Abstract
Sulforaphane (SFN) is abundant in cruciferous plants, providing significant protection against many chronic diseases. With the aim of clarifying the efficacy of sulforaphane in diabetic retinopathy (DR), a series of systematic studies were carried out in the present study. Male Sprague Dawley rats were intraperitoneally injected with streptozotocin (STZ, 65 mg/kg), and those with confirmed diabetes mellitus were given different doses of SFN (0.5 and 1 mg/kg/d) for 12 weeks. In vitro, Müller cells exposed to 25 mM glucose were treated with 2.5 µM SFN. The results indicated that SFN significantly reduced the generation of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and enhanced the activity of antioxidant enzymes (GSH, SOD, and CAT) in the retina of STZ rats. Further, SFN enhanced the nuclear accumulation of Nrf2 and increased the expression of HO-1 and NQO1, two major antioxidants downstream to Nrf2, in the injured retina. In addition, retinal expression levels of NLRP3, cleaved caspase-1 p20, IL-1β p17, and ASC were dramatically increased in STZ-induced DR, and this was abolished by SFN intervention. In vitro, high glucose-induced inflammation and oxidative stress damage in Müller cells were attenuated by SFN. SFN also exerted antioxidant effects, activated the Nrf2 pathway, and inhibited the NLRP3 inflammasome in Müller cells. In conclusion, our work demonstrates that SFN attenuates retinal inflammation and oxidative stress induced by high glucose and activates the antioxidative Nrf2 pathway and inhibits the formation of the NLRP3 inflammasome in vivo and in vitro.
Keywords: diabetic retinopathy, inflammasome, Nrf2 signaling pathway, oxidative stress, sulforaphane
Introduction
Diabetic retinopathy (DR), a complication of both type 1 and type 2 diabetes mellitus, arises from long-term elevated blood glucose levels [1]. In the initial stages of DR, patients are often asymptomatic. Once visual problems appear, they are almost irreversible and lead to blindness [32]. It has been reported that inflammation, hypoxia, and oxidative stress can accelerate DR development [3, 16]. However, the pathogenesis of DR is very complex and multifactorial. Therefore, there is an urgent demand for developing more effective therapeutic agents to interdict the progression of DR.
Oxidative stress can be triggered by circulating high glucose, and it directly or indirectly induces over-generation of inflammatory mediators that contribute to the retinal cell damage in DR [28]. Studies have demonstrated that reactive oxygen species (ROS) are a major causative factor involved in DR development [15, 17]. The more ROS accumulate, the more severe the damage is. Subsequently, the levels of inflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-6, and IL-1β, will increase [14]. Nuclear erythroid type 2 factor (Nrf2) is a major transcription factor that responds to oxidative stress-induced cytotoxicity, and it exerts anti-antioxidant effects by inducing phase II detoxification (glutathione S-transferases) and/or promoting the expression of antioxidant enzymes (superoxide dismutase, SOD; heme oxygenase-1, HO-1; and NAD (P) H quinone oxidoreductase 1, NQO1) [11, 19]. Loss of Nrf2 has been shown to exacerbate oxidative damage to retinas [4]. Moreover, it has been reported that the nucleotide binding domain, leucine-rich repeat-containing receptor (NLR), pyrin domain-containing 3 (NLRP3) inflammasome could be triggered by oxidative stress and could be involved in the pathogenesis of DR [21, 39]. So, it is necessary to clarify whether drugs can regulate retinal Nrf2 signaling and the NLRP3 inflammasome during DR.
Several clinical trials have confirmed the advantages of using natural products derived from functional foods for hyperglycemia management [5]. For instance, flavonoids and phenolic compounds prevent oxidative damage directly by scavenging ROS or indirectly by activating the transcription of cytoprotective genes [30]. Sulforaphane (SFN) is an isothiocyanate which is found abundantly in cruciferous vegetables including cabbage, broccoli, and radish [10, 29]. It displays strong antioxidant, anti-inflammatory, and anti-tumor activities [13]. Although SFN has already been reported to attenuate experimental diabetic nephropathy and diabetic cardiomyopathy [12, 33], how it prevents DR development is unclear. Further investigation of how SFN affects DR development will help to fully evaluate the effects of SFN on treatment of diabetes mellitus.
Therefore, in this study, rats with streptozotocin (STZ)-induced DR were given SFN, and their retinal pathological changes were analyzed. In vitro, Müller cells exposed to high glucose were treated with SFN. Our data indicated that SFN could attenuate high glucose-associated DR in vivo and in vitro.
Materials and Methods
Animal experiments
All animal experiments were performed taking the welfare of all animals into consideration, conformed to the policies of the guide for the care and use of laboratory animals of Shengjing Hospital of China Medical University, and were approved by the hospital. Healthy male Sprague Dawley rats (200–250 g) were kept in plastic cages under standard conditions with a light/dark cycle of 12 h, a temperature of 21°C, and controlled humidity (55%). They were allowed to access to water and food freely. SFN (ab141969) and STZ (s110910) were obtained from Abcam (Cambridge, MA, USA) and Aladdin (Shanghai, China), respectively.
