Abstract
Background: Enhanced recovery after surgery (ERAS) protocols are well known for reducing post-operative complications, facilitating early recovery and reducing hospitalization. In this study, we developed ERAS protocols involving immediate ice cream intake for checking postoperative chylothorax and subsequent early ambulation in order to investigate whether these methods have postoperative benefits.
Methods: We retrospectively evaluated 500 patients who underwent thoracoscopic segmentectomy and/or lobectomy (TSL) between January 2014 and September 2017. The patients were divided into two groups: 271 patients for Phase I and 229 for Phase II. Ice cream intake commenced during Phase I. Phase I patients were made to walk on the following day, whereas Phase II ambulate within 4 hrs after immediate ice-cream intake.
Results: The mean ice cream intake was significantly higher in Phase II than in Phase I (81.6% vs 56.1%). In Phase II, 91.2% and 94.0% were able to ambulate within 4 and 6 hrs, respectively. Minor postoperative complications (Clavien–Dindo I–II classification) were lower in Phase II (3.1%) than in Phase I (10.4%); however, we found no statistical significance (p=0.08). Multivariate analysis showed that ice cream intake and removal of chest drainage tube within 4–6 hrs significantly contributed to the reduction of hospitalization to ≤3 postoperative days (p=0.03 and p<0.01).
Conclusions: The results of this study suggested that our ERAS protocol represented by immediate ice cream intake, and early ambulation is feasible and can help in reducing postoperative complications, chest drainage duration, and hospitalization after TSL.
Keywords: ice cream, ERAS, thoracoscopy, lobectomy, segmentectomy, chylothorax
Introduction
Clinical recovery protocols are indispensable, with the aim to encourage patients by improving their physical condition and restoring their willpower. Throughout the world, the clinical impact of enhanced recovery after surgery (ERAS) protocols for various major surgeries has been notable.1,2 However, the number of studies on the clinical use of ERAS protocols for pulmonary resection is limited, and most of the retrospective studies are based on experience.3–5
Compared to open thoracotomy, thoracoscopic segmentectomy or lobectomy (TSL) is less invasive as it involves less post-operative pain, a low complication rate, less time to begin ambulation, and early discharge of patients. Therefore, as a synergistic effect, improvement in patient performance during post-TSL recovery is better than expected compared to conventional thoracotomy. Salati et al, implemented a fast-track program after lobectomy, which was effective and safe because it reduced postoperative hospitalization without an increase in the readmission rate.3 Similarly, Khandhar et al, reported that ambulation of 250 feet within 1 hr of extubation after TSL led to low morbidity.4
ERAS comprises four major factors and 24 core elements. When the efficacy of ERAS in postoperative recovery of patients was tangibly verified by evidence-based treatments, its worldwide reliance rapidly increased.5 In 2010, it was announced worldwide that ERAS could be used to improve short-term patient outcomes, including a reduction of postoperative complications, early discharge of patients and cost-effectiveness.6 However, it is impossible to achieve full compliance, and the mean compliance rate ranges from 60% to 80%.7
In this retrospective study, we developed and implemented an ERAS protocol which included immediate ice cream intake postoperatively and subsequent early ambulation for patients who underwent TSL at a cancer-specific hospital in Japan.
Materials and methods
In this study, we retrospectively evaluated 500 patients from a single institution between January 2014 and September 2017. Of the original 503 patients, 3 who underwent thoracotomy conversion (0.6%) were excluded because of intraoperative bleeding. The final 500 patients were divided into two groups, 271 in Phase I ERAS and 229 in Phase II ERAS. Table 1 shows the relevant patient characteristics. The concept of ERAS was explained to the patients (Table 2). The institutional review board at the Aichi Cancer Center, Aichi Prefecture, Japan, approved the study. All protocols were performed in accordance with the relevant local guidelines. Written informed consent was obtained from all eligible patients. Patients underwent TSL with systemic lymph node dissection and sampling.
Table 1.
