Abstract
Purpose: Cancer mortality is relatively high in the elderly population. Folate receptor-positive circulating tumor cell (FR+CTC) has proven an effective biomarker for diagnosis of lung cancer and bladder cancer and may be suitable for other cancer types accompanied with a high expression of FR. To date, the diagnostic efficiency of FR+CTC in the elderly population has not been systematically studied. Herein, we sought to investigate the utility of FR+CTC in cancer diagnosis in the elderly population and the influence of comorbidities on FR+CTC levels in such a population.
Patients and methods: A total of 35 cancer patients (including 23 lung cancers, 8 colorectal cancers, and 4 other cancers) and 40 noncancer participants, aged between 80 and 110, were recruited in this study. Three milliliters of pretreatment peripheral blood was collected from each participant for FR+CTC analysis.
Results: Compared to previous studies, the FR+CTC level was slightly higher in the elderly population (median FR+CTC levels in cancer patients versus noncancer participants were 14.3 versus 9.2 CTC U/3 mL, respectively, P=0.0002). With 10.0 CTC U/3 mL as the cut-off value, the sensitivity and specificity of FR+CTC were 85.7% and 65.0%, respectively. In combination with established serum tumor biomarkers, the diagnostic efficiency of FR+CTC further improved (sensitivity=87.9%, specificity=71.8%). Clinical factors including diabetes, cardiovascular diseases, respiratory diseases, cerebral infarction, and cardiac, liver, and kidney function were not associated with the FR+CTC level (P>0.05).
Conclusion: In this exploratory study, we showed that FR+CTC is an effective biomarker for cancer diagnosis in the elderly population. The presence of comorbidities did not affect the diagnostic efficiency of FR+CTC.
Keywords: biomarker, cancer, circulating tumor cell, elderly, folate receptor
Introduction
Cancer mortality is relatively high in the elderly population due at least in part to usually late diagnosis and the presence of comorbidities.1 Elderly cancers are usually identified at late stage where treatment outcome is poor and survival rate is low.2 Hence, the early-diagnosis of elderly cancer has the possibility of prolonging lifespan. Further, elderly patients typically suffer from poor performance status and cannot withstand more radical or invasive diagnosis, which underscores the need for a more sensitive and reliable non-invasive diagnostic method for cancer in the elderly population.
Recent studies have suggested that the vascular invasion of tumor cells may happen as early as at the precancerous stage, leading to the release of circulating tumor cells (CTCs) into the circulatory system.3,4 In the last decade, technology advancements have enabled reliable detection of CTCs in routine clinical practice.5 CTCs, as a liquid biopsy approach, possess a great potential in assisting cancer management.6 Compared with bone marrow or lymph nodes, peripheral blood specimens are easy to obtain, less invasive, and can be repeatedly taken, which makes CTC an ideal specimen for routine clinical assessment.
Folate receptor (FR), a membrane glycoprotein that shows a high positivity in a range of cancer types, has emerged as a potential drug target for lung cancers.7 In the peripheral blood, only a few FR-expressing cells are present, including CTCs and a rare subtype of activated monocytes which are seldom detected in nonmalignant individuals (note that the α-isoform of FR which is the target of the CTC detection platform used in our study is absent from all hematopoietic cells).8,9 In contrast, FR expression was reported to be upregulated in over 75% of non-small cell lung cancer.10 Thus, FR is theoretically an effective target for labeling CTCs, especially for lung cancers. Several studies had demonstrated the diagnostic efficiency of folate receptor-positive CTCs (FR+CTC) in lung cancers and bladder cancers, as well as for predicting patient’s prognosis and chemotherapy sensitivity in small-cell lung cancer.11–16 The related FR+CTC detection kit has been approved by the CFDA for clinical use. For lung cancer diagnosis, the sensitivity and specificity of FR+CTC were reported to be 72.5–81.8% and 82.4–93.2%, respectively.11–14 FR+CTC may also be suitable for other cancer types such as pancreatic cancer, breast cancer, ovarian cancer, and so on, which are accompanied by a high expression of FR.17,18
To date, the utility of FR+CTC in the elderly cancer population has not been systematically studied. Herein, we sought to explore the feasibility of applying FR+CTC in cancer diagnosis in the elderly population and the influence of comorbidities on FR+CTC levels. Building on those previous studies,11–16 we conducted this single-center, cross-sectional clinical trial.
Material and methods
Study design
From September 2017 to June 2018, 75 subjects aged between 80 and 110 were enrolled in this clinical trial conducted at China–Japan Friendship Hospital. In the study cohort, 35 patients were diagnosed with cancers (lung cancers, colorectal cancers, prostate cancers, pancreatic cancer, and lymphoma) and the other 40 were noncancer participants and had no cancer history. Cancer patients did not undergo any anticancer therapy before sample collection. The diagnosis of cancer was based on pathologic assessment, computed tomography (CT) imaging features, and clinical laboratory test. Major comorbidities included respiratory diseases (pneumonia, bronchitis, and chronic obstructive pulmonary disease), cardiovascular diseases (coronary heart diseases, heart failure, and hypertension), diabetes, and cerebral infarction.