Animals were randomly divided into control, SFN1, STZ, SFN0.5+STZ, and SFN1+STZ groups, respectively. To induce DR, rats were intraperitoneally (i.p.) injected with STZ (65 mg/kg body weight) dissolved in freshly prepared sodium citrate buffer (50 mM, pH 4.5). After 3 days, blood samples were collected to measure glucose levels. Rats with a blood glucose level over 16.7 mM for three consecutive days were considered diabetic rats and were used as the animal model in subsequent experiments. Rats in the SFN1, SFN0.5+STZ, and SFN1+STZ groups were given different doses of SFN (1 mg/kg/d, 0.5 mg/kg/d, and 1 mg/kg/d, respectively) for 12 weeks. Then they were euthanized by anesthesia (pentobarbital sodium: 200 mg/kg), and retinal tissue samples were isolated for further biochemical measurements.
Histological analysis and ganglion cell counting
Retinal tissue samples removed from rats were fixed in 4% paraformaldehyde and embedded in paraffin. Paraffin sections were then cut to a thickness of 5 µm and stained with hematoxylin and eosin (H&E) for histological evaluation. These slices were selected at a distance of 1 mm from the optic disc. The number of ganglion cells in the retinal ganglion cell layer was counted in three random fields per eye (5 rats/group) under a light microscope (200×; Olympus, Tokyo, Japan) and averaged.
Measurement of TNF-α, IL-6, IL-1β, GSH, SOD, and CAT
TNF-α, IL-6, and IL-1β concentrations in the retinal tissue samples were analyzed using commercially available kits from USCN life science (Wuhan, China). The principle of assay was ELISA. Absorbance was taken at 450 nm.
GSH, SOD, and CAT were measured by kits purchased from Jiancheng Bioengineering Institute (Nanjing, China). All procedures followed were in accordance with standard protocols described in the kits. OD values were measured by microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Total RNA isolation and real-time PCR
Total RNA was extracted with TRIpure (BioTeke, Beijing, China). Super M-MLV Reverse Transcriptase (BioTeke, Beijing, China) and SYBR Green (Solarbio, Beijing, China) were used to synthesize cDNA and amplify target genes, respectively. All procedures were in accordance with the manufacturer’s protocol. Amplification was performed using an ExicyclerTM 96 (Bioneer, Daejeon, South Korea). The levels of genes of interest were standardized to the level of actin. β-actin was used as a reference gene. The mRNA relative expression levels of target genes were normalized to β-actin and calculated using the 2-ΔΔCT method. The real-time PCR primers are listed in Table 1.
Table 1. Real-time PCR primers.
| Gene | Primer sequence | Product length |
|---|---|---|
| HO-1, NM_012580.2 | AGCGAAACAAGCAGAACCCA | 196 bp |
| GCCACCAGCAGCTCAGGATG | ||
| NQO1, NM_017000.3 | GCGGTGAGAAGAGCCCTGAT | 115 bp |
| ATTCGACCACCTCCCATCCT |
Western blot analysis
A regular western blot protocol was performed. Primary antibodies included NRF2 (1:1,000), HO-1 (1:1,000), NQO1 (1:500), NLRP3 (1:400), cleaved caspase-1 p20 (1:500), IL-1β p17 (1:1,000), ASC (1:500), histone H3 (1:500) and β-actin (1:500). Primary antibodies were detected using secondary antibodies according to the origin of the primary antibody (anti-rabbit, anti-mouse, or anti-goat).
Electrophoretic mobility shift assay (EMSA)
To determine the Nrf2-ARE binding activity, an EMSA was performed as previously described [23]. Briefly, the nuclear proteins from the retinal tissue were mixed with biotin-labeled probes containing the Nrf2 sequence for 10 min at room temperature. The DNA-protein complexes were electrophoresed.
Cell lines and cell culture
The rat Müller cell line was purchased from Chi Scientific (Wuxi, China) and maintained in Dulbecco’s modified Eagle’s medium (DMEM)/F12 with 10% (v/v) fetal bovine serum (FBS). DMEM/F12 and FBS were bought from Gibco BRL (Gaithersburg, MD, USA) and Hyclone (SH30084.03, Logan, Utah, USA), respectively. Cells (1 × 105) were placed on 6-well plates. Cells were randomly divided into LG, SFN, HG, and HG+SFN groups, respectively. When the cell density was about 80%, cells of the LG group were incubated in 5.5 mM glucose plus 19.5 mM mannitol for 48 h. Cells of the HG group were incubated in 25 mM glucose for 48 h. Cells of the SFN group were incubated in 2.5 µM SFN for 48 h. Cells of the HG+SFN group were pretreated with 2.5 µM SFN for 2 h and then incubated in 25 mM glucose for an additional 48 h.
Statistical analysis
All data were expressed as the mean ± SD. One-way analysis of variance (ANOVA) was performed with the IBM SPSS ver. 20.0 software for multiple comparisons followed by Bonferroni’s post hoc test. A value of P<0.05 was considered statistically significant.