Patient characteristics
| Variables | Phase I (n=271) | Phase II (n=229) | p |
|---|---|---|---|
| Age (years) | 66 (19–87) | 67 (16–88) | 0.14 |
| Gender (Male/Female) | 133/139 | 109/119 | 0.81 |
| Smoke (pack/year) | 20.7±29.8 | 21.7±30.6 | 0.59 |
| BMI (kg/mm2) | 22.4±3.9 | 22.1±3.2 | 0.56 |
| Histology (LC/MT/Benign) | 233/37/2 | 203/21/4 | 0.29 |
| Operative time (mins) | 218.9±66.9 | 204.3±53.2 | <0.01* |
| Bleeding (mL) | 32.2±107.8 | 23.7±39.0 | 0.83 |
| Respiratory function | |||
| %Vital capacity | 101.2±14.3 | 99.3±13.7 | 0.12 |
| FEV% in 1 s | 79.2±7.6 | 78.0±8.8 | 0.10 |
| Procedures | |||
| Segmentectomy/lobectomy | 89/183 | 84/144 | 0.34 |
| Any preoperative Comorbidity | |||
| Cardiovascular | 17 (6.3) | 15 (6.6) | 0.88 |
| Pulmonary | 25 (9.) | 24 (10.5) | 0.62 |
| Diabetes | 26 (9.6) | 30 (13.2) | 0.20 |
Note: *p<0.05.
Abbreviations: BMI, body mass index; LC, lung cancer; MT, metastasized tumor; FEV1, forced expiratory volume in 1 s.
Table 2.
ERAS society guidelines
| Postoperative elements | Periods | |
|---|---|---|
| Phase I | Phase II | |
| Early mobilization | 1 POD | On the day |
| Early intake of oral fluids and solids | On the day ±ice cream |
On the day +ice cream |
| Early removal of urinary catheters and intravenous fluids | 1 POD | On the day |
| Use of chewing gums and laxatives and peripheral opioid-blocking agents (when using opioids) | No | No |
| Intake of protein and energy-rich nutritional supplements | Diet | Diet |
| Multimodal approach to control opioid-sparing pain control | Yes | Yes |
| Multimodal approach to control nausea and vomiting | Yes | Yes |
| Prepare for early discharge | Yes | Yes |
| Audit of outcomes and process in multiprofessional, multidisciplinary team on a regular basis | Doctor and Nursing staffs | Yes |
Abbreviations: ERAS, enhanced recovery after surgery; POD, postoperative day.
Our TSL was performed using an upside-down monitor setting with four ports.8 Postoperative pain was managed using a multimodal analgesic regimen, including a single intercostal nerve block (ICB) and oral nonsteroidal anti-inflammatory drugs and weak opioids, as reported in the previous studies.9 Immediately after surgery, the patients were forced to ingest fat-rich (16.3 g/piece) vanilla ice cream (Häagen-Dazs and Meiji, both Tokyo, Japan) in order to determine the presence of chylothorax after having eaten more than the quantity that was equal to low fat diet (6 mg per one meal during a day). The judgment of postoperative complications was evaluated according to the report of Clavien–Dindo classification.10
Phase I ERAS
On the basis of ERAS Society guidelines, we established the Phase I ERAS protocol, depending on compliance with the postoperative elements specified in the guidelines. Phase I fulfilled six elements (66.7%), managed with surgeons only (Table 2).6 The patients rested in the recovery room for 20 mins after extubation and were then transferred to the thoracic surgery-specific ward. Immediately after recovery from anesthesia, the patients were not allowed any water in order to examine whether recurrent nerve paralysis developed. The patients were made to walk on the following day after surgery.
