Abstract
Background and Objectives
Although current guidelines recommend early initiation of statin in patients with acute myocardial infarction (AMI), there is no consensus for optimal timing of statin initiation.
Methods
A total of 3,921 statin-naïve patients undergoing percutaneous coronary intervention were analyzed, and divided into 3 groups according to statin initiation time: group 1 (statin initiation <24 hours after admission), group 2 (24–48 hours) and group 3 (≥48 hours). We also made 3 stratified models to reduce bias: model 1 (<24 hours vs. ≥24 hours), model 2 (<48 hours vs. ≥48 hours) and model 3 (<24 hours vs. 24–48 hours). The endpoint was major adverse cardiac events (MACE; composite of cardiac death, myocardial infarction and target-vessel revascularization) during median 3.8 years.
Results
During follow-up, incidence of MACE was lower in early statin group in both model 1 (14.3% vs. 18.4%, hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.66–0.91; p=0.002) and model 2 (14.6% vs. 19.7%, HR, 0.81; 95% CI, 0.67–0.97; p=0.022). After propensity-score matching, results remained unaltered. Statin initiation <24 hours reduced MACE compared to statin initiation ≥24 hours in model 1. Statin initiation <48 hours also reduced MACE compared to statin initiation later in model 2. However, there was no difference in incidence of MACE between statin initiation <24 hours and 24–48 hours) in model 3.
Conclusions
Early statin therapy within 48 hours after admission in statin-naïve patients with AMI reduced long-term clinical outcomes compared with statin initiation later.
Trial Registration
ClinicalTrials.gov Identifier: NCT02385682
Keywords: Myocardial infarction, Hydroxymethylglutaryl-CoA reductase inhibitors, Percutaneous coronary intervention
INTRODUCTION
Statins are essential for patients with acute myocardial infarction (AMI). Current guidelines recommend early initiation of statin therapy in statin-naïve patients with AMI unless there is a contraindication to statins.1),2),3),4) Although many clinical studies support the initiation of statin therapy before discharge after AMI, few studies indicate the optimal timing of statin initiation in statin-naïve patients with AMI.5),6),7),8),9) While the current guidelines recommend that statin therapy be started as early as possible after admission, the optimal timing of statin initiation in patients with AMI is still unknown. The timing of statin initiation varied among these studies, and several meta-analyses and large-sized observational cohort studies showed no correlation between early statin initiation and improved clinical outcomes among AMI patients.10),11)
In the present study, we aimed to determine whether early statin initiation could reduce long-term clinical adverse events in statin-naïve AMI patients using a large, multi-center Korean registry.
METHODS
Study population
The Convergent Registry of Catholic and Chonnam University for AMI (COREA-AMI) is a prospective, multi-center, web-based observational cohort registry. It was designed to reflect real-world practice as it pertains to Korean AMI patients registered at nine centres with facilities for primary percutaneous coronary intervention (PCI) representing two universities between January 2004 and December 2009.12),13) We selected 3,921 statin-naïve patients undergoing successful PCI from among 4,748 consecutive AMI patients in the COREA-AMI registry. The exclusion criteria were as follows: in-hospital death (n=116), patients who were not prescribed a statin at discharge (n=613), patients with no data regarding statin initiation time (n=3), and patients who had already taken a statin before admission (n=95). Based on prior studies, subjects were divided into three groups according to the timing of statin initiation after admission: group 1 (statin initiation <24 hours, n=2,665), group 2 (24–48 hours, n=480) and group 3 (≥48 hours, n=776).5),6),7),9) We also generated three stratified models according to the timing of statin initiation to reduce bias: model 1 (<24 hours vs. ≥24 hours), model 2 (<48 hours vs. ≥48 hours) and model 3 (<24 hours vs. 24–48 hours). The study protocols were approved by the ethics committee at each participating center and adhered to the principles of the Declaration of Helsinki. This registry has been registered on ClinicalTrials.gov (study ID: NCT02385682). All patients provided written informed consent for participation in the registry. Trained study coordinators at each center collected data according to a standardised format. Standardised definitions of all variables were determined by the steering committee board of COREA-AMI.
Study definitions
The diagnosis of AMI was based on the criteria for a third universal definition of myocardial infarction (MI). Chronic kidney disease was defined as a glomerular filtration rate below 60 mL/min/1.73 m2, or when the patient had already received renal replacement therapy. Laboratory data were obtained on admission, except for lipid profiles; these were obtained after at least 9 hours of fasting, within 24 hours of hospitalisation. The baseline left ventricular ejection fraction (LVEF) was measured by two-dimensional echocardiography before or immediately after PCI. The timing of statin initiation, medications, and use of coronary stents, thrombi aspiration, intravascular ultrasound, and intra-aortic balloon pumps were at the operators' discretion. All patients underwent PCI within 48 hours after admission. The extent of coronary blood flow before and after PCI was graded using the thrombolysis in myocardial infarction (TIMI) flow grade, and the complexity of coronary lesions was based on the definitions of the American College of Cardiology (ACC)/American Heart Association (AHA). Patients who underwent PCI received 300 mg aspirin and 300 or 600 mg clopidogrel as a loading dose prior to PCI. Unfractionated heparin (50–70 U/kg) was given before or during PCI to maintain the activated clotting time at 250–300 seconds. After PCI, 100–300 mg aspirin and 75 mg clopidogrel daily were prescribed as the maintenance dose.