Results
Histological evaluations
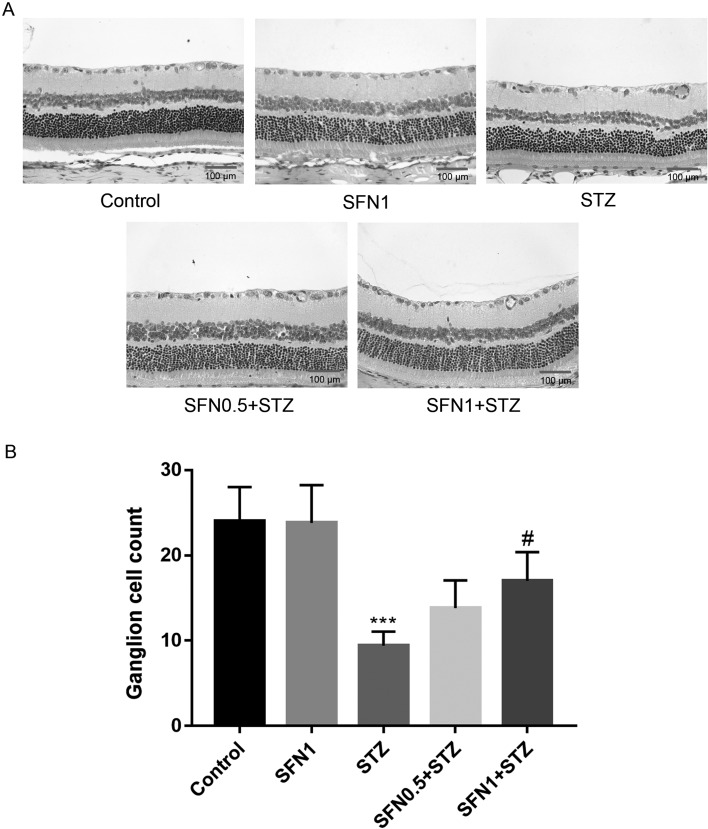
The protective effects of SFN on DR were confirmed histologically. The retina of the control group had a clear structure and smooth inner membrane, and the retinal cells were normal in appearance and arranged regularly (Fig. 1A). There was no significant difference between the control and SFN1 group in histological evaluations. The retina of the STZ group had a rough inner membrane; the cells were arranged loosely and even seemed messy; the number of ganglion cells was reduced. As shown in Fig. 1B, the amount of ganglion cells was counted, which demonstrated that SFN increased the number of ganglion cells in a dose-dependent manner (P<0.05).
Fig. 1.
Pathological observation of SFN in retinas of STZ-induced DR rats (A) and the number of ganglion cells (B). ***P<0.001 vs. control. #P<0.05 vs. STZ group. Values are expressed as the mean ± SD (n=5).
Effects of SFN on inflammatory cytokines and oxidative stress in vivo
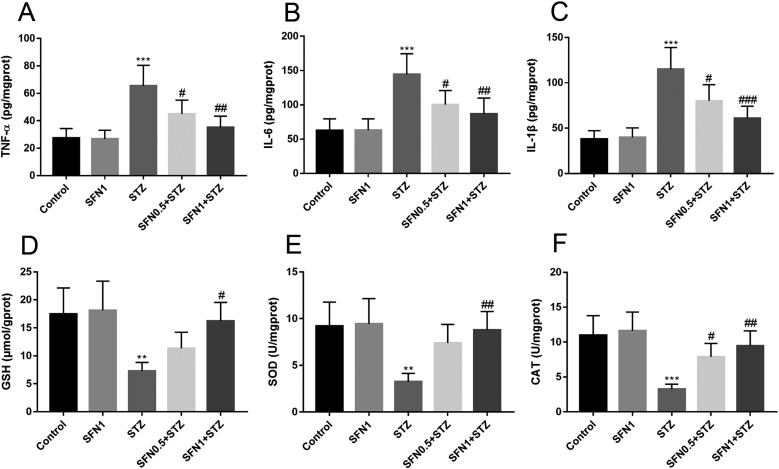
The TNF-α, IL-6, IL-1β, GSH, SOD, and CAT levels in retinal tissue treated with SFN are shown in Fig. 2. To evaluate the levels of proinflammatory cytokines in the retina, TNF-α, IL-6, and IL-1β were measured in experimental groups of rats using ELISA kits. As shown in Figs. 2A–C, markedly increased levels of TNF-α, IL-6, and IL-1β were detected in the retina of the STZ group (P<0.001), whereas significantly reduced levels were detected in the SFN0.5+STZ and SFN1+STZ groups (P<0.05, P<0.01, P<0.001, respectively). The TNF-α, IL-6, and IL-1β values in the STZ group were nearly twice those in the control group. Based on analysis and comparison with the other three sets of data, it was not difficult to determine that the antioxidant capacity in the STZ group was decreased. SFN, especially at the higher concentration (1 mg/kg), could upregulate the antioxidant capacity effectively such that it was almost equal to that of the control group.
Fig. 2.
The levels of TNF-α, IL-6, IL-1β, GSH, SOD, and CAT in rat retina tissue. **P<0.01 vs. control. ***P<0.001 vs. control. #P<0.05 vs. STZ group. ##P<0.01 vs. STZ group. ###P<0.001 vs. STZ group. Values are expressed as the mean ± SD (n=5).