Phase II ERAS
After reviewing the patient outcomes in Phase I and referring to published, successful ERAS protocols, we developed the Phase II ERAS protocol, depending on compliance with criteria of preadmission patient education and post-operative elements. Phase II fulfilled all elements (100%), managed jointly with surgeons, the nursing staff and a couple of experts (Table 2). In addition, we experienced a postoperative chylothorax after thoracoscopic wedge resection, and succeed in prevention using preoperative ice cream intake.11 The patients were educated on the Phase II ERAS protocol, emphasizing the brief perioperative management based on Phase I analyses and focussing on improving patients’ psychological stability and physical mobility. In contrast to Phase I, however, within 0.5 hrs after ice cream intake after evaluating whether patients were examined recurrent nerve paralysis or not at the thoracic surgery-specific ward, the patients were asked to stand up by the bedside and, if possible, walk around the thoracic surgery-specific ward for 10 mins always accompanied by thoracic surgeons and the nursing staff within 4 hrs after extubation. After early ambulation, the chest drainage tube was removed in the absence of air leakage, bleeding and chylothorax and drainage ≤100 mL within 4 hrs after extubation. We enforced the drain tube removal under the condition of fat more than 6.0 g from ice cream or from diet on post-operative day. In addition, the urinary catheter was removal and intravenous crystalloid administration discontinued. After discharge, the patients were re-educated on the ERAS protocol, and institutional informational pamphlets containing brief programs with a focus on retrieving the preoperative quality of life were distributed.
Statistical analyses
All computations were done using standard SPSS v17.0 software (SPSS Inc., IBM, Chicago, IL, USA). Mann–Whitney U tests were used to compare the two groups of patients. The Cox proportional hazards regression model was used to perform univariate and multivariate analyses of potential correlates of early discharge of patients. Hazard ratios and median survival rates were presented with 95% confidence intervals (CIs). p<0.05 was considered statistically significant.
Results
In this study, the mean operation time was 212.3 mins (range: 55–487 mins) and the mean blood loss was 28.3 g (range: 0–1670 g); we found no 90-day mortality. These findings were compatible with previous studies reporting best post-TSL performance in Japan.8 Phase II showed a shorter operative time compared to Phase I, which was statistically significant (p<0.01) (Table 1). Chronic preoperative comorbidities (eg, chronic cardiac conditions, diabetes, smoking status, and pulmonary disorder) were equally represented in the two phases.
The mean ice cream intake was significantly higher in Phase II (81.6%; fat: 13.3 g) than in Phase I (56.1%; fat: 9.1 g) as was the mean feeding (39.4% vs 21.5%). In both phases, the mean numerical rating scale (NRS) score was higher on ambulation (2.75±1.98) than at rest (1.84±1.45) (p<0.01), and the mean NRS score on ambulation was equivalent in both Phase II and Phase I (2.84±1.75 vs 2.64±2.22; p=0.03). We did not record the morphine consumption in both phases; also studies have shown that postoperative administration of weak opioids, such as tramadol, is clinically considerable for patients undergoing TSL.9 The percentage of tramadol use was equivalent in Phase II and Phase I (40.3% vs 37.7%; p=0.57). In Phase II, 91.2% and 94.0% of the patients were able to ambulate on the day of surgery within 4 and 6 hrs of ice cream intake.
The percentage of major postoperative complications (Clavien–Dindo III–IV classification) was equivalent in both phases, while minor postoperative complications (Clavien–Dindo I–II classification) were lower in Phase II (3.1%) compared to Phase I (10.4%) but without statistical significance (p=0.08; Table 3).12 The rate of readmission within 90 days of surgery was the same in both phases (Table 3). In addition, early ambulation contributed significantly to the reduction of chest drainage duration (Phase I, 1.4 days; Phase II, 0.4 days; p<0.01) and postoperative hospitalization (Phase I, 4.8 days; Phase II, 3.8 days; p<0.01).
Table 3.
Short-term (30-day) morbidity of patients
| Characteristic | Phase I (n=271) | Phase II (n=229) | p |
|---|---|---|---|
| Any complications (%) | 19 (7.0) | 11 (4.8) | 0.31 |
| Minor (Clavien-Dingo I–II) | 16 (5.9) | 7 (3.1) | 0.08 |
| Tachyarrhythmia | 7 (2.6) | 0 (0) | |
| Prolonged air leak (>7 days) | 4 (1.5) | 0 (0) | |
| Atelectasis | 1 (0.4) | 1 (0.4) | |
| Surgical site infection | 0 (0) | 2 (0.9) | |
| Clostridium difficile colitis | 1 (0.4) | 1 (0.4) | |
| Othersa | 3 (1.1) | 3 (1.3) | |
| Major (Clavien-Dingo III–IV) | 3 (1.1) | 4 (1.8) | 0.33 |
| Pneumothorax requiring drainage | 2 (0.7) | 3 (1.3) | |
| Chylothorax requiring drainage | 1 (0.4) | 0 (0) | |
| Pleural effusion requiring drainage | 0 (0) | 1 (0.4) | |
| Mortality | 0 (0) | 0 (0) | – |
| Readmission | 4 (1.5) | 3 (1.3) | 0.88 |
Notes: a Phase I includes delirium, temporary (reversible) paralysis of the recurrent nerve and chest wall bleeding. Phase II includes Clostridium difficile colitis, acute laryngitis, and thrombosis at the stump of the left upper superior vein.