Study endpoints
The primary endpoint was major adverse cardiac events (MACE; including cardiac death, non-fatal spontaneous MI and target-vessel revascularization [TVR]) during a median follow-up period of 3.8 years (interquartile range: 2.6–5.1 years). We also analysed the incidence of all-cause death, cardiac death, nonfatal spontaneous MI, target-lesion revascularisation (TLR), TVR, non-TVR, stroke, and definite or probable stent thrombosis. Nonfatal spontaneous MI was defined as the development of recurrent angina symptoms accompanied by changes in the 12-lead electrocardiogram, or increased levels of cardiac-specific biomarkers. TLR was defined as PCI for restenosis or other lesion complications within the treated segment 5 mm proximal and 5 mm distal to the stent. TVR was defined as repeated PCI for any segment of the entire coronary artery proximal and distal to the target lesion. Stent thrombosis was defined as definite and probable stent thrombosis, according to the Academic Research Consortium's definition.14)
Patient outcomes were recorded at 1, 6, and 12 months, and annually thereafter at hospital visits or via telephone contact. Briefly, all clinical outcomes of interest were confirmed by source documents and were centrally adjudicated by the local events committee of the Cardiovascular Center, Seoul St Mary's Hospital, and by an independent group of clinicians who were unaware of patient status. Information about death was matched with records from the National Population Registry of the Korea National Statistical Office using a unique personal identification number to validate mortality follow-up data.12)
Statistical analysis
Continuous variables are presented as means±standard deviation (SD) and were compared using the unpaired t-test if the data were normally distributed. Non-normally distributed continuous data are summarised as medians with interquartile ranges and compared using the Mann-Whitney rank-sum test. Comparisons among the three groups were performed using one-way analysis of variance. Discrete variables are expressed as counts with percentages and were analysed by the Pearson χ2 test or Fisher's exact test. We constructed Kaplan-Meier curves to compare primary endpoints between the different statin initiation timing groups in each propensity-matched model (models 1, 2 and 3); the differences were assessed using the log-rank test. Cox's proportional hazards regression model (with adjustment for covariates) was used to assess clinical outcomes. Variables significant for end-points in the univariate analysis (p<0.05) were included in the multivariate analysis. The following variables were included in the multivariate Cox regression analysis: age ≥65 years, male gender, hypertension, diabetes mellitus, chronic kidney disease, Killip class ≥3, peak level of troponin-I, glucose level, high-sensitivity C-reactive protein (hsCRP) level, LVEF, use of an angiotensin-converting enzyme inhibitor or angiotensin-II receptor blocker, multivessel disease, ACC/AHA B2/C lesion, coronary stenting, total number of stents, total stent length, mean stent diameter, periprocedural shock, ventricular tachycardia or fibrillation, and use of intra-aortic balloon pump. The propensity score was estimated by logistic regression analysis using the variable shown in Tables 1 and 2.15) The Hosmer-Lemeshow goodness-of-fit p value was 0.185 in model 1, 0.357 in model 2 and 0.119 in model 3. The C-statistic values for the logistic models were 0.637 in model 1, 0.635 in model 2 and 0.660 in model 3. Finally, we performed 1:1 propensity score-matching without replacement using the nearest neighbour method. A calliper width of 0.1 SD was used for matching. Data and standardized mean differences before and after matching were presented in the Supplementary Tables 1, 2, 3, 4, 5, 6. The value of standardized mean differences was within 0.1 in nearly all variables of 3 models (0.103 in ST-segment elevation myocardial infarction [STEMI] and 0.144 in creatine kinase-myocardial band isozyme after matching of model 3).
Table 1. Baseline clinical characteristics.
| Variables | Time from admission to statin initiation (hours) | p value | |||
|---|---|---|---|---|---|
| Group I: <24 (n=2,665) | Group II: 24–48 (n=480) | Group III: ≥48 (n=776) | |||
| Demographics | |||||
| Age (years) | 61.4±12.5 | 61.9±12.1 | 63.3±12.5 | 0.001 | |
| Male | 1,993 (74.8) | 354 (73.8) | 531 (68.4) | 0.001 | |
| Initial vital signs | |||||
| Systolic blood pressure (mmHg) | 130.8±28.8 | 129.7±27.4 | 127.3±30.3 | 0.012 | |
| Heart rate (/minute) | 75.9±18.4 | 75.7±18.4 | 76.7±20.6 | 0.523 | |
| Medical history | |||||
| Current or ex-smoking | 1,564 (58.7) | 297 (61.9) | 419 (54.0) | 0.061 | |
| Hypertension | 1,264 (47.4) | 240 (50.0) | 410 (52.8) | 0.007 | |
| Diabetes mellitus | 791 (29.7) | 151 (31.5) | 254 (32.7) | 0.091 | |
| Familial history of coronary artery disease | 153 (5.7) | 28 (5.8) | 42 (5.4) | 0.760 | |
| Chronic kidney disease | 83 (3.1) | 16 (3.3) | 33 (4.3) | 0.134 | |
| Cerebrovascular accident | 114 (4.3) | 16 (3.3) | 39 (5.0) | 0.530 | |
| Previous myocardial infarction | 100 (3.8) | 14 (2.9) | 30 (3.9) | 0.947 | |
| Previous PCI | 105 (3.9) | 16 (3.3) | 30 (3.9) | 0.816 | |
| STEMI | 1,739 (65.3) | 255 (53.1) | 426 (54.9) | <0.001 | |
| Killip class ≥3 | 223 (8.4) | 38 (7.9) | 107 (13.8) | <0.001 | |
| Laboratory findings | |||||
| Serum creatinine (mg/dL) | 0.96 (0.80–1.16) | 1.00 (0.80–1.20) | 1.00 (0.80–1.20) | 0.080 | |
| Peak level of troponin-I (mg/dL) | 25.0 (5.1–50.0) | 13.5 (4.2–50.0) | 16.4 (3.9–50.0) | 0.374 | |
| Peak level of CK-MB (mg/dL) | 60.6 (14.8–161.1) | 27.7 (10.8–92.4) | 36.8 (11.2–115.6) | <0.001 | |
| Total cholesterol (mg/dL) | 180 (155–207) | 185 (159–205) | 181 (157–208) | 0.286 | |
| Triglyceride (mg/dL) | 102 (70–148) | 111 (75–156) | 108 (76–145) | 0.061 | |
| HDL-cholesterol (mg/dL) | 42 (36–49) | 41 (36–49) | 41 (35–48) | 0.065 | |
| LDL-cholesterol (mg/dL) | 115 (94–140) | 120 (99–140) | 116 (96–141) | 0.167 | |
| Serum glucose (mg/dL) | 145 (118–191) | 144 (112–194) | 150 (119–207) | 0.066 | |
| NT pro BNP (pg/mL) | 492 (110–1,672) | 551 (154–1,559) | 849 (198–2,947) | 0.001 | |
| High-sensitivity CRP (mg/L) | 5.6 (1.7–20.1) | 6.5 (2.1–26.5) | 9.3 (2.9–31.6) | <0.001 | |
| LVEF (%) | 53.9±11.1 | 56.1±11.8 | 53.1±11.7 | <0.001 | |
| Medications at discharge | |||||
| Aspirin | 2,664 (100) | 475 (99.0) | 776 (100) | 0.336 | |
| Clopidogrel | 2,656 (99.7) | 480 (100) | 773 (99.6) | 0.937 | |
| Beta-blocker | 2,075 (77.9) | 384 (80.0) | 597 (76.9) | 0.784 | |
| ACE inhibitor or ARB | 2,158 (81.0) | 383 (79.8) | 600 (77.3) | 0.079 | |
Values are presented as mean±standard deviation, median (interquartile range) or number (percentage).
ACE = angiotensin-converting enzyme; ARB = angiotensin-II receptor blocker; CK-MB = creatine kinase-myocardial band isoenzyme; CRP = C-reactive protein; HDL = high-density lipoprotein; LDL = low-density lipoprotein; LVEF = left ventricular ejection fraction; NT pro BNP = N-terminal pro B-type natriuretic peptide; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction.