Effects of SFN on Nrf2 signaling-related proteins and genes
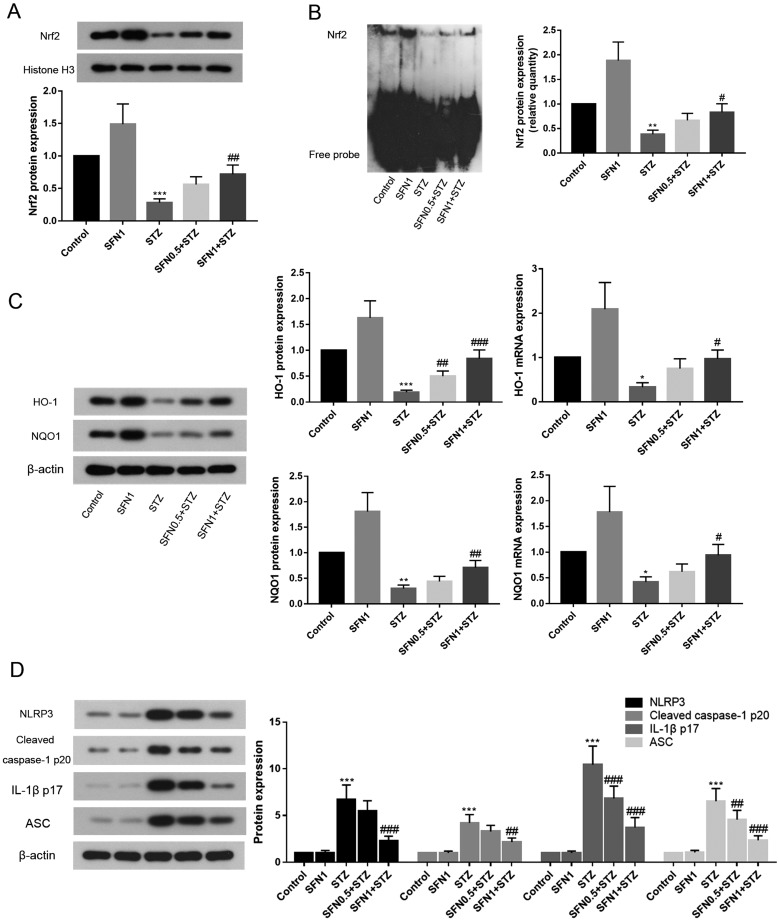
Nrf2 binds to AREs and positively regulates the expression of HO-1 and NQO1. In the present study, the protein level of NRF2 in the nucleus was quantified by western blotting (Fig. 3A) and found to be lower than that in the STZ group (P<0.001). On the other hand, SFN caused an observable increase in this level in a dose-dependent manner. To elucidate the binding activity between Nrf2 and ARE, an EMSA was subsequently performed. As shown in Fig. 3B, SFN improved the Nrf2-ARE binding activity and appeared to be dose dependent to some extent. To further find out the relationship between Nrf2 and its downstream ARE-driven target antioxidative defence enzyme, the mRNA and protein levels of HO-1 and NQO1 were examined through biochemical analysis. Whether at the mRNA or protein level, the transcriptions of HO-1 and NQO1 were all upregulated when rats were given SFN (Fig. 3C).
Fig. 3.
The protein expression of NRF2 in rat retina tissue (A); nuclear levels of Nrf2 as assessed by EMSA in nuclear extract (B); activation of HO-1/NQO1 signaling-related genes and proteins (C) in retinal tissue of rats after diabetes induction with STZ; and inhibition of NLRP3, cleaved caspase-1 p20, IL-1β p17, and ASC protein expression after treatment with SFN (D). *P<0.05 vs. control. **P<0.01 vs. control. ***P<0.001 vs. control. #P<0.05 vs. STZ group. ##P<0.01 vs. STZ group. ###P<0.001 vs. STZ group. Values are expressed as the mean ± SD (n=5).
Assessment of NLRP3 inflammasome components in the retina of rats
To understand the mechanism of the inflammation indicated by STZ disturbances, we further investigated the role of the NLRP3 inflammasome via assessment of the expression levels of its components including the NLRP3, cleaved caspase-1 p20, IL-1β p17, and the adaptor protein apoptosis associated speck-like protein (ASC). As shown in Fig. 3D, the expression levels of all four inflammatory components were significantly upregulated in STZ rats compared with those in control rats. Specifically, the IL-1β p17 level in the STZ group increased to 10.4-fold that in control rats; the NLRP3 and ASC levels in the STZ group increased to 6.7- and 6.5-fold those in control rats; and the cleaved caspase-1 p20 level in the STZ group was upregulated to 4.2-fold that in control rats. Notably, the expression levels of all four inflammatory components were downregulated in diabetic rats which received SFN treatment.