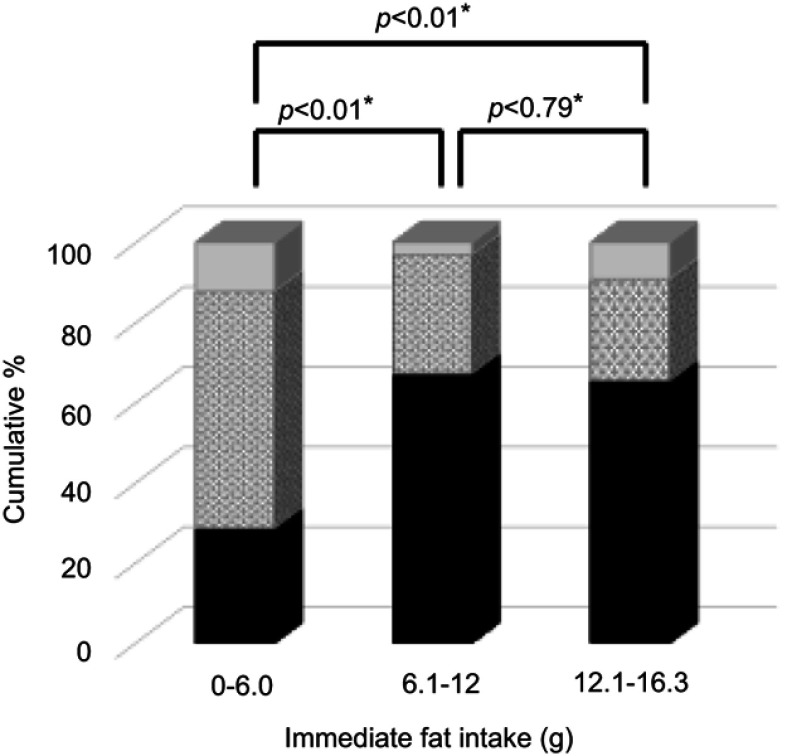
Univariate and multivariate analyses of potential correlates of early discharge of patients without postoperative complications, ice cream intake and removal of the chest drainage tube on the day of surgery statistically affected the early discharge of patients (≤3 days hospitalization postoperatively; Table 4). We classified ice cream intake (n=321) into three categories according to fat consumption, as follows: Low (less than 6.0 g, n=22), Medium (6.1 to 12.0 g; n=64), and Large (more than 12.0 g; n=208). The percentages of removal of drainage tube within 24 hrs was not significant (p=0.18), and those after Small, Medium, and Large were as follows: 87.8%, 96.9%, and 90.9%, respectively. The significant differences between Small and Medium were found, but not between Medium and Large in those within 4–6 hrs of ice cream intake (p<0.01 and p=0.79, respectively) (Figure 1).
Table 4.
