- 21.LeBlanc J., Ducharme M.B.2007. Plasma dopamine and noradrenaline variations in response to stress. Physiol. Behav. 91: 208–211. doi: 10.1016/j.physbeh.2007.02.011 [DOI] [PubMed] [Google Scholar]
- 22.Li Q., Han Y., Dy A.B.C., Hagerman R.J.2017. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 11: 120. doi: 10.3389/fncel.2017.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumachi F., Motta R., Cecchin D., Ave S., Camozzi V., Basso S.M., Luisetto G.2011. Calcium metabolism & hypercalcemia in adults. Curr. Med. Chem. 18: 3529–3536. doi: 10.2174/092986711796642599 [DOI] [PubMed] [Google Scholar]
- 24.Lutz C., Well A., Novak M.2003. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am. J. Primatol. 60: 1–15. doi: 10.1002/ajp.10075 [DOI] [PubMed] [Google Scholar]
- 25.Mallapur A., Choudhury B.C.2003. Behavioral abnormalities in captive nonhuman primates. J. Appl. Anim. Welf. Sci. 6: 275–284. doi: 10.1207/s15327604jaws0604_2 [DOI] [PubMed] [Google Scholar]
- 26.Menard C., Pfau M.L., Hodes G.E., Kana V., Wang V.X., Bouchard S., Takahashi A., Flanigan M.E., Aleyasin H., LeClair K.B., Janssen W.G., Labonté B., Parise E.M., Lorsch Z.S., Golden S.A., Heshmati M., Tamminga C., Turecki G., Campbell M., Fayad Z.A., Tang C.Y., Merad M., Russo S.J.2017. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20: 1752–1760. doi: 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Research Council2011. Guide for the care and use of laboratory animals, 8th ed., The national academies press, Washington, D.C. [Google Scholar]
- 28.Nishimoto A., Tachibana Y., Takaura K., Ochi T., Koyama H.2015. Effect of new training technique on affinity of cynomolgus monkeys for animal care personnel. Exp. Anim. 64: 369–373. doi: 10.1538/expanim.14-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak M.A., Hamel A.F., Kelly B.J., Dettmer A.M., Meyer J.S.2013. Stress, the HPA axis, and nonhuman primate well-being: A review. Appl. Anim. Behav. Sci. 143: 135–149. doi: 10.1016/j.applanim.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak M.A., Hamel A.F., Ryan A.M., Menard M.T., Meyer J.S.2017. The role of stress in abnormal behavior and other abnormal conditions such as hair loss. pp.75–94. In: Handbook of primate behavioral management (Schapiro, S.J. eds.), CRC press, NW. [Google Scholar]
- 31.O’Farrell K., Harkin A.2017. Stress-related regulation of the kynurenine pathway: Relevance to neuropsychiatric and degenerative disorders. Neuropharmacology 112:(Pt B): 307–323. doi: 10.1016/j.neuropharm.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 32.Payne M.E., Pierce C.W., McQuoid D.R., Steffens D.C., Anderson J.J.2013. Serum ionized calcium may be related to white matter lesion volumes in older adults: a pilot study. Nutrients 5: 2192–2205. doi: 10.3390/nu5062192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perlman J.E., Bloomsmith M.A., Whittaker M.A., McMillan J.L., Minier D.E., McCowan B.2012. Implementing positive reinforcement animal training programs at primate laboratories. Appl. Anim. Behav. Sci. 137: 114–126. doi: 10.1016/j.applanim.2011.11.003 [DOI] [Google Scholar]
- 34.Persico A.M., Napolioni V.2013. Urinary p-cresol in autism spectrum disorder. Neurotoxicol. Teratol. 36: 82–90. doi: 10.1016/j.ntt.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 35.Pomerantz O., Paukner A., Terkel J.2012. Some stereotypic behaviors in rhesus macaques (Macaca mulatta) are correlated with both perseveration and the ability to cope with acute stressors. Behav. Brain Res. 230: 274–280. doi: 10.1016/j.bbr.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinhardt V.2005. Environmental enrichment and refinement of handling procedures. p.209–219. In: The laboratory primate (Wolffe-Coote, S. eds.), Elsevier academic press, San Diego, California. [Google Scholar]