The exclusion criteria included: 1) patients with white blood cell count >1.2×107/mL or <2×106/mL (as extreme white blood cell count could affect the enumeration of CTCs); 2) patients who took folic acid tablets long term stopped taking <3 days (as high folic acid concentration has reported lowering the expression of FR).19
The clinical trial has been approved by the Research Ethics Committee of China–Japan Friendship Hospital and was carried out in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent before inclusion into this study.
Demographic information (gender and age) and clinical factors (cancer, cardiovascular disease, diabetes, respiratory disease, and so on) were collected after admission. All participants were required to fast overnight before blood collection.
CTC enrichment and labeling
FR+CTC analysis was performed using CytoploRare® kit provided by GenoSaber Biotech Co. Ltd. (Nantong, China).14 Three milliliters of whole blood sample was collected into anticoagulant tubes from each participating subject before treatment. Samples were stored at 4–8°C and analyzed within 24 hrs. According to the manufacturer’s instruction, CTCs were negatively enriched where erythrocytes were lyzed by lysis buffer and leukocytes were depleted using anti-CD45 immunomagnetic beads. Next, CTCs were incubated with a proprietary probe which consists of conjugates of a folic acid and a synthesized oligonucleotide. Then, the unbound probes were washed off. The bound probes were removed with stripping buffer and collected by centrifugation and neutralized.
FR+CTC quantification
FR+CTC was quantified by qPCR analysis.14 First, the probe was annealed and extended before amplification. Then, the extended probe was analyzed using a Taqman probe on ABI StepOneTM system (ThermoFisher, Waltham, MA, USA). The primer sequences and reaction conditions were as described by Lou et al.14 “CTC unit” in this study represents the number of CTCs detected in 3 mL of blood, for example, 10 CTC units stands for 10 CTCs in 3 mL of blood. It should be noted that as a PCR-based CTC detection method, background noise could lead to the virtual positive value which did not exactly represent the presence of CTCs. Only FR+CTC levels higher than the determined cutoff threshold are considered to be positive for FR+CTC.
A serial of standards containing oligonucleotides ranging from 10−14 to 10−9 M is used for CTC quantification, which corresponds to 2–2×105 CTC U/3 mL blood. Samples from each patient were tested in duplicates with 6 standards and 3 quality controls.
Tumor biomarker tests
Three milliliters of coagulated blood samples was obtained from each participating subject. Serum was collected by centrifugation and tumor biomarkers (including CA125 [cancer antigen 125], CA199 [cancer antigen 19-9], CEA [carcinomaembryonic antigen], CYFRA21-1 [cytokeratin 19-fragments], CA724 [cancer antigen 724], and NSE [neuron-specific enolase]) were tested by chemiluminescence method (Roche, Rotkreuz, Switzerland).
Routine blood examinations
The routine blood examinations, plasma B-type natriuretic peptide (BNP) levels, and biochemical markers were tested with an automatic clinical chemistry analyzer (Sysmex, Beckman DXL800 and SIEMENS ADVIA2400, Hyogo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) and Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Wilcoxon test, Mann–Whitney test, and Kruskal–Wallis test were used for comparison between groups, as appropriate. P<0.05 was considered to indicate a statistically significant difference. Receiver operating characteristic (ROC) curve was used to examine the diagnostic efficiency, revealed by the area under the ROC curves (AUC). Youden index was used to determine the optimal cutoff threshold. Logistic regression was used to establish a joint diagnostic model of different biomarkers for cancer diagnosis. Pearson’s correlation was used to analyze the association between FR+CTC levels and clinical factors.
Results
Patients’ characteristics
Seventy-five participants were recruited into this single-center clinical trial, in which 23 patients had lung cancers, 8 patients had colorectal cancers, 2 patients had prostate cancers, 1 patient had pancreatic cancer, and 1 patient had lymphoma. The remaining 40 noncancer participants served as controls. The clinical characteristics of the participants are presented in Table 1. Patient demographics were similar for the cancer and the noncancer groups.
Table 1.
