Abstract
Background
Bevacizumab, a humanized monoclonal antibody against vascular epithelial growth factor, plays a significant role in first-line, second-line, beyond-progression, and maintenance treatment of patients with metastatic colorectal carcinoma (mCRC). Nevertheless, there are currently no biomarkers available to predict patient response or resistance to bevacizumab, which would be useful in clinical trials.
Methods
Using PRISMA guidelines, we conducted a systematic review and meta-analysis of the association between serum lactate dehydrogenase (LDH) level and progression-free survival (PFS), overall survival (OS), and objective response rate in mCRC patients treated with bevacizumab-based first-line chemotherapy. A comprehensive, computerized literature search of PubMed, the Web of Science, Scopus, Ovid, and the gray literature was performed. Only studies conforming to specific eligibility criteria were included. Pooled hazard ratios (HRs) were estimated using random-effects or fixed-effects models according to heterogeneity between studies. Sensitivity analysis was conducted to evaluate the stability of the results by removing each individual study from the meta-analysis.
Results
Seven eligible studies of 1,219 total patients were included in the analysis. Meta-analysis of all studies revealed that high serum LDH level is associated with shorter PFS (HR: 1.43, 95% CI: 1.05–1.94; P=0.023) and OS (HR: 1.667, 95% CI: 1.230–2.259; P=0.001) times in mCRC patients treated with bevacizumab-based first-line chemotherapy. However, there was no significant association between serum LDH and objective response rate.
Conclusions
High serum LDH level is significantly associated with shorter PFS and OS time and may have utility as a prognostic factor for mCRC patients receiving bevacizumab as first-line chemotherapy and as a predictive factor for those receiving bevacizumab-based therapy at other times.
Keywords: Lactate dehydrogenase (LDH), colorectal cancer, bevacizumab
Introduction
Bevacizumab is a humanized anti-vascular endothelial growth factor A (anti-VEGF) monoclonal antibody that is frequently employed as first-line therapy for patients with metastatic colorectal carcinoma (mCRC) (1). In addition, phase III randomized clinical trials have confirmed the efficacy of bevacizumab as second-line, beyond-progression, and maintenance treatments in this patient population (2,3). However, at present, no biomarkers exist that can predict either the response or resistance to bevacizumab therapy. There is thus an urgent need to identify such predictive biomarkers to enable identification of subjects most likely to benefit from bevacizumab.
Recently, several studies have reported that a high serum lactate dehydrogenase (LDH) level is associated with poor prognosis in patients with CRC (4) and other tumors (5,6). LDH has been identified as a potential signaling molecule for angiogenesis, suggesting a potential mechanistic link between LDH levels and prognosis. High LDH levels inhibit the formation of 2-oxoglutarate, which leads to stabilization of the transcription factor hypoxia-inducible factor α and, subsequently, to increased expression of its target gene vascular endothelial growth factor (VEGF) (7). Azuma et al. also demonstrated that patients with high serum LDH levels have increased intratumoral expression of VEGFA and VEGF1 genes (8).
Two retrospective studies have explored a potential predictive role for serum LDH in anti-angiogenic therapy of mCRC patients. Both studies found a significant association between high serum LDH level and poor outcome and response rate (RR) in patients treated with bevacizumab-based first-line chemotherapy (9,10). However, a recent prospective phase II clinical trial (CENTRAL) of a similarly treated mCRC patient cohort confirmed a significant relationship between serum LDH level and overall survival (OS), but not RR (11).
Thus, the association between serum LDH level, outcome, and RR of mCRC patients treated with bevacizumab-based first-line chemotherapy is controversial, and a large prospective study will be required to resolve this issue. In the mean time, we report here the results of a meta-analysis conducted to evaluate the prognostic and predictive role of LDH in mCRC patients treated with bevacizumab-based first-line chemotherapy.