Univariate and multivariate analyses for ≤3 days’ hospitalization after surgery
| Univariate analysis | Multivariate analysis | ||
|---|---|---|---|
| p | p | HR (95% CI) |
|
| Age (equal or less than 60 years old) | 0.53 | ||
| Gender (male) | 0.20 | ||
| Smoking (S.I >800) | 0.11 | 0.22 | 0.76 (0.48–1.19) |
| BMI (>24) | 0.45 | ||
| Any comorbidity | 0.83 | ||
| Procedures (Segmentectomy) | 0.04 | 0.25 | 1.28 (0.84–1.94) |
| Lymphadenectomy (vs Sampling) | 0.63 | ||
| Operative time (equal or less than 180 mins) | <0.01 | 0.11 | 1.43 (0.93–2.21) |
| Bleeding/Weight ×103<1 | 0.04 | 0.27 | 1.44 (0.75–2.76) |
| Ambulation (yes) | <0.01 | 0.29 | 0.73 (0.40–1.31) |
| Ice (more than half) | <0.01 | 0.03 | 1.80 (1.07–3.03) |
| Light pain (NRS <3) on ambulation | 0.43 | ||
| The removal of drainage tube (<1 day) | 0.04 | <0.01 | 2.90 (1.68–4.96) |
Abbreviations: BMI, body mass index; NRS, numerical rating scale.
Figure 1.
Removal of the drainage tube within 4–6 hrs (the black bar area), within 24 hrs (the gray-dotted area), and after two or more postoperative days (the gray area). *A p-value <0.05 was considered significant.
Discussion
Our multidisciplinary, evidence-based, enhanced ERAS protocol for patients undergoing TSL led to a reduction of chest drainage duration and hospitalization postoperatively but not the readmission rate. ERAS program developers, with a decade of experience and abundant data accumulation, need to ensure that common compliances are shared. Compliance with 70–80% or more of the ERAS protocol elements seems important to improve patient outcomes.5 In this retrospective study, we examined patient outcomes after implementation of our ERAS protocol and hypothesized that immediate ice cream intake postoperatively and early ambulation within 4–6 hrs of ice cream intake are associated with early removal of the chest drainage tube, consequently followed by early discharge of patients.
Early ambulation within 24 hrs is beneficial in terms of early discharge of patients and low morbidity.5 Das-Neves-Pereira reported that early ambulation after lobectomy was possible in 99 patients (90.4%) but not in 10 patients (9.6%) – early ambulation was defined as the patient starting ambulation before the first postoperative hour.12 One reason for this improvement in the rate of ambulation could be that hypnosis due to anesthesia decreases with the progress of the time. In our study, too, the patients could not ambulate within 4 hrs because of nausea (n=7, 36.8%), hypnosis (n=6, 31.6%), orthostatic hypotension (n=5, 26.3%), and bleeding (n=1, 5.3%).
In this study, the patients were discharged by POD 3, similar to other studies.12,13 Decreased hospitalization is a necessary condition for patients doing better and is enough to fulfill discharge criteria sooner. In contrast, studies have shown that because of the selection bias associated with several non-clinical factors (the attending physician’s preference, patient anxiety and distress, insurance system, etc.), the length of hospitalization does not reflect the efficacy of ERAS.14 A Brazilian report showed that by fast-track rehabilitation, >90% of the patients who underwent lung cancer lobectomy were discharged by POD 2. However, few studies have focused on patient psychology, especially in lung resection. We feel it will be possible to realize 1-day hospitalization after lung resection by accumulating promising patient data.
ERAS protocols can reduce postoperative complications by 10–20% or more by supporting institutes in the adoption of evidence-based care.15,16 In addition, thoracoscopy is associated with the reduction of postoperative complications, which are related to increased hospitalization.17 Some retrospective single-institute studies have reported decreased hospital costs and fewer postoperative complications with thoracoscopy lobectomy compared to thoracotomy lobectomy. In our study, the percentage of minor postoperative complications (Clavien–Dindo I–II classification) was less in Phase II compared to Phase I. This difference was compatible with previous data of different organs.4,5,11 Khandhar et al, reported that in patients who underwent thoracoscopy lobectomy, early postoperative ambulation is feasible and is considered key in achieving low morbidity (pneumonia in 0.7% and atrial fibrillation in 4.0% of the patients).4 The surgeon’s technical development and patient recovery due to early ambulation should be associated with short-term patient outcomes.