Table 2. Procedural characteristics.
| Variables | Time from admission to statin initiation (hours) | p value | |||
|---|---|---|---|---|---|
| Group I: <24 (n=2,665) | Group II: 24–48 (n=480) | Group III: ≥48 (n=776) | |||
| Infarct-related artery | |||||
| Left anterior descending artery | 1,311 (49.2) | 202 (42.1) | 361 (46.5) | 0.061 | |
| Right coronary artery | 882 (33.1) | 186 (38.8) | 266 (34.3) | 0.057 | |
| Left circumflex artery | 409 (15.3) | 84 (17.5) | 137 (17.7) | 0.088 | |
| Left main coronary artery | 51 (1.9) | 8 (1.7) | 12 (1.5) | 0.475 | |
| Multivessel disease | 1,348 (50.6) | 235 (49.0) | 441 (56.8) | 0.007 | |
| ACC/AHA B2/C lesion | 2,094 (78.6) | 369 (76.9) | 606 (78.1) | 0.648 | |
| Pre-PCI TIMI flow grade 0 | 1,161 (45.5) | 195 (41.8) | 282 (38.1) | <0.001 | |
| Coronary stenting | 2,617 (98.2) | 467 (97.3) | 759 (97.9) | 0.463 | |
| Total No. of stents | 1.66±0.93 | 1.60±0.87 | 1.71±0.93 | 0.096 | |
| Total stent length (mm) | 30 (20–49) | 28 (23–48) | 32 (20–51) | 0.472 | |
| Mean stent diameter (mm) | 3.21±0.43 | 3.18±0.41 | 3.18±0.41 | 0.052 | |
| Use of intravascular ultrasound | 722 (27.1) | 139 (29.0) | 195 (25.1) | 0.413 | |
| Post-PCI TIMI flow grade 3 | 2,420 (92.6) | 438 (93.0) | 704 (93.4) | 0.490 | |
| Procedural complications | |||||
| Periprocedural shock | 62 (2.3) | 25 (5.2) | 31 (4.0) | 0.003 | |
| No-reflow phenomenon | 144 (5.4) | 27 (5.6) | 36 (4.6) | 0.462 | |
| Ventricular tachycardia or fibrillation | 33 (1.2) | 11 (2.3) | 19 (2.4) | 0.010 | |
| Use of intra-aortic balloon pump | 100 (3.8) | 24 (5.0) | 42 (5.4) | 0.088 | |
| Thrombi aspiration | 115 (4.3) | 26 (5.4) | 43 (5.5) | 0.118 | |
Values are presented as mean±standard deviation, median (interquartile range) or number (percentage).
ACC = American College of Cardiology; AHA = American Heart Association; PCI = percutaneous coronary intervention; TIMI = thrombolysis in myocardial infarction.
All analyses were two-tailed, and a p value <0.05 was considered to reflect statistical significance. All statistical analyses were performed using SPSS for Windows (ver. 21.0; SPSS Inc., Chicago, IL, USA) and R software (ver. 2.13.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline and procedural characteristics
Statins were prescribed 48 hours after admission in 19.8% of all patients. Group 3 contained older patients and more females. Although the prevalence of hypertension was highest in group 3, other atherosclerotic risk factors were similarly prevalent among the three groups. The frequency of STEMI was highest in group 1, while group 3 had the most patients with a high Killip class (3 or 4). In laboratory examinations, lipid profiles were comparable among the three groups; however, levels of hsCRP and N-terminal pro B-natriuretic peptide were higher in group 3 than in the other groups. Evidence-based medications were prescribed at similar rates among the three groups (Table 1). The frequency of infarct-related artery was comparable among the groups; however, multivessel coronary artery disease was seen most frequently in group 3. There were no differences among the three groups in any other procedural characteristic, except for a higher prevalence of pre-PCI TIMI flow grade 0 in group 1, and a higher frequency of periprocedural cardiogenic shock in group 2 (Table 2). Among the three propensity score-matched models, there were no significant differences in baseline or procedural characteristics (Tables 3 and 4).
Table 3. Baseline clinical characteristics in propensity score matched populations according to different statin initiation timing.
| Variables | Model 1 (group I vs. II or III) | Model 2 (group I or II vs. III) | Model 3 (group I vs. II) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <24 hours (n=1,248) | ≥24 hours (n=1,248) | p value | <48 hours (n=768) | ≥48 hours (n=768) | p value | <24 hours (n=476) | 24–48 hours (n=476) | p value | ||
| Demographics | ||||||||||
| Age (years) | 62.2±12.5 | 62.8±12.3 | 0.212 | 62.6±12.3 | 63.2±12.4 | 0.365 | 61.5±12.4 | 61.9±12.0 | 0.536 | |
| Male | 906 (72.6) | 881 (70.6) | 0.267 | 545 (71.0) | 529 (68.9) | 0.373 | 341 (71.6) | 351 (73.7) | 0.467 | |
| Initial vital signs | ||||||||||
| Systolic BP (mmHg) | 130.3±28.5 | 128.3±29.0 | 0.093 | 130.1±28.5 | 127.6±29.9 | 0.089 | 128.7±26.9 | 129.6±27.4 | 0.635 | |
| Heart rate (/minute) | 76.0±17.9 | 76.3±19.8 | 0.660 | 77.0±19.2 | 76.6±20.3 | 0.661 | 75.8±18.5 | 75.7±18.5 | 0.853 | |
| Medical history | ||||||||||
| Current or ex-smoking | 716 (57.4) | 711 (57.0) | 0.840 | 434 (56.5) | 417 (54.3) | 0.383 | 273 (57.4) | 294 (61.8) | 0.166 | |
| Hypertension | 617 (49.4) | 647 (51.8) | 0.230 | 388 (50.5) | 404 (52.6) | 0.414 | 250 (52.5) | 239 (50.2) | 0.476 | |
| Diabetes mellitus | 382 (30.6) | 403 (32.3) | 0.365 | 247 (32.2) | 250 (32.6) | 0.870 | 155 (32.6) | 151 (31.7) | 0.781 | |
| Familial history of CAD | 61 (4.9) | 70 (5.6) | 0.419 | 41 (5.3) | 42 (5.5) | 0.910 | 23 (4.8) | 28 (5.9) | 0.472 | |
| CKD | 43 (3.4) | 49 (3.9) | 0.524 | 29 (3.8) | 30 (3.9) | 0.894 | 11 (2.3) | 16 (3.4) | 0.329 | |
| CVA | 52 (4.2) | 55 (4.4) | 0.767 | 3 (4.8) | 39 (5.1) | 0.814 | 19 (4.0) | 16 (3.4) | 0.605 | |
| Previous MI | 48 (3.8) | 42 (3.4) | 0.519 | 37 (4.8) | 30 (3.9) | 0.382 | 8 (1.7) | 12 (2.5) | 0.366 | |
| Previous PCI | 49 (3.9) | 44 (3.5) | 0.597 | 40 (5.2) | 30 (3.9) | 0.221 | 9 (1.9) | 15 (3.2) | 0.215 | |
| STEMI | 712 (57.1) | 677 (54.2) | 0.158 | 428 (55.7) | 424 (55.2) | 0.837 | 276 (58.0) | 252 (52.9) | 0.118 | |