SFN alleviated the high glucose-induced impairments in vitro
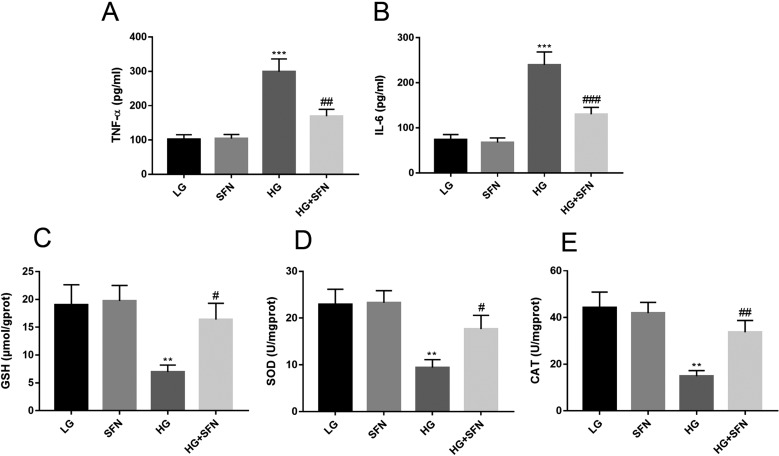
It is recognized that inflammation and oxidative stress play a vital role in the development of diabetic complications. In the present study, the effect of SFN on high glucose-induced impairments was investigated using ELISA kits (Fig. 4). It was revealed that the levels of TNF-α and IL-6 in the HG group increased nearly three times but that those in the HG+SFN group was reduced by half. Meanwhile, a sharp decline of antioxidant activities (GSH, SOD, and CAT) was also discovered in the HG group (P<0.05). Expectedly, antioxidant activities were significantly improved after treatment with SFN. Therefore, SFN protected cells against high glucose-induced oxidative insults by decrease of TNF-α and IL-6 levels, and increase of GSH, SOD, and CAT activities.
Fig. 4.
The levels of TNF-α, IL-6, GSH, SOD, and CAT in Müller cell. **P<0.01 vs. LG group. ***P<0.001 vs. LG group. #P<0.05 vs. HG group. ##P<0.01 vs. HG group. ###P<0.001 vs. HG group. Values are expressed as the mean ± SD (n=3).
SFN activated the antioxidative Nrf2 pathway and inhibited NLRP3 inflammasome formation in high glucose-induced Müller cells
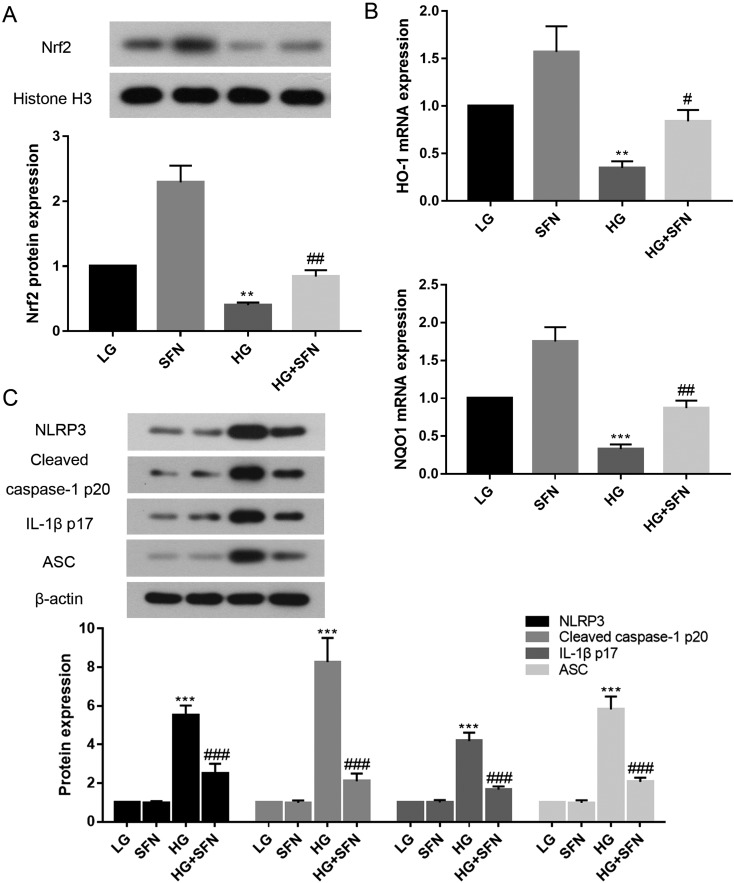
In order to further evaluate the regulation of the Nrf2 pathway by SFN, western blotting and real-time PCR were applied to monitor the protein expression of NRF2 (Fig. 5A) and the mRNA expression of Ho-1 and Nqo1 (Fig. 5B), respectively. The results demonstrated that SFN indeed upregulated the NRF2 expression in high glucose-induced Müller cells. Furthermore, the mRNA expression levels of Ho-1 and Nqo1 in the HG+SFN group also increased compared with those in the HG group. As expected, the protective effects of SFN were derived from the induction of HO-1, NQO1, and its transcription factor Nrf2.
Fig. 5.
The protein expression of NRF2 in Müller cell (A); effect of SFN on Ho-1 and Nqo1 mRNA expression in Müller cell (B); and the expression of NLRP3, cleaved caspase-1 p20, IL-1β p17, and ASC protein in Müller cell (C). **P<0.01 vs. LG group. ***P<0.001 vs. LG group. #P<0.05 vs. HG group. ##P<0.01 vs. HG group. ###P<0.001 vs. HG group. Values are expressed as the mean ± SD (n=3).
We then examined the ability of SFN to inhibit the activation of NLRP3, cleaved caspase-1 p20, IL-1β p17, and ASC. The expression levels of all four inflammatory components were significantly increased in the HG group (Fig. 5C, P<0.001), while treatment with SFN showed an obvious reduction compared with the expression in the HG group (P<0.001).