- 37.Russel W.M.S., Burch R.L.1959. The principles of Humane experimental technique. Methuen and Co., London. [Reissued: 1992, Universities federation for animal welfare, Herts, UK.]. [Google Scholar]
- 38.Schapiro S.J.2002. Effects of social manipulations and environmental enrichment on behavior and cell-mediated immune responses in rhesus macaques. Pharmacol. Biochem. Behav. 73: 271–278. doi: 10.1016/S0091-3057(02)00779-7 [DOI] [PubMed] [Google Scholar]
- 39.Schapiro S.J., Bloomsmith M.A., Porter L.M., Suarez S.A.1996. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Appl. Anim. Behav. Sci. 48: 159–171. doi: 10.1016/0168-1591(96)01038-6 [DOI] [Google Scholar]
- 40.Schapiro S.J., Nehete P.N., Perlman J.E., Sastry K.J.2000. A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Appl. Anim. Behav. Sci. 68: 67–84. doi: 10.1016/S0168-1591(00)00090-3 [DOI] [PubMed] [Google Scholar]
- 41.Schapiro S.J., Perlman J.E., Boudreau B.A.2001. Manipulating the affiliative interactions of group-housed rhesus macaques using positive reinforcement training techniques. Am. J. Primatol. 55: 137–149. doi: 10.1002/ajp.1047 [DOI] [PubMed] [Google Scholar]
- 42.Sharma A., Schray A., Bartolovic M., Roesch-Ely D., Aschenbrenner S., Weisbrod M.2017. Relationship between serum calcium and neuropsychological performance might indicate etiological heterogeneity underlying cognitive deficits in schizophrenia and depression. Psychiatry Res. 252: 80–86. doi: 10.1016/j.psychres.2017.01.101 [DOI] [PubMed] [Google Scholar]
- 43.Simpson J., Kelly J.P.2011. The impact of environmental enrichment in laboratory rats--behavioural and neurochemical aspects. Behav. Brain Res. 222: 246–264. doi: 10.1016/j.bbr.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 44.Smith A.L., Corrow D.J.2005. Modifications to husbandry and housing conditions of laboratory rodents for improved well-being. ILAR J. 46: 140–147. doi: 10.1093/ilar.46.2.140 [DOI] [PubMed] [Google Scholar]
- 45.Smith E.A., Macfarlane G.T.1997. Formation of phenolic and indolic compounds by anaerobic bacteria in the human large intestine. Microb. Ecol. 33: 180–188. doi: 10.1007/s002489900020 [DOI] [PubMed] [Google Scholar]
- 46.Suzuki J., Ohkura S., Terao K.2002. Baseline and stress levels of cortisol in conscious and unrestrained Japanese macaques (Macaca fuscata). J. Med. Primatol. 31: 340–344. doi: 10.1034/j.1600-0684.2002.01011.x [DOI] [PubMed] [Google Scholar]
- 47.Vandeleest J.J., McCowan B., Capitanio J.P.2011. Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta). Appl. Anim. Behav. Sci. 132: 81–89. doi: 10.1016/j.applanim.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Velenosi T.J., Hennop A., Feere D.A., Tieu A., Kucey A.S., Kyriacou P., McCuaig L.E., Nevison S.E., Kerr M.A., Urquhart B.L.2016. Untargeted plasma and tissue metabolomics in rats with chronic kidney disease given AST-120. Sci. Rep. 6: 22526. doi: 10.1038/srep22526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vuong H.E., Hsiao E.Y.2017. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiatry 81: 411–423. doi: 10.1016/j.biopsych.2016.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]