| Killip class ≥3 | 119 (9.5) | 143 (11.5) | 0.117 | 81 (10.5) | 101 (13.2) | 0.114 | 43 (9.0) | 38 (8.0) | 0.561 | |
| Laboratory findings | ||||||||||
| Serum creatinine (mg/dL) | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 0.335 | 1.0 (0.8–1.2) | 1.0 (0.8–1.2) | 0.894 | 0.9 (0.8–1.2) | 1.0 (0.8–1.2) | 0.834 | |
| Peak troponin-I (mg/dL) | 38 (7–50) | 29 (6–42) | 0.822 | 38 (7–50) | 35 (6–42) | 0.545 | 37 (7–50) | 22 (6–42) | 0.876 | |
| Peak CK-MB (mg/dL) | 45 (13–126) | 35 (11–104) | 0.193 | 44 (14–141) | 39 (12–112) | 0.383 | 44 (12–127) | 28 (11–94) | 0.052 | |
| Total cholesterol (mg/dL) | 182 (156–207) | 184 (159–207) | 0.191 | 184 (157–208) | 182 (157–207) | 0.559 | 182 (157–150) | 184 (160–204) | 0.325 | |
| Triglyceride (mg/dL) | 103 (71–146) | 110 (77–150) | 0.098 | 107 (74–152) | 109 (77–144) | 0.786 | 105 (74–150) | 111 (76–156) | 0.286 | |
| HDL-cholesterol (mg/dL) | 42 (36–48) | 41 (35–48) | 0.132 | 42 (36–48) | 41 (35–48) | 0.648 | 42 (37–48) | 41 (36–48) | 0.900 | |
| LDL-cholesterol (mg/dL) | 115 (95–140) | 118 (97–139) | 0.179 | 118 (96–142) | 116 (96–139) | 0.696 | 116 (95–140) | 119 (99–139) | 0.275 | |
| Serum glucose (mg/dL) | 151 (120–186) | 151 (119–196) | 0.322 | 151 (118–193) | 152 (121–200) | 0.880 | 150 (118–187) | 148 (113–192) | 0.616 | |
| NT pro BNP (pg/mL) | 1,643 (248–2,270) | 2,157 (369–2,270) | 0.096 | 1,969 (311–2,270) | 2,270 (471–2,270) | 0.668 | 1,779 (229–2,270) | 1,283 (279–2,270) | 0.895 | |
| hsCRP (mg/L) | 7.6 (2.2–21.8) | 11.9 (3.0–24.3) | 0.089 | 6.8 (1.9–21.8) | 13.9 (3.5–24.6) | 0.104 | 7.9 (2.3–21.8) | 8.3 (2.5–23.2) | 0.929 | |
| LVEF (%) | 54.5±11.1 | 54.2±11.8 | 0.594 | 53.4±11.5 | 53.2±11.6 | 0.764 | 55.3±10.8 | 55.9±11.7 | 0.330 | |
| Medications at discharge | ||||||||||
| Aspirin | 1,248 (100) | 1,248 (100) | 1.000 | 768 (100) | 768 (100) | 1.000 | 475 (99.8) | 475 (99.8) | 1.000 | |
| Clopidogrel | 1,244 (99.7) | 1,245 (99.8) | 0.705 | 763 (99.3) | 765 (99.6) | 0.478 | 476 (100) | 476 (100) | 1.000 | |
| Beta-blocker | 977 (78.3) | 974 (78.0) | 0.884 | 595 (77.5) | 592 (77.1) | 0.855 | 381 (80.0) | 380 (79.8) | 0.935 | |
| ACE inhibitor or ARB | 1,004 (80.4) | 975 (78.1) | 0.152 | 617 (80.3) | 596 (77.6) | 0.189 | 377 (79.2) | 379 (79.6) | 0.873 | |
ACE = angiotensin-converting enzyme; ARB = angiotensin-II receptor blocker; BP = blood pressure; CAD = coronary artery disease; CK-MB = creatine kinase-myocardial band isoenzyme; CKD = chronic kidney disease; CVA = cerebrovascular accident; HDL = high-density lipoprotein; hsCRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NT pro BNP = N-terminal pro B-type natriuretic peptide; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction.
Table 4. Procedural characteristics in propensity score matched populations according to different statin initiation timing.
| Variables | Model 1 (group I vs. II or III) | Model 2 (group I or II vs. III) | Model 3 (group I vs. II) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <24 hours (n=1,248) | ≥24 hours (n=1,248) | p value | <48 hours (n=768) | ≥48 hours (n=768) | p value | <24 hours (n=476) | 24–48 hours (n=476) | p value | ||
| Infarct-related artery | ||||||||||
| Left anterior descending | 573 (45.9) | 559 (44.8) | 0.572 | 352 (45.8) | 357 (46.5) | 0.798 | 217 (45.6) | 199 (41.8) | 0.240 | |
| Right coronary artery | 445 (35.7) | 449 (36.0) | 0.867 | 264 (34.4) | 263 (34.2) | 0.957 | 180 (37.8) | 185 (38.9) | 0.739 | |
| Left circumflex artery | 208 (16.7) | 220 (17.6) | 0.524 | 135 (17.6) | 136 (17.7) | 0.947 | 72 (15.1) | 84 (17.6) | 0.293 | |
| Left main coronary artery | 22 (1.8) | 20 (1.6) | 0.756 | 17 (2.2) | 12 (1.6) | 0.349 | 7 (1.5) | 8 (1.7) | 0.795 | |
| Multivessel disease | 671 (53.8) | 672 (53.8) | 0.968 | 411 (53.5) | 435 (56.6) | 0.218 | 230 (48.3) | 234 (49.2) | 0.795 | |
| ACC/AHA B2/C lesion | 972 (77.9) | 969 (77.6) | 0.885 | 607 (79.0) | 599 (78.0) | 0.619 | 368 (77.3) | 366 (76.9) | 0.877 | |
| Pre-PCI TIMI flow 0 | 510 (40.9) | 475 (38.1) | 0.152 | 301 (39.2) | 281 (36.6) | 0.293 | 217 (45.6) | 194 (40.8) | 0.132 | |
| Coronary stenting | 1,219 (97.7) | 1,221 (97.8) | 0.787 | 753 (98.0) | 753 (98.0) | 1.000 | 465 (97.7) | 463 (97.3) | 0.679 | |
| Total No. of stents | 1.69±0.94 | 1.67±0.90 | 0.645 | 1.70±0.94 | 1.71±0.92 | 0.854 | 1.58±0.86 | 1.60±0.87 | 0.625 | |
| Total stent length (mm) | 31 (23–48) | 30 (23–49) | 0.473 | 30 (20–49) | 32 (21–51) | 0.924 | 28 (20–47) | 28 (23–48) | 0.762 | |
| Mean stent diameter (mm) | 3.19±0.39 | 3.18±0.41 | 0.240 | 3.19±0.39 | 3.18±0.42 | 0.860 | 3.18±0.41 | 3.18±0.41 | 0.900 | |
| Use of IVUS | 323 (25.9) | 332 (26.6) | 0.682 | 201 (26.2) | 194 (25.3) | 0.683 | 141 (29.6) | 137 (28.8) | 0.776 | |
| Post-PCI TIMI flow 3 | 1,175 (94.2) | 1,165 (93.3) | 0.408 | 717 (93.4) | 719 (93.6) | 0.836 | 448 (94.1) | 443 (93.1) | 0.508 | |
| Procedural complications | ||||||||||
| Periprocedural shock | 39 (3.1) | 54 (4.3) | 0.113 | 22 (2.9) | 29 (3.8) | 0.319 | 16 (3.4) | 25 (5.3) | 0.151 | |
| No-reflow phenomenon | 65 (5.2) | 62 (5.0) | 0.785 | 36 (4.7) | 36 (4.7) | 1.000 | 22 (4.6) | 27 (5.7) | 0.463 | |
| VT or VF | 22 (1.8) | 29 (2.3) | 0.322 | 14 (1.8) | 16 (2.1) | 0.712 | 7 (1.5) | 10 (2.1) | 0.463 | |
| Use of IABP | 54 (4.3) | 65 (5.2) | 0.301 | 31 (4.0) | 37 (4.8) | 0.457 | 19 (4.0) | 24 (5.0) | 0.435 | |
| Thrombi aspiration | 60 (4.8) | 68 (5.4) | 0.468 | 29 (3.8) | 43 (5.6) | 0.091 | 23 (4.8) | 25 (5.3) | 0.767 | |
Values are presented as mean±standard deviation, median (interquartile range) or number (percentage).