Methods
The systematic review and meta-analysis were conducted according to PRISMA criteria. We performed a comprehensive, computerized search of PubMed, the Web of Science, Scopus, Ovid, and the gray literature for publications up to December 31st, 2018, using the search terms “colorectal cancer”, “colorectal carcinoma”, “colorectal neoplasm”, “lactate dehydrogenase”, and “bevacizumab”. In addition, reference lists in the identified primary papers and review articles were searched. Eligibility criteria for inclusion in this meta-analysis were: (I) the study evaluated correlations between serum LDH level and survival in mCRC patients receiving bevacizumab-based first-line therapy, (II) the study provided sufficient information for estimation of hazard ratios (HRs) and corresponding 95% confidence intervals (CIs), and (III) the study was published in English. Reviews and studies using animals or cell lines were excluded. Study quality was assessed using the Newcastle-Ottawa Scale.
Data extraction and outcomes
To guarantee homogeneity and to prevent subjectivity in the data collection and entry, the studies were independently assessed by two of the authors (Feng and Wang). The following information was independently extracted: first author names, year of publication, study location, sample size, median/mean age, stage of disease, cut-off value of serum LDH concentration, adjuvant chemotherapy, and survival outcome (Table 1).
Table 1. Baseline characteristics in concluded studies.
| Study | Country | Sample size | Median age, years | Tumor stage | Treatment | LDH | Outcome report | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Colon | Rectum | Cut-off | Detection method | ||||||
| M Jary [2016] | France | 177 | 120 | 57 | NR | mCRC | First-line chemotherapy and bevacizumab | 350 U/L | Serum | OS |
| N Silvestris [2015] | Italy | 139 | NR | NR | NR | mCRC | First-line chemotherapy and bevacizumab | NR | Serum | ORR, PFS, OS |
| M Scartozzi [2012] | Italy | 82 | NR | NR | 61 | mCRC | First-line chemotherapy and bevacizumab | 588 mg/dL | Serum | ORR, PFS, OS |
| A Passardi [2015] | Italy | 176 | NR | NR | 67 | mCRC | First-line chemotherapy and bevacizumab | UNL | Serum | ORR, PFS, OS |
| B Cetin [2012] | Turkey | 170 | 100 | 70 | NR | mCRC | First-line chemotherapy and bevacizumab | UNL | Serum | OS |
| R Giampieri [2017] | Italy | 81 | 61 | 20 | 65 | mCRC | FOLFIRI and bevacizumab | 1.2 ULN | Serum | ORR, PFS, OS |
| E Diaz-Rubio [2012] | US | 394 | 165 | 229 | 63 | mCRC | XELOX and bevacizumab | ULN | Serum | PFS, OS |
mCRC, metastatic colorectal carcinoma; LDH, lactate dehydrogenase; UNL, upper normal limit; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Statistical analysis
Heterogeneity between studies was assessed by the I2 statistic and Q test, with I2>57% or P<0.05, respectively, being considered statistically significant. The extracted HRs were then assessed using a fixed-effects model or, if heterogeneity could not be explained by the fixed-effects model, a random-effects model was applied. In this study, heterogeneity between studies was not significant for OS (I2=25.8%, P=0.232), and a fixed-effects model was therefore used to analyze the relationship between serum LDH level and OS. However, heterogeneity was significant for progression-free survival (PFS; I2=59.5%, P=0.043) and for clinical tumor response (complete response, partial response, stable disease, progressive disease; I2=78.4%, P=0.003), and a random-effects model was applied for these analyses. Next, we performed a sensitivity analysis to evaluate the stability of the results by removing each individual study from the meta-analysis. Finally, Begg’s funnel plot or Egger’s linear regression test were used to assess publication bias. All reported P values were two-sided, and P<0.05 was considered statistically significant. All analyses were performed using Stata software version 12.0 (Stata Corp., College Station, TX, USA).