Immediate ice cream intake postoperatively can lead to the removal of the chest drainage tube on the day of surgery and is significantly related to the early discharge of patients. In other words, the cold environment in the oral cavity relaxes breathing.18 It is indispensable to verify no chylothorax. In Phase I, it was a problem that there were few patients who could ingest enough fat by meal to remove the drainage tube. Ice cream is usually provided for the patients with anorexia after chemotherapy or cancer cachexia in our cancer-specific hospital. Phase II ERAS was made in reference to this information, and the percentages removal of drainage tube within 4–6 hrs and 24 hrs after ice cream intake successfully resulted in 60.3% and 91.6%, respectively (Figure 1). Additionally, after early ambulation, those percentages in Phase II only rose more in 78.2% and 93.3%, respectively. A previous retrospective study has revealed that the chest drainage volume is not associated with increased morbidity when the chest drainage tube is removed on POD 1.19 In this study, a chest drainage tube was required because of pleural effusion after early removal in one patient (0.2%) during hospitalization and in four patients (0.8%) 90 days after TLS.
In this study, the mild to severe pain on ambulation (NRS score >4) could not affect early discharge of patients. Reduction of pain-related postoperative complications and speedy recovery are indispensable for ERAS implementation.20 In a previous study, we reported that the incidence of mild pain was 75.6% on post-TS ambulation, and we managed acute postthoracoscopic pain with intraoperative use of a single-shot ICB and postoperative use of tramadol.9,21 Wurnig et al, reported that compared to thoracic epidural anesthesia and a paravertebral block, an ICB seems to offer poorer pain control, yet it is favored for its lower incidence of adverse events.22 Multimodal analgesic management should contribute to ERAS post-TS because of analgesic efficacy and fewer side effects.
This study had a few limitations. Firstly, although the two groups were comparable and uniform with little differences, the clinical data were from a single institute. Secondly, we excluded three patients because of thoracotomy conversion due to intraoperative bleeding (2/273 [0.74%] patients from Phase I and 1/230 [0.43%] patients from Phase II); however, we still had a relatively large sample. Thirdly, the self-produced nature of our ERAS protocol might potentially result in selection bias. The before-and-after design did not comply with conventional ERAS criteria and depended on information analyses based on our clinical results. Fortunately, the balanced and invariable patient characteristics might ensure more efficient use of our ERAS protocol. Fourthly, the rate of complications in this study might be less compared to other studies, because, sometimes, patients were referred to affiliated community hospitals rather than our institution.4,5,10 However, surgical outcomes in this study seem compatible with those from a similar TS surgical system setting in Japan.8 Fifthly, the mean operative time was shorter in Phase I than that in Phase II. This seemed to be a relevant bias for this study. It is true that the accumulation of surgical experience showed shortening of the operative time. However, there was no significant difference in the incidence of post-operative complications between Phase I and Phase II. Therefore, we subjected to only the patients with no complications. By multi-variate analyses, the operative time did not influence the ≤3 days’ hospitalization after surgery in the patients with no complications. Since, we perceived that it was not more likely to be a relevant bias.
Conclusions
ERAS can be used to improve short-term patient outcomes, including a reduction of postoperative complications, and hospitalization. Immediate ice cream intake and early ambulation within 4 hrs of ice cream intake are associated with early removal of the chest drainage tube, consequently followed by early discharge of patients. Multicenter and prospective randomized studies following quasi-unified ERAS protocols should be conducted in routine clinical practice so that it becomes possible to realize 1-day hospitalization after lung resection.