ACC = American College of Cardiology; AHA = American Heart Association; IABP = intra-aortic balloon pump; IVUS = intravascular ultrasound; PCI = percutaneous coronary intervention; TIMI = thrombolysis in myocardial infarction; VF = ventricular fibrillation; VT = ventricular tachycardia.
Clinical outcomes
In model 1 (<24 hours vs. ≥24 hours), more MACE occurred in the early statin group (<24 hours) (14.3% vs. 18.4%, adjusted hazard ratio [HR], 0.77; 95% confidence interval [CI], 0.66–0.91; p=0.002). Early statin was also associated with lower incidence rates of nonfatal spontaneous MI, TVR and stent thrombosis. Results were similar in model 2 (<48 hours vs. ≥48 hours). Statin initiation <48 hours reduced MACE (14.6% vs. 19.7%, adjusted HR, 0.81; 95% CI, 0.67–0.97; p=0.022), cardiac death and nonfatal spontaneous MI compared to statin initiation at a later time. However, there were no differences in the incidence of MACE (14.3% vs. 16.3%, adjusted HR, 0.87; 95% CI, 0.68–1.11; p=0.268) or other secondary endpoints between the early (<24 hours) and later statin initiation (24–48 hours) groups in model 3 (Table 5).
Table 5. Clinical outcomes according to different statin initiation timing.
| Variables | Time (hours) | Unadjusted HR | Adjusted HR | Propensity-score-adjusted HR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <24 (n=2,665) | ≥24 (n=1,256) | p value | 95% CI | p value | 95% CI | p value | 95% CI | p value | ||
| Model 1 (group I vs. II or III) | ||||||||||
| Cardiac death, MI or TVR | 381 (14.3) | 231 (18.4) | 0.001 | 0.75 (0.64–0.88) | 0.001 | 0.77 (0.66–0.91) | 0.002 | 0.78 (0.64–0.94) | 0.011 | |
| All-cause death | 356 (13.4) | 201 (16.0) | 0.027 | 0.81 (0.68–0.96) | 0.017 | 0.87 (0.73–1.08) | 0.108 | 0.87 (0.71–1.07) | 0.179 | |
| Cardiac death | 115 (4.3) | 74 (5.9) | 0.032 | 0.71 (0.53–0.95) | 0.021 | 0.79 (0.59–1.06) | 0.115 | 0.74 (0.52–1.05) | 0.090 | |
| Nonfatal spontaneous MI | 52 (2.0) | 41 (3.3) | 0.012 | 0.59 (0.39–0.89) | 0.011 | 0.60 (0.40–0.91) | 0.015 | 0.48 (0.28–0.82) | 0.007 | |
| TLR | 215 (8.1) | 121 (9.6) | 0.102 | 0.82 (0.65–1.02) | 0.074 | 0.82 (0.66–1.03) | 0.082 | 0.82 (0.63–1.07) | 0.137 | |
| TVR | 262 (9.8) | 150 (11.9) | 0.044 | 0.80 (0.66–0.98) | 0.031 | 0.81 (0.66–0.99) | 0.036 | 0.83 (0.65–1.05) | 0.122 | |
| Non-TVR | 155 (5.8) | 80 (6.4) | 0.496 | 0.90 (0.69–1.18) | 0.439 | 0.90 (0.69–1.18) | 0.456 | 0.96 (0.71–1.32) | 0.812 | |
| Stroke | 55 (2.1) | 34 (2.7) | 0.207 | 0.75 (0.49–1.15) | 0.184 | 0.76 (0.49–1.17) | 0.207 | 0.69 (0.41–1.16) | 0.164 | |
| Definite or probable stent thrombosis | 72 (2.7) | 49 (3.9) | 0.043 | 0.68 (0.47–0.98) | 0.037 | 0.69 (0.48–0.99) | 0.045 | 0.51 (0.31–0.83) | 0.006 | |
| Model 2 (group I or II vs. III) | <48 (n=3,145) | ≥48 (n=776) | ||||||||
| Cardiac death, MI or TVR | 459 (14.6) | 153 (19.7) | <0.001 | 0.72 (0.60–0.87) | 0.001 | 0.81 (0.67–0.97) | 0.022 | 0.69 (0.54–0.89) | 0.005 | |
| All-cause death | 425 (13.5) | 132 (17.0) | 0.012 | 0.78 (0.64–0.95) | 0.012 | 0.87 (0.72–1.06) | 0.173 | 0.89 (0.69–1.15) | 0.363 | |
| Cardiac death | 135 (4.3) | 54 (7.0) | 0.002 | 0.60 (0.44–0.83) | 0.002 | 0.72 (0.52–0.99) | 0.042 | 0.74 (0.49–0.98) | 0.047 | |
| Nonfatal spontaneous MI | 65 (2.1) | 28 (3.6) | 0.011 | 0.57 (0.37–0.89) | 0.014 | 0.59 (0.38–0.92) | 0.021 | 0.47 (0.24–0.91) | 0.024 | |
| TLR | 257 (8.2) | 79 (10.2) | 0.073 | 0.79 (0.61–1.02) | 0.065 | 0.79 (0.61–1.02) | 0.074 | 0.69 (0.49–0.98) | 0.039 | |
| TVR | 319 (10.1) | 93 (12.0) | 0.134 | 0.84 (0.66–1.05) | 0.127 | 0.84 (0.67–1.06) | 0.141 | 0.72 (0.52–0.98) | 0.039 | |
| Non-TVR | 180 (5.7) | 55 (7.1) | 0.152 | 0.80 (0.59–1.08) | 0.150 | 0.84 (0.67–1.06) | 0.141 | 1.05 (0.72–1.52) | 0.815 | |
| Stroke | 69 (2.2) | 20 (2.6) | 0.521 | 0.85 (0.52–1.40) | 0.531 | 0.87 (0.53–1.44) | 0.598 | 0.86 (0.45–1.65) | 0.651 | |