Discussion
The number of people with diabetes worldwide has exploded in the past several years and is expected to sustain rapid growth. Long-term and uncontrollable high blood glucose generates various diabetic complications, including DR [34]. A global epidemiological analysis showed that 35% of people with diabetes have some form of DR [36], which is characterized by a progressive alteration in retinal microvasculature, leading to capillary closure [24]. The pathogenesis of DR has been understudied for decades, and it is still not yet fully understood. Therefore, this study focused on DR, researched the therapeutic effect of SFN on it, and revealed its potential mechanism. This study provided new evidence showing a significant preventive effect of SFN on the development of DR.
In this paper, the protective effect of SFN on DR was studied in vivo and in vitro. Apoptotic retinal cell death causes reduction in the thickness of various layers and in the number of ganglion cells in experimental animal models [26]. We found that SFN made the morphometric thicknesses of the retinal layers move towards the basal levels in STZ-induced diabetic rats (Fig. 1). The histological results scientifically confirmed the traditional uses of SFN as an effective protective agent for DR.
To further investigate the underlying mechanism of the protective effects of SFN, we first paid attention to retina antioxidant activation. Oxidative stress plays an important role in the etiology of DR. Although the retina contains a robust antioxidant defense system with antioxidant molecules (GSH, vitamins C and E) and endogenous enzymes (SOD, CAT, and glutathione peroxidase), an imbalance between prooxidants and antioxidants may lead to oxidative stress [16]. So, the detection of these related index parameters reflects the correlation with oxidative stress. On the other hand, oxidative stress has been associated with cellular inflammation and release of inflammatory cytokines. For instance, TNF-α, IL-6, and IL-1β are important mediators of diabetes-induced retinal neuroinflammation [18]. Increased levels of these cytokines have been observed in the vitreous of diabetic patients with proliferative DR as well as in diabetic rat retinas [8]. It was found that levels of inflammatory cytokines were increased in diabetic retinas, yet SFN-treated retinas displayed lower levels of cytokines. At a dose of 1 mg/kg, the downregulations of TNF-α and IL-6 were equal to those in the control group. There are similar reports demonstrating that dh404 [6] has the same effect, but the dose of dh404 (3 mg/kg) is more than that of SFN. This also illustrated the effectiveness of SFN.
It is known that Nrf2 expression and transcription in tissues in vivo and in cells in vitro are increased in response to oxidative stress [25, 31]. Activation of the Nrf2/ARE signaling pathway plays a part in the protection of cells against oxidative stress, carbonyl compounds, and electrophilic agents in the human body [38]. In vivo, Nrf2 was activated by SFN in a dose-dependent manner. According to experimentation in vitro, to prevent oxidative stress-mediated damage caused by high glucose, cells have evolved the Nrf2-mediated defense mechanism to cope with adverse environmental conditions. Moreover, it was reported that inflammation, ROS production, protein kinase C, and the hexosamine pathways were inhibited by Nrf2 activation under high glucose conditions [35]. Thus, it was suggested that activation of Nrf2 before disease development or during the early stage of DR is the key for an intervention to prevent oxidative stress-induced damage as well as DR progression [20]. HO-1 is a stress-responsive enzyme that maintains homeostasis under pathological conditions [9]. NQO1 is an enzyme responsible for two-electron reduction of quinones, which converts these highly reactive molecules into hydroquinones and further produces a stable water-soluble conjugate that can be excreted in the urine [22]. Consistent with related studies [9], the expression levels of HO-1 and NQO1 were increased by SFN treatment in the retinas of diabetic rats, and the data were also similar to those of studies on ischemic retinopathy [7].
In the present study, we also became aware of retina inflammasome activation, which is important for the inflammation induced by the cell injury in DR [2]. The NLRP3 inflammasome is a key initiator of the inflammatory cascade and is best characterized as a protein scaffold upon which an ASC adaptor assembles and acts to recruit pro-caspase-1. We found that inflammasome components, NLRP3, cleaved caspase-1 p20, IL-1β p17, and ASC, were upregulated whether in the retinas of STZ-induced diabetic rats or in those of HG-induced Müller cells. Müller cells are recognized as an important mediator and source of the increased levels of these pro-inflammatory factors in DR [27, 37]. These outcomes agreed with those of previous studies [21] and indicated that NLRP3 inflammasome effectors were important determinants of inflammatory progress in DR. It was proved that SFN not only activated the Nrf2 signaling pathway but also inhibited the NLRP3 inflammasome, which further clarified the possible molecular mechanism of SFN.
Conclusions
Based on the data, SFN appears to have a protective action against DR via reduction of TNF-α, IL-6, and IL-1β levels, improvement of antioxidant capacities (GSH, SOD, and CAT), promotion of mRNA and protein levels (Nrf2, HO-1, and NQO1), and downregulation of the expression of inflammasome components (NLRP3, cleaved caspase-1 p20, IL-1β p17, and ASC). Our data indicates that inflammation, oxidative stress, and inflammasome activation played a pathogenic role in DR. Activation of Nrf2 signaling in diabetic rats could be regarded as a compensatory response to combat oxidative stress during DR. The inhibition of NLRP3 inflammasome formation attenuated damage in DR, and this finding enriched our understanding of the mechanisms behind the anti-inflammatory activity of SFN. This study should provide some hints and help for treatment of DR in the future. Moreover, our group will study the effect of SFN from different angles in future research, in order to clarify the therapeutic value of SFN for this disease more comprehensively and thoroughly.
Conflicts of Interest
The authors declare no competing financial interest.