We concluded that immediate ice cream intake post-operatively and early ambulation within 4 hrs of ice cream intake are associated with early removal of the chest drainage tube inserted due to chylothorax, consequently followed by early discharge of patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–477. doi: 10.1016/j.clnu.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 2.Lassen K, Soop M, Nygren J, et al., Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969. doi: 10.1001/archsurg.2009.170 [DOI] [PubMed] [Google Scholar]
- 3.Salati M, Brunelli A, Xiumè F, Refai M, Pompili C, Sabbatini A. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardiothorac Surg. 2012;41(5):1083–7;discussion 1087. doi: 10.1093/ejcts/ezr171 [DOI] [PubMed] [Google Scholar]
- 4.Khandhar SJ, Schatz CL, Collins DT, et al. Thoracic enhanced recovery with ambulation after surgery: a 6-year experience. Eur J Cardiothorac Surg. 2018;53(6):1192–1198. doi: 10.1093/ejcts/ezy061 [DOI] [PubMed] [Google Scholar]
- 5.Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg. 2018;155(4):1843–1852. doi: 10.1016/j.jtcvs.2017.10.151 [DOI] [PubMed] [Google Scholar]
- 6.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–298. doi: 10.1001/jamasurg.2016.4952 [DOI] [PubMed] [Google Scholar]
- 7.Pisarska M, Pędziwiatr M, Małczak P, et al. Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. Int J Surg. 2016;36(Pt A):377–382. doi: 10.1016/j.ijsu.2016.11.088 [DOI] [PubMed] [Google Scholar]
- 8.Mun M, Ichinose J, Matsuura Y, Nakao M, Okumura S. Video-assisted thoracoscopic surgery lobectomy via confronting upside-down monitor setting. J Vis Surg. 2017;3:129. doi: 10.21037/jovs.2017.07.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuroda H, Mizuno H, Dejima H, et al. A retrospective study on analgesic requirements for thoracoscopic surgery postoperative pain. J Pain Res. 2017;15(10):2643–2648. doi: 10.2147/JPR.S147691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seto K, Kuroda H, Mizuno T, Sakakura N, Sakao Y. Postoperative chylothorax after pulmonary wedge resection in two patients who underwent radical neck dissection: a case report. Asian J Endosc Surg. 2016;9(4):322–324. doi: 10.1111/ases.12305 [DOI] [PubMed] [Google Scholar]
- 12.Das-Neves-Pereira JC, Bagan P, Coimbra-Israel AP, et al. Fast-track rehabilitation for lung cancer lobectomy: a five-year experience. Eur J Cardiothorac Surg. 2009;36(2):383–391, discussion 391–392. doi: 10.1016/j.ejcts.2009.02.020 [DOI] [PubMed] [Google Scholar]
- 13.Madani A, Fiore JF Jr, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery. 2015;158(4):899–908, discussion 908–910. doi: 10.1016/j.surg.2015.04.046 [DOI] [PubMed] [Google Scholar]
- 14.Fiore JF Jr, Faragher IG, Bialocerkowski A, Browning L, Denehy L. Time to readiness for discharge is a valid and reliable measure of short-term recovery after colorectal surgery. World J Surg. 2013;37(12):2927–2934. doi: 10.1007/s00268-013-2208-1 [DOI] [PubMed] [Google Scholar]
- 15.ERAS Compliance Group. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg. 2015;261(6):1153–1159. doi: 10.1097/SLA.0000000000001029 [DOI] [PubMed] [Google Scholar]
- 16.Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38(6):1531–1541. doi: 10.1007/s00268-013-2416-8 [DOI] [PubMed] [Google Scholar]
- 17.Cho S, Do YW, Lee EB. Comparison of costs for video-assisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surg Endosc. 2011;25(4):1054–1061. doi: 10.1007/s00464-010-1315-4 [DOI] [PubMed] [Google Scholar]
- 18.Hanna JN, McN Hill P, Sinclair JD. Human cardiorespiratory responses to acute cold exposure. Clin Exp Pharmacol Physiol. 1975;2(3):229–238. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi R, Fujino Y, Yamashita T, Oka S. A prospective study of the association between drainage volume within 24 hrs after thoracoscopic lobectomy and postoperative morbidity. J Thorac Cardiovasc Surg. 2009;137(6):1394–1399. doi: 10.1016/j.jtcvs.2008.10.035 [DOI] [PubMed] [Google Scholar]
- 20.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691–697. doi: 10.1001/jamasurg.2017.0898 [DOI] [PubMed] [Google Scholar]
- 21.Kuroda H, Sakao Y. Analgesic management after thoracoscopic surgery: recent studies and our experience. J Thorac Dis. 2018;10(Suppl 9):S1050–S1054. doi: 10.21037/jtd.2018.04.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wurnig PN, Lackner H, Teiner C, et al. Is intercostal block for pain management in thoracic surgery more successful than epidural anaesthesia? Eur J Cardiothorac Surg. 2002;21(6):1115–1119. [DOI] [PubMed] [Google Scholar]