| Definite or probable stent thrombosis | 91 (2.9) | 30 (3.9) | 0.161 | 0.74 (0.49–1.12) | 0.159 | 0.76 (0.50–1.15) | 0.194 | 0.56 (0.31–1.02) | 0.059 | |
| Model 3 (group I vs. II) | <24 (n=2,665) | 24–48 (n=480) | ||||||||
| Cardiac death, MI or TVR | 381 (14.3) | 78 (16.3) | 0.264 | 0.84 (0.66–1.07) | 0.160 | 0.87 (0.68–1.11) | 0.268 | 0.84 (0.61–1.16) | 0.290 | |
| All-cause death | 356 (13.4) | 69 (14.4) | 0.549 | 0.90 (0.69–1.16) | 0.402 | 0.89 (0.69–1.15) | 0.368 | 0.94 (0.67–1.32) | 0.723 | |
| Cardiac death | 115 (4.3) | 20 (4.2) | 0.883 | 1.00 (0.62–1.61) | 0.997 | 0.99 (0.61–1.59) | 0.953 | 1.06 (0.57–1.95) | 0.856 | |
| Nonfatal spontaneous MI | 52 (2.0) | 13 (2.7) | 0.283 | 0.69 (0.38–1.27) | 0.237 | 0.67 (0.37–1.24) | 0.203 | 0.45 (0.17–1.19) | 0.107 | |
| TLR | 215 (8.1) | 42 (8.8) | 0.615 | 0.89 (0.64–1.24) | 0.500 | 0.94 (0.67–1.31) | 0.697 | 0.94 (0.61–1.45) | 0.775 | |
| TVR | 262 (9.8) | 57 (11.9) | 0.172 | 0.79 (0.59–1.06) | 0.116 | 0.82 (0.61–1.09) | 0.177 | 0.80 (0.55–1.18) | 0.269 | |
| Non-TVR | 155 (5.8) | 25 (5.2) | 0.598 | 1.09 (0.72–1.67) | 0.683 | 1.07 (0.70–1.64) | 0.756 | 0.99 (0.57–1.73) | 0.974 | |
| Stroke | 55 (2.1) | 14 (2.9) | 0.240 | 0.68 (0.38–1.22) | 0.196 | 0.68 (0.38–1.22) | 0.190 | 0.77 (0.35–1.70) | 0.517 | |
| Definite or probable stent thrombosis | 72 (2.7) | 19 (4.0) | 0.131 | 0.66 (0.39–1.10) | 0.108 | 0.65 (0.39–1.09) | 0.100 | 0.57 (0.27–1.20) | 0.136 | |
Propensity-score adjusted HRs were calculated in 1,248 matched pair in model 1, 767 matched pair in model 2, and 475 matched pair in model 3.
CI = confidence interval; HR = hazard ratio; MI = myocardial infarction; TLR = target-lesion revascularization; TVR = target-vessel revascularization.
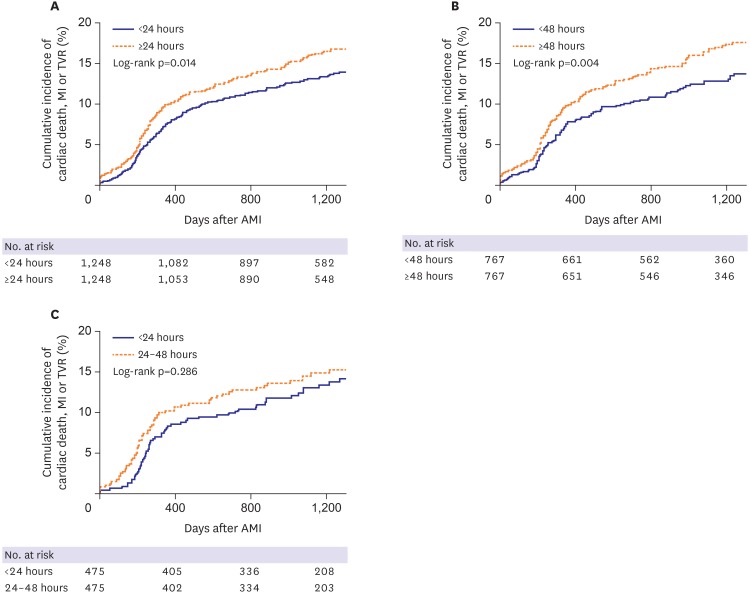
Results were comparable after propensity score-matching (Table 5). Early statin initiation (<24 hours) was associated with lower cumulative incidences of MACE (propensity-score adjusted HR, 0.78; 95% CI, 0.64–0.95; p=0.011, Figure 1A), nonfatal spontaneous MI and stent thrombosis in model 1. In model 2, MACE also occurred less frequently in the early statin initiation <48 hours group (propensity-score adjusted HR, 0.69; 95% CI, 0.54–0.89; p=0.005, Figure 1B); similar results were seen in nonfatal spontaneous MI, TLR and TVR. There was no significant difference in MACE incidence (propensity-score adjusted HR, 0.84; 95% CI, 0.61–1.16; p=0.290, Figure 1C) or other secondary outcomes between the early (<24 hours) and later statin initiation (24–48 hours) groups in model 3 following propensity score-matching.
Figure 1. Kaplan-Meier curves for cumulative incidence of cardiac death, myocardial infarction or TVR in patients who received statin <24 hours vs. ≥24 hours (A), <48 hours vs. ≥48 hours (B), and <24 hours vs. 24–48 hours (C) after admission.
AMI = acute myocardial infarction; MI = myocardial infarction; TVR = target-vessel revascularization.
Subgroup analysis and independent predictors of delay of statin initiation ≥48 hours after admission
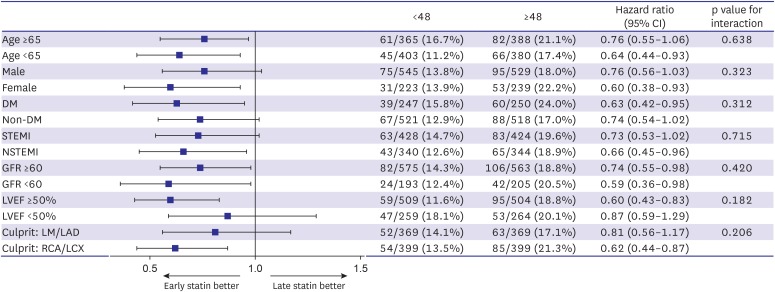
Subgroup analysis of propensity score-matched populations in model 2 was performed (Figure 2). The subgroups showed similar rates of MACE, and did not significantly interact with the early statin initiation <48 hours group. We also performed multivariate logistic regression analysis to identify independent predictors of delayed statin initiation (≥48 hours) using variables that had a p<0.05 in the univariate analysis (Table 6). Among these variables, female gender and Killip class 3 or 4 were identified as independent predictors of delayed statin initiation. On the contrary, statins were prescribed earlier in patients with STEMI.
Figure 2. Subgroup analysis of major adverse cardiac events in model 2 (<48 hours vs. ≥48 hours).