Results
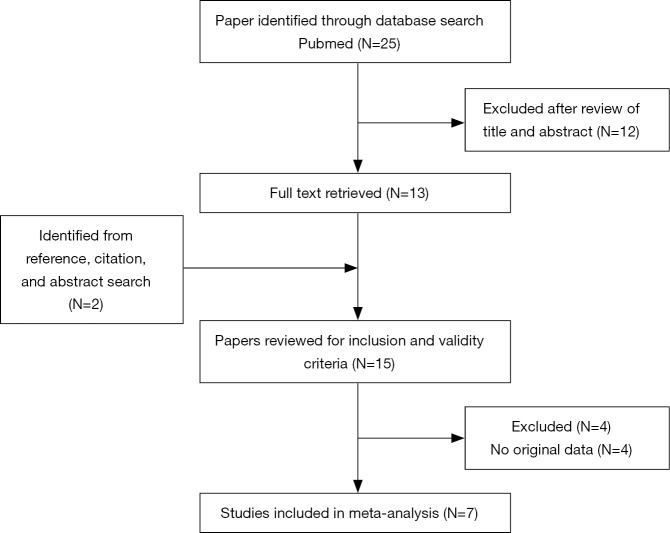
A flowchart of the literature search, study selection, and the results obtained at each step is shown in Figure 1. In total, seven studies with a sample size of 1,219 patients were deemed eligible and included in the analysis. The main characteristics of the studies, which were published between 2012 and 2018, are shown in Table 1. HRs were obtained for OS and PFS from seven (9-15) and five (9-11,13,15) studies, respectively, whereas data for evaluation of objective RR were obtained from four studies (9,10,13,15). Among the seven studies, two were prospective phase II clinical trials and five were retrospective cohort studies.
Figure 1.
The literature search process. Twenty-five studies were identified in the primary literature search, and seven studies were finally included in the analysis according to the inclusion criteria.
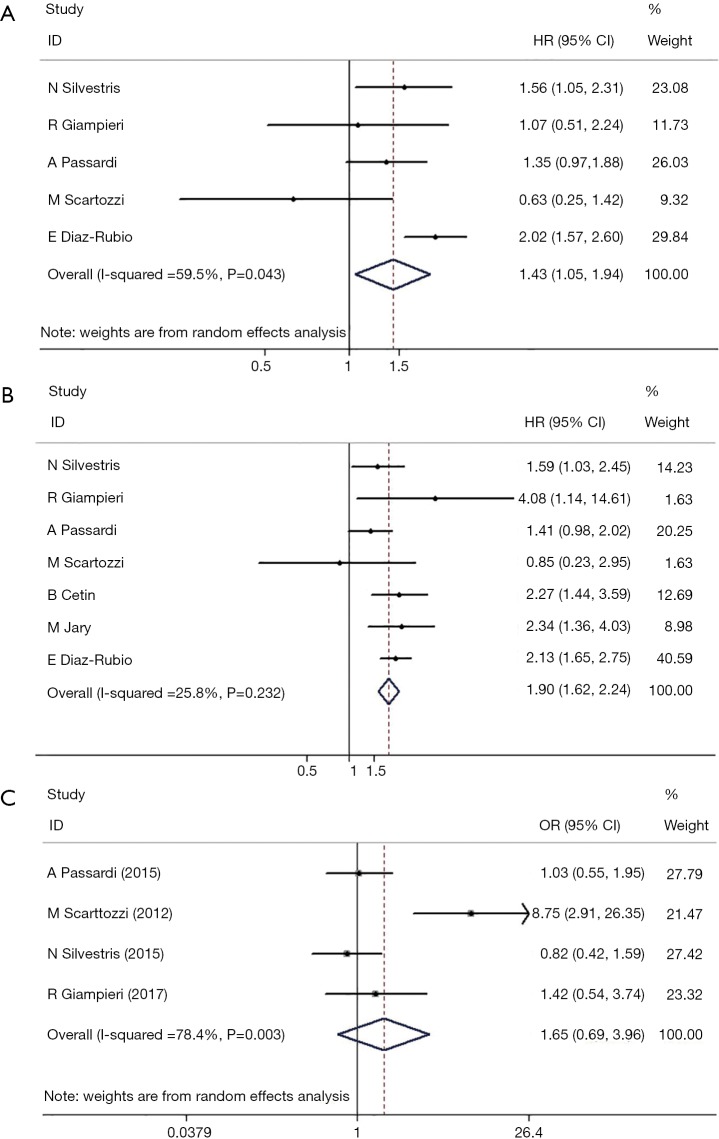
A forest plot, showing HRs, 95% CIs, and the weight of each study in the analysis, was constructed to illustrate the correlation between serum LDH level and PFS (Figure 2). A random-effects model was applied because the heterogeneity between studies was significant (I2=59.5%, P=0.043). The overall HR was 1.43 (95% CI: 1.05–1.94, P=0.023). No significant publication bias was detected, as analyzed by Egger’s and Begg’s tests (P=0.142, Figure 3). These results indicate that high serum LDH level is significantly associated with shorter PFS time in mCRC patients treated with bevacizumab-based first-line chemotherapy.