Reference
- 1.Abu El-Asrar A.M., Midena E., Al-Shabrawey M., Mohammad G.2013. New developments in the pathophysiology and management of diabetic retinopathy. J. Diabetes Res. 2013: 424258. doi: 10.1155/2013/424258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Shabrawey M., Zhang W., McDonald D.2015. Diabetic retinopathy: mechanism, diagnosis, prevention, and treatment. BioMed Res. Int. 2015: 854593. doi: 10.1155/2015/854593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonetti D.A., Barber A.J., Bronson S.K., Freeman W.M., Gardner T.W., Jefferson L.S., Kester M., Kimball S.R., Krady J.K., LaNoue K.F., Norbury C.C., Quinn P.G., Sandirasegarane L., Simpson I.A., JDRF Diabetic Retinopathy Center Group2006. Diabetic retinopathy: seeing beyond glucose-induced microvascular disease. Diabetes 55: 2401–2411. doi: 10.2337/db05-1635 [DOI] [PubMed] [Google Scholar]
- 4.Chen W.J., Wu C., Xu Z., Kuse Y., Hara H., Duh E.J.2017. Nrf2 protects photoreceptor cells from photo-oxidative stress induced by blue light. Exp. Eye Res. 154: 151–158. doi: 10.1016/j.exer.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu J., Khan Z.A., Farhangkhoee H., Chakrabarti S.2009. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-kappaB. Nutrition 25: 964–972. doi: 10.1016/j.nut.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 6.Deliyanti D., Alrashdi S.F., Tan S.M., Meyer C., Ward K.W., de Haan J.B., Wilkinson-Berka J.L.2018. Nrf2 Activation Is a Potential Therapeutic Approach to Attenuate Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci. 59: 815–825. doi: 10.1167/iovs.17-22920 [DOI] [PubMed] [Google Scholar]
- 7.Deliyanti D., Lee J.Y., Petratos S., Meyer C.J., Ward K.W., Wilkinson-Berka J.L., de Haan J.B.2016. A potent Nrf2 activator, dh404, bolsters antioxidant capacity in glial cells and attenuates ischaemic retinopathy. Clin. Sci. (Lond.) 130: 1375–1387. doi: 10.1042/CS20160068 [DOI] [PubMed] [Google Scholar]
- 8.Demircan N., Safran B.G., Soylu M., Ozcan A.A., Sizmaz S.2006. Determination of vitreous interleukin-1 (IL-1) and tumour necrosis factor (TNF) levels in proliferative diabetic retinopathy. Eye (Lond.) 20: 1366–1369. doi: 10.1038/sj.eye.6702138 [DOI] [PubMed] [Google Scholar]
- 9.Fan J., Xu G., Jiang T., Qin Y.2012. Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Invest. Ophthalmol. Vis. Sci. 53: 6541–6556. doi: 10.1167/iovs.11-9241 [DOI] [PubMed] [Google Scholar]
- 10.Fimognari C., Hrelia P.2007. Sulforaphane as a promising molecule for fighting cancer. Mutat. Res. 635: 90–104. doi: 10.1016/j.mrrev.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 11.Ganesh Yerra V., Negi G., Sharma S.S., Kumar A.2013. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 1: 394–397. doi: 10.1016/j.redox.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J., Cheng Y., Wu H., Kong L., Wang S., Xu Z., Zhang Z., Tan Y., Keller B.B., Zhou H., Wang Y., Xu Z., Cai L.2017. Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy. Diabetes 66: 529–542. doi: 10.2337/db15-1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang J.H., Lim S.B.2014. Antioxidant and Anti-inflammatory Activities of Broccoli Florets in LPS-stimulated RAW 264.7 Cells. Prev. Nutr. Food Sci. 19: 89–97. doi: 10.3746/pnf.2014.19.2.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jialal I., Kaur H.2012. The role of toll-like receptors in diabetes-induced inflammation: implications for vascular complications. Curr. Diab. Rep. 12: 172–179. doi: 10.1007/s11892-012-0258-7 [DOI] [PubMed] [Google Scholar]
- 15.Kanwar M., Chan P.S., Kern T.S., Kowluru R.A.2007. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest. Ophthalmol. Vis. Sci. 48: 3805–3811. doi: 10.1167/iovs.06-1280 [DOI] [PubMed] [Google Scholar]
- 16.Kowluru R.A., Chan P.S.2007. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007: 43603. doi: 10.1155/2007/43603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowluru R.A., Tang J., Kern T.S.2001. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 50: 1938–1942. doi: 10.2337/diabetes.50.8.1938 [DOI] [PubMed] [Google Scholar]
- 18.Kumar B., Gupta S.K., Nag T.C., Srivastava S., Saxena R., Jha K.A., Srinivasan B.P.2014. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 125: 193–202. doi: 10.1016/j.exer.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 19.Liu C.M., Ma J.Q., Xie W.R., Liu S.S., Feng Z.J., Zheng G.H., Wang A.M.2015. Quercetin protects mouse liver against nickel-induced DNA methylation and inflammation associated with the Nrf2/HO-1 and p38/STAT1/NF-κB pathway. Food Chem. Toxicol. 82: 19–26. doi: 10.1016/j.fct.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y.W., Cheng Y.Q., Liu X.L., Hao Y.C., Li Y., Zhu X., Zhang F., Yin X.X.2017. Mangiferin Upregulates Glyoxalase 1 Through Activation of Nrf2/ARE Signaling in Central Neurons Cultured with High Glucose. Mol. Neurobiol. 54: 4060–4070. doi: 10.1007/s12035-016-9978-z [DOI] [PubMed] [Google Scholar]