CI = confidence interval; DM = diabetes mellitus; GFR = glomerular filtration rate; LAD = left anterior descending; LCX = left circumflex; LM = left main; LVEF = left ventricular ejection fraction; NSTEMI = non-ST-segment elevation myocardial infarction; RCA = right coronary artery; STEMI = ST-segment elevation myocardial infarction.
Table 6. Independent predictors of delay of statin initiation ≥48 hours after admission.
| Variables | OR (95% CI) | p value |
|---|---|---|
| Age ≥65 years | 1.16 (0.98–1.37) | 0.089 |
| Female | 1.22 (1.02–1.47) | 0.032 |
| History of cerebrovascular accident | 1.67 (0.93–3.03) | 0.089 |
| Killip class 3 or 4 | 1.61 (1.25–2.07) | <0.001 |
| Chronic kidney disease or end-stage renal disease | 1.23 (0.82–1.84) | 0.328 |
| STEMI | 0.71 (0.61–0.83) | <0.001 |
| Development of procedural complications | 1.21 (0.89–1.64) | 0.223 |
Procedural complications include periprocedural shock, no-reflow phenomenon, or ventricular arrhythmia.
CI = confidence interval; OR = odds ratio; STEMI = ST-segment elevation myocardial infarction.
DISCUSSION
The main purpose of the present study was to explore the impact of the timing of statin initiation on long-term clinical outcomes in statin-naïve patients with AMI. Our principal findings were as follows: 1) approximately 20% of statin-naïve patients with AMI received a statin after 48 hours following admission; and 2) early statin initiation within 48 hours after admission could reduce long-term adverse cardiac events, including cardiac death, nonfatal MI, TLR and TVR compared to statin initiation at a later time. This study provides important insights into the long-term outcomes of AMI patient through the utilization of a multi-center registry that reflects real-life practice.
The current clinical guidelines do not specify the optimal timing for statin initiation in AMI patients after admission.1),2),3),4) This could be due to the heterogeneity of the timing of statin initiation in previous studies, and to the lack of randomized trials on this topic. In fact, several small single-center retrospective studies reported no significant difference in outcomes regarding the timing of statin initiation.5),6) One study indicated that early statin initiation (within 2 days after admission) does not reduce adverse cardiac outcomes, and concluded that in-hospital statin initiation is sufficient for treating patients with acute coronary syndrome.6) In contrast, in the large-scale study using the National Registry of Myocardial Infarction 4 database, early statin therapy (within 24 hours of hospitalization) for AMI was associated with a significantly lower rate of in-hospital mortality.7) Using the Euro Heart Survey registry, Lenderink et al.9) also showed that early statin therapy (within 24 hours after admission) in 8,000 STEMI patients was associated with reduced 1-month mortality. Although they demonstrated the efficacy of early statin therapy (within 24 hours of hospitalization) in the treatment of AMI patients, their study focused only on short-term cardiovascular outcomes. In the current study, we showed that early statin therapy (within 48 hours after admission) was associated with a lower incidence of MACE in the long-term compared to later statin initiation (> 48 hours after admission). In our cohort, almost 20% of statin-naïve patients with AMI received a statin within 48 hours after admission. Independent predictors of delayed statin initiation after 48 hours were female gender and Killip class 3 or 4. However, no evidence indicates a delay in statin therapy in female or unstable patients, such as those experiencing cardiogenic shock. Although AMI cases complicated by cardiogenic shock may show higher levels of liver enzymes, we suggest that statins should be prescribed for all AMI patients as early as possible, unless there is a contraindication. In Korea, both the statin non-prescription rate during hospitalisation and the discontinuation rate after discharge remain high in patients with AMI.13),16) Therefore, education emphasizing the importance of early statin therapy is needed for both medical teams and patients. Furthermore, we believe that criteria for deciding the optimal timing of statin initiation should be implemented to increase the statin prescription rate in the acute setting.
Numerous factors related to statins can affect clinical outcomes in patients with AMI. These include statin intensity,17) dosage,18) timing, concentration of low-density lipoprotein19),20) and patient status. However, the mechanism by which early statin initiation (within 48 hours after admission) confers benefits for statin-naïve patients with AMI is unclear. In one study, late statin initiation (48 hours after admission in STEMI patients) produced a milder attenuation of inflammation burst compared to early statin initiation (i.e., on admission).21) This finding suggests that the timing of statin therapy during AMI may be a key determinant of its clinical benefits. Many pleiotropic effects of statins aside from reducing low-density lipoprotein, such as plaque stabilization, improvement of endothelial dysfunction, decreased thrombogenicity, and reduced inflammation, could affect patient outcomes.22) These effects can be achieved when statin therapy is initiated as early as possible after MI. PCI timing in our study may also have influenced the endpoints, as all patients in our cohort received PCI within 48 hours after admission; therefore, not all patients received a pre-procedural statin. However, controversy exists as to whether pre-procedural statin use is associated with in-hospital or long-term complications. Moreover, most studies on pre-procedural statin treatment focused on stable coronary artery disease, not AMI.23),24),25) However, there were 3 studies which investigated the clinical impact of pre-procedural statin use and pre-procedural high-dose statin in patients with acute coronary syndrome. Although these studies showed the clinical benefits, improved microvascular myocardial perfusion and reducing infarct size by pre-procedural statin in patients with acute coronary syndrome, they only focused on the pre-procedural statin, not a timing of statin initiation.26),27),28) More investigations utilizing larger real-world registries and randomized designs are needed to further explore this issue.
The current study has several limitations. First, this study used a non-randomised, observational design, despite being based on a large, multi-center registry, which resulted in differences in baseline clinical and angiographic findings among the groups. Although we performed propensity-matched analysis of stratified groups, other variables not included in our registry may also have influenced the study outcomes. Second, data on dosage and type of statin, as well as patient compliance, were not considered. Unfortunately, there was no exact information about statin dosages and types in our registry. There is a possibility that statin dosage, type and compliance might affect clinical outcomes, although this remains controversial. There was a study which investigated the impact of statin intensity in Korean AMI patients using the Korean Acute Myocardial Infarction Registry, and high-intensity statin therapy did not show clinical benefits over low-to-moderate intensity statin therapy after AMI.29) Although this issue needs more study results, the impact of statin intensity in Korean AMI patients should be compared to those in western AMI patients. Third, all patients in the current study received clopidogrel as a P2Y12 inhibitor instead of potent P2Y12 inhibitors such as prasugrel or ticagrelor because of study period. The current registry registered patients between 2004 and 2009, therefore it is not available to prescribe prasugrel or ticagrelor in the market. Finally, we could not perform a serial check of the laboratory findings within the registry, and therefore inflammatory state and post-procedural myocardial necrosis data were not examined.
In conclusion, the timing of statin therapy initiation is an important factor in patient outcomes in the case of AMI. This study was not a randomised controlled trial and the timing of statin initiation was at the discretion of individual operators. Using a large, real-world registry, we found that early statin therapy (within 48 hours after admission) reduced long-term clinical adverse events in statin-naïve patients with AMI compared to later statin initiation. While we support the current guidelines on early statin initiation in AMI patients, we propose that initiation <48 hours after admission could represent the optimal timing for statin therapy.