Figure 2.
Meta-analysis of the association between lactate dehydrogenase serum level and (A) progression-free survival, (B) overall survival and (C) objective response rate.
Figure 3.
Test of publication bias of the analysis of lactate dehydrogenase serum level and (A) progression-free survival, (B) overall survival and (C) objective response rate.
The heterogeneity between studies with respect to OS was not statistically significant (I2=25.8%, P=0.232) and a fixed-effects model was used to analyze the correlation between serum LDH level and OS. The corresponding forest plot (Figure 2) indicates an overall HR of 1.667 (95% CI: 1.230–2.259, P=0.001). Egger’s or Begg’s tests indicated no significant publication bias (P=0.881, Figure 3). These results indicate that high serum LDH level is significantly associated with shorter OS time in mCRC patients treated with bevacizumab-based first-line chemotherapy.
In contrast to OS and PFS, however, objective RR was not significantly associated with serum LDH in our meta-analysis using a random-effects model (HR: 1.651, 95% CI: 0.689–3.957; P=0.261; Figure 2). In addition, no significant publication bias was detected by Egger’s or Begg’s tests (P=0.308, Figure 3).
Discussion
The systematic review and meta-analysis reported here showed that high serum LDH level is significantly associated with shorter PFS and OS times in mCRC patients treated with bevacizumab-based first-line chemotherapy, indicating that this parameter may be useful as a prognostic biomarker for this patient cohort. This result differs in some aspects from a similar meta-analysis of mCRC patients by Li et al., which found that serum LDH level had prognostic value for OS but not PFS (6). However, the meta-analysis inclusion criteria differed between the two studies. First, we included only mCRC patients who were treated with bevacizumab as first-line chemotherapy, whereas Li et al. did not specify treatment with bevacizumab. Second, the method of LDH detection in the seven included studies here were to measure the LDH concentration in plasma, whereas two of the eligible studies in the analysis by Li et al. used immunohistochemical techniques, suggesting that the detection methods may have contributed to the discrepancy between studies. Therefore, we can conclude only that serum LDH may be a prognostic factor for PFS specifically in mCRC patients who were treated with bevacizumab.
The relationship between LDH and the outcome of anti-angiogenic treatment has been analyzed in several clinical trials. Two randomized phase III trials examined the efficacy of vatalanib (PTK/ZK), an oral angiogenesis inhibitor, in combination with FOLFOX (leucovorin, fluorouracil, oxaliplatin) for first-line (CONFIRM-1) and second-line (CONFIRM-2) therapy in mCRC patients (16,17). Although both studies failed to reach their primary end points, subgroup analysis showed that vatalanib improved the median PFS in patients with high serum LDH levels (16,17), supporting the possibility that LDH may be a predictive biomarker for anti-angiogenic therapy. In another study, Bar et al. retrospectively evaluated serum LDH levels obtained in the HORIZON I study, which was a randomized phase II head-to-head comparison of bevacizumab and cediranib, an oral VEGF receptor inhibitor, in mCRC patients. That analysis identified a significant correlation between high serum LDH and shorter PFS. Thus, the results of our meta-analysis are consistent with the conclusions of three previous studies identifying a correlation between serum LDH level and outcome for mCRC patients treated with bevacizumab.
Our study has several limitations. First, the number of eligible studies was small, and most of them were retrospective, which indicates a lower level in the hierarchy of evidence-based medicine. Second, our meta-analysis was based only on studies meeting our inclusion criteria, whereas many others studies failed to meet these criteria. Third, we could not obtain updated data on individual patients, which would further enhance the accuracy and reduce the uncertainty of our estimates.
Conclusions
Our meta-analysis provides evidence that high serum LDH level is significantly associated with shorter PFS and OS time in mCRC patients treated with bevacizumab-based first-line chemotherapy. Serum LDH may thus be useful as a prognostic factor for patients receiving bevacizumab as first-line chemotherapy and as a predictive factor for those receiving bevacizumab-based therapy at other times.