- 21.Loukovaara S., Piippo N., Kinnunen K., Hytti M., Kaarniranta K., Kauppinen A.2017. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 95: 803–808. doi: 10.1111/aos.13427 [DOI] [PubMed] [Google Scholar]
- 22.Matusheski N.V., Jeffery E.H.2001. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J. Agric. Food Chem. 49: 5743–5749. doi: 10.1021/jf010809a [DOI] [PubMed] [Google Scholar]
- 23.Meyer T., Münch C., Völkel H., Booms P., Ludolph A.C.1998. The EAAT2 (GLT-1) gene in motor neuron disease: absence of mutations in amyotrophic lateral sclerosis and a point mutation in patients with hereditary spastic paraplegia. J. Neurol. Neurosurg. Psychiatry 65: 594–596. doi: 10.1136/jnnp.65.4.594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagai N., Deguchi S., Otake H., Hiramatsu N., Yamamoto N.2017. Therapeutic Effect of Cilostazol Ophthalmic Nanodispersions on Retinal Dysfunction in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 18: 18. doi: 10.3390/ijms18091971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palsamy P., Subramanian S.2011. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim. Biophys. Acta 1812: 719–731. doi: 10.1016/j.bbadis.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 26.Park S.H., Park J.W., Park S.J., Kim K.Y., Chung J.W., Chun M.H., Oh S.J.2003. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia 46: 1260–1268. doi: 10.1007/s00125-003-1177-6 [DOI] [PubMed] [Google Scholar]
- 27.Portillo J.C., Lopez Corcino Y., Miao Y., Tang J., Sheibani N., Kern T.S., Dubyak G.R., Subauste C.S.2017. CD40 in Retinal Müller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes 66: 483–493. doi: 10.2337/db16-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy S., Kern T.S., Song B., Stuebe C.2017. Mechanistic Insights into Pathological Changes in the Diabetic Retina: Implications for Targeting Diabetic Retinopathy. Am. J. Pathol. 187: 9–19. doi: 10.1016/j.ajpath.2016.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sestili P., Fimognari C.2015. Cytotoxic and Antitumor Activity of Sulforaphane: The Role of Reactive Oxygen Species. BioMed Res. Int. 2015: 402386. doi: 10.1155/2015/402386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surh Y.J., Kundu J.K., Na H.K.2008. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 74: 1526–1539. doi: 10.1055/s-0028-1088302 [DOI] [PubMed] [Google Scholar]
- 31.Tan Y., Ichikawa T., Li J., Si Q., Yang H., Chen X., Goldblatt C.S., Meyer C.J., Li X., Cai L., Cui T.2011. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 60: 625–633. doi: 10.2337/db10-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ting D.S., Cheung G.C., Wong T.Y.2016. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin. Exp. Ophthalmol. 44: 260–277. doi: 10.1111/ceo.12696 [DOI] [PubMed] [Google Scholar]
- 33.Wu H., Kong L., Cheng Y., Zhang Z., Wang Y., Luo M., Tan Y., Chen X., Miao L., Cai L.2016. Corrigendum to “Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2” [Free Radic. Biol. Med. 89 (2015) 431-42]. Free Radic. Biol. Med. 97: 621. doi: 10.1016/j.freeradbiomed.2016.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J., Li X., Fang H., Yi Y., Chen D., Long Y., Gao X., Wei X., Chen C.Y.2016. Investigation of synergistic mechanism and identification of interaction site of aldose reductase with the combination of gigantol and syringic acid for prevention of diabetic cataract. BMC Complement. Altern. Med. 16: 286. doi: 10.1186/s12906-016-1251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue M., Qian Q., Adaikalakoteswari A., Rabbani N., Babaei-Jadidi R., Thornalley P.J.2008. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes 57: 2809–2817. doi: 10.2337/db06-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yau J.W., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., Haffner S., Hamman R.F., Ikram M.K., Kayama T., Klein B.E., Klein R., Krishnaiah S., Mayurasakorn K., O’Hare J.P., Orchard T.J., Porta M., Rema M., Roy M.S., Sharma T., Shaw J., Taylor H., Tielsch J.M., Varma R., Wang J.J., Wang N., West S., Xu L., Yasuda M., Zhang X., Mitchell P., Wong T.Y., Meta-Analysis for Eye Disease (META-EYE) Study Group2012. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35: 556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yong P.H., Zong H., Medina R.J., Limb G.A., Uchida K., Stitt A.W., Curtis T.M.2010. Evidence supporting a role for N-(3-formyl-3,4-dehydropiperidino)lysine accumulation in Müller glia dysfunction and death in diabetic retinopathy. Mol. Vis. 16: 2524–2538. [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng H., Whitman S.A., Wu W., Wondrak G.T., Wong P.K., Fang D., Zhang D.D.2011. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60: 3055–3066. doi: 10.2337/db11-0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J.2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11: 136–140. doi: 10.1038/ni.1831 [DOI] [PubMed] [Google Scholar]