Footnotes
Funding: This study was supported by a grant of the Bio & Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Korean government, MSIP (2017M3A9E8023001).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kim MC, Ahn Y.
- Data curation: Kim MC, Cho JY, Lee KH.
- Formal analysis: Kim MC, Ahn Y.
- Methodology: Kim MC, Ahn Y, Sim DS.
- Project administration: Ahn Y, Chang K, Seung KB.
- Resources: Ahn Y, Chang K.
- Supervision: Yoon NS, Yoon HJ, Kim KH, Park HW, Cho JG, Park JC.
- Validation: Sim DS, Hong YJ, Kim JH, Jeong MH.
- Visualization: Ahn Y, Jeong MH.
- Writing - original draft: Kim MC, Ahn Y.
- Writing - review & editing: Kim MC, Ahn Y, Sim DS, Hong YJ, Kim JH, Jeong MH.
SUPPLEMENTARY MATERIALS
Baseline clinical characteristics before and after propensity-score matching in model 1
Procedural characteristics before and after propensity-score matching in model 1
Baseline clinical characteristics before and after propensity-score matching in model 2
Procedural characteristics before and after propensity-score matching in model 2
Baseline clinical characteristics before and after propensity-score matching in model 3
Procedural characteristics before and after propensity-score matching in model 3
References
- 1.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 2.Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 3.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Kim MC, Ahn Y, Cho KH, et al. Early statin therapy within 48 hours decreased one-year major adverse cardiac events in patients with acute myocardial infarction. Int Heart J. 2011;52:1–6. doi: 10.1536/ihj.52.1. [DOI] [PubMed] [Google Scholar]
- 6.Li YH, Wu HL, Yang YH, Tsai HS, Chao TH. Effect of early versus late in-hospital initiation of statin therapy on the clinical outcomes of patients with acute coronary syndrome. Int Heart J. 2007;48:677–688. doi: 10.1536/ihj.48.677. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Wright RS, Spencer FA, et al. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol. 2005;96:611–616. doi: 10.1016/j.amjcard.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Aronow HD, Novaro GM, Lauer MS, et al. In-hospital initiation of lipid-lowering therapy after coronary intervention as a predictor of long-term utilization: a propensity analysis. Arch Intern Med. 2003;163:2576–2582. doi: 10.1001/archinte.163.21.2576. [DOI] [PubMed] [Google Scholar]
- 9.Lenderink T, Boersma E, Gitt AK, et al. Patients using statin treatment within 24 h after admission for ST-elevation acute coronary syndromes had lower mortality than non-users: a report from the first Euro Heart Survey on acute coronary syndromes. Eur Heart J. 2006;27:1799–1804. doi: 10.1093/eurheartj/ehl125. [DOI] [PubMed] [Google Scholar]
- 10.Newby LK, Kristinsson A, Bhapkar MV, et al. Early statin initiation and outcomes in patients with acute coronary syndromes. JAMA. 2002;287:3087–3095. doi: 10.1001/jama.287.23.3087. [DOI] [PubMed] [Google Scholar]
- 11.Briel M, Schwartz GG, Thompson PL, et al. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA. 2006;295:2046–2056. doi: 10.1001/jama.295.17.2046. [DOI] [PubMed] [Google Scholar]
- 12.Choo EH, Chang K, Ahn Y, et al. Benefit of β-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart. 2014;100:492–499. doi: 10.1136/heartjnl-2013-305137. [DOI] [PubMed] [Google Scholar]
- 13.Kim MC, Cho JY, Jeong HC, et al. Impact of postdischarge statin withdrawal on long-term outcomes in patients with acute myocardial infarction. Am J Cardiol. 2015;115:1–7. doi: 10.1016/j.amjcard.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 14.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 15.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Lee SH, Park JS, et al. Impact of statin usage patterns on outcomes after percutaneous coronary intervention in acute myocardial infarction: Korea Working Group on Myocardial Infarction registry (KorMI) study. J Geriatr Cardiol. 2014;11:93–99. doi: 10.3969/j.issn.1671-5411.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenson RS, Farkouh ME, Mefford M, et al. Trends in use of high-intensity statin therapy after myocardial infarction, 2011 to 2014. J Am Coll Cardiol. 2017;69:2696–2706. doi: 10.1016/j.jacc.2017.03.585. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Xu Y, Hao H, et al. Efficacy of high intensity atorvastatin versus moderate intensity atorvastatin for acute coronary syndrome patients with diabetes mellitus. Int J Cardiol. 2016;222:22–26. doi: 10.1016/j.ijcard.2016.07.140. [DOI] [PubMed] [Google Scholar]
- 19.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 20.Lee KH, Jeong MH, Kim HM, et al. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2011;58:1664–1671. doi: 10.1016/j.jacc.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 21.Sposito AC, Santos SN, de Faria EC, et al. Timing and dose of statin therapy define its impact on inflammatory and endothelial responses during myocardial infarction. Arterioscler Thromb Vasc Biol. 2011;31:1240–1246. doi: 10.1161/ATVBAHA.110.218685. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 23.Kenaan M, Seth M, Aronow HD, et al. Preprocedural statin use in patients undergoing percutaneous coronary intervention. Am Heart J. 2014;168:110–116.e3. doi: 10.1016/j.ahj.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Ko DT, Wijeysundera HC, Yun L, Austin PC, Cantor WJ, Tu JV. Effectiveness of preprocedural statin therapy on clinical outcomes for patients with stable coronary artery disease after percutaneous coronary interventions. Circ Cardiovasc Qual Outcomes. 2011;4:459–466. doi: 10.1161/CIRCOUTCOMES.111.960740. [DOI] [PubMed] [Google Scholar]
- 25.Greque GV, Serrano CV, Jr, Strunz CM, et al. Preprocedural statin therapy, inflammation, and myocardial injury in low-risk stable coronary artery disease patients submitted to coronary stent implantation. Catheter Cardiovasc Interv. 2016;87:222–229. doi: 10.1002/ccd.24937. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Kim J, Choi D, et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv. 2010;3:332–339. doi: 10.1016/j.jcin.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Yun KH, Jeong MH, Oh SK, et al. The beneficial effect of high loading dose of rosuvastatin before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol. 2009;137:246–251. doi: 10.1016/j.ijcard.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–1278. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Hwang D, Kim HK, Lee JM, et al. Effects of statin intensity on clinical outcome in acute myocardial infarction patients. Circ J. 2018;82:1112–1120. doi: 10.1253/circj.CJ-17-1221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline clinical characteristics before and after propensity-score matching in model 1
Procedural characteristics before and after propensity-score matching in model 1
Baseline clinical characteristics before and after propensity-score matching in model 2
Procedural characteristics before and after propensity-score matching in model 2
Baseline clinical characteristics before and after propensity-score matching in model 3
Procedural characteristics before and after propensity-score matching in model 3