Acknowledgements
Funding: The National Key Research and Development Program of China (Award Number: 2017YFC1308900).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. 10.1200/JCO.2007.14.9930 [DOI] [PubMed] [Google Scholar]
- 2.Iwamoto S, Takahashi T, Tamagawa H, et al. FOLFIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer after first-line bevacizumab plus oxaliplatin-based therapy: the randomized phase III EAGLE study. Ann Oncol 2015;26:1427-33. 10.1093/annonc/mdv197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masi G, Salvatore L, Boni L, et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol 2015;26:724-30. 10.1093/annonc/mdv012 [DOI] [PubMed] [Google Scholar]
- 4.Wu XZ, Ma F, Wang XL. Serological diagnostic factors for liver metastasis in patients with colorectal cancer. World J Gastroenterol 2010;16:4084-8. 10.3748/wjg.v16.i32.4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brizel DM, Schroeder T, Scher RL, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;51:349-53. 10.1016/S0360-3016(01)01630-3 [DOI] [PubMed] [Google Scholar]
- 6.Li G, Wang Z, Xu J, et al. The prognostic value of lactate dehydrogenase levels in colorectal cancer: a meta-analysis. BMC Cancer 2016;16:249. 10.1186/s12885-016-2276-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin ML, Gladden LB, Nijsten MW, et al. Lactate and cancer: revisiting the warburg effect in an era of lactate shuttling. Front Nutr 2015;1:27. 10.3389/fnut.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azuma M, Shi M, Danenberg KD, et al. Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmacogenomics 2007;8:1705-13. 10.2217/14622416.8.12.1705 [DOI] [PubMed] [Google Scholar]
- 9.Silvestris N, Scartozzi M, Graziano G, et al. Basal and bevacizumab-based therapy-induced changes of lactate dehydrogenases and fibrinogen levels and clinical outcome of previously untreated metastatic colorectal cancer patients: a multicentric retrospective analysis. Expert Opin Biol Ther 2015;15:155-62. 10.1517/14712598.2015.986452 [DOI] [PubMed] [Google Scholar]
- 10.Scartozzi M, Giampieri R, Maccaroni E, et al. Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer 2012;106:799-804. 10.1038/bjc.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giampieri R, Puzzoni M, Daniele B, et al. First-line FOLFIRI and bevacizumab in patients with advanced colorectal cancer prospectively stratified according to serum LDH: final results of the GISCAD (Italian Group for the Study of Digestive Tract Cancers) CENTRAL (ColorEctalavastiNTRiAlLdh) trial. Br J Cancer 2017;117:1099-104. 10.1038/bjc.2017.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jary M, Lecomte T, Bouche O, et al. Prognostic value of baseline seric Syndecan-1 in initially unresectable metastatic colorectal cancer patients: a simple biological score. Int J Cancer 2016;139:2325-35. 10.1002/ijc.30367 [DOI] [PubMed] [Google Scholar]
- 13.Passardi A, Scarpi E, Tamberi S, et al. Impact of Pre-Treatment Lactate Dehydrogenase Levels on Prognosis and Bevacizumab Efficacy in Patients with Metastatic Colorectal Cancer. PLoS One 2015;10:e0134732. 10.1371/journal.pone.0134732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cetin B, Kaplan MA, Berk V, et al. Prognostic Factors for Overall Survival in Patients With Metastatic Colorectal Carcinoma Treated With Vascular Endothelial Growth Factor-Targeting Agents. Asian Pacific Journal of Cancer Prevention 2012;13:1059-63. 10.7314/APJCP.2012.13.3.1059 [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Rubio E, Gomez-Espana A, Massuti B, et al. Role of Kras status in patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab: a TTD group cooperative study. PLoS One 2012;7:e47345. 10.1371/journal.pone.0047345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecht JR, Trarbach T, Hainsworth JD, et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol 2011;29:1997-2003. 10.1200/JCO.2010.29.4496 [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Bajetta E, Valle J, et al. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol 2011;29:2004-10. 10.1200/JCO.2010.29.5436 [DOI] [PubMed] [Google Scholar]