Patients’ characteristics
| Lung cancer (n=23) |
Colorectal cancer (n=8) | Prostate cancer (n=2) | Pancreatic cancer (n=1) | Lymphoma (n=1) | Noncancer (n=40) | P-value | |
|---|---|---|---|---|---|---|---|
| Gender | 1.0000 | ||||||
| Male | 17 | 5 | 2 | 0 | 1 | 29 | |
| Female | 6 | 3 | 0 | 1 | 0 | 11 | |
| Age, years | 0.0514 | ||||||
| Median | 85 | 87.5 | 86.5 | 83 | 80 | 89.5 | |
| Range | 80–98 | 80–98 | 83–90 | / | / | 80–110 | |
| Biomarker expression level, median (IQR) | |||||||
| FR+CTC, CTC U/3 mL | 14.1 (11.8–18.4) |
18.8 (9.7–22.8) |
12.1 (4.6–19.6) |
8.8 | 16.1 | 9.2 (7.1–12.5) |
0.0002 |
| CA125, U/mL | 68.9 (27.7–167.2) |
14.7 (8.2–67.9) |
67.1 (8.2–126.0) |
135.7 | 28.2 | 16.8 (8.3–33.2) |
0.0028 |
| CA199, U/mL | 18.8 (8.7–30.6) |
25.4 (14.6–87.7) |
109.2 (12.4–206.0) |
239.7 | 14.2 | 15.4 (9.8–23.8) |
0.1284 |
| CEA, ng/mL | 5.2 (2.6–13.3) |
4.2 (3.5–7.6) |
12.7 (2.2–23.4) |
6.3 | 2.5 | 4.2 (2.5–5.5) |
0.1257 |
| CYFRA21-1, ng/mL | 5.6 (5.0–25.4) |
3.2 (2.2–5.8) |
5.7 (1.4–10.0) |
3.1 | ND | 3.3 (2.5–5.5) |
0.0102 |
| CA724, U/mL | 2.9 (0.9–8.8) |
2.6 (1.0–8.5) |
1.5 | 1.1 | ND | 1.9 (0.9–3.5) |
0.2024 |
| NSE, ng/mL | 15.0 (11.0–26.0) |
12.7 (10.1–14.0) |
15.4 (15.0–15.8) |
16.9 | ND | 10.2 (8.4–12.2) |
<0.0001 |
Notes: CYFRA21-1, CA724, and NSE tests had not performed for the lymphoma patient. P-value: comparison between the cancer group and the noncancer group.
Abbreviation: IQR, interquartile range; FR+CTC, folate receptor-positive circulating tumor cell; CTC U, circulating tumor cell unit; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CYFRA21-1, cytokeratin 19 fragment antigen; NSE, neuron-specific enolase.
FR+CTC levels in elderly population
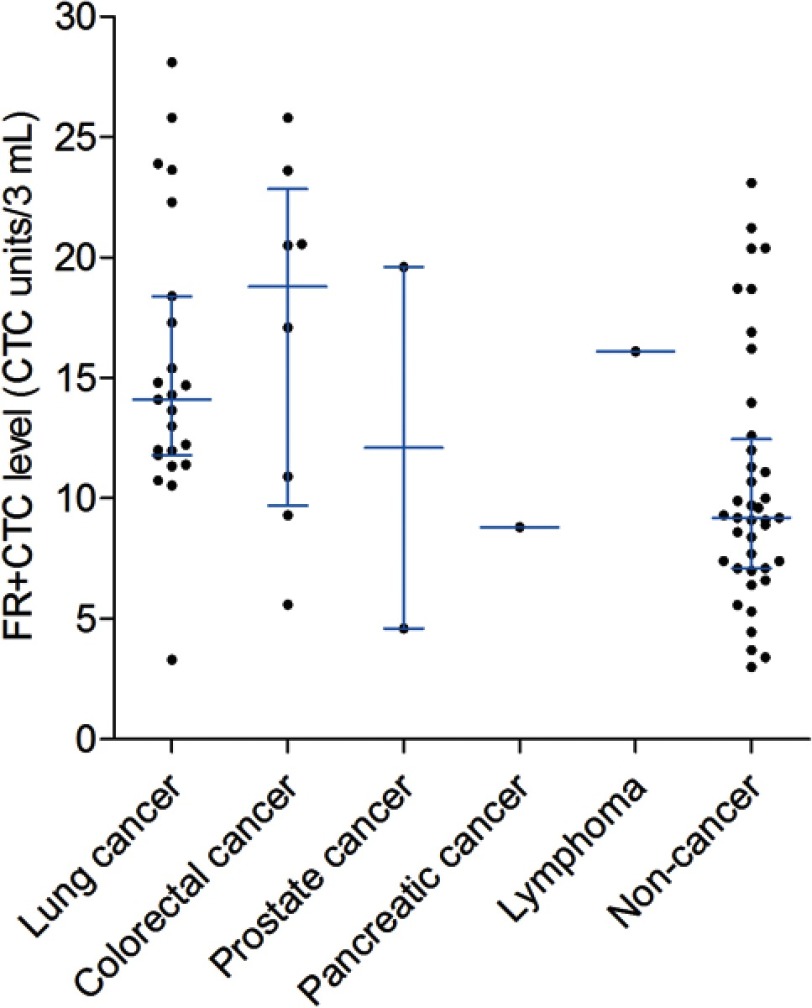
We compared the FR+CTC levels between patients with lung cancers, colorectal cancers, all cancers, and noncancer participants (Figure 1). As shown in Table 1, the FR+CTC levels in lung cancer patients (median 14.1 CTC U/3 mL) were significantly higher than that of noncancer participants (median 9.2 CTC U/3 mL, P<0.0001). Similarly, the FR+CTC levels in colorectal cancer patients (median 18.8 CTC U/3 mL, P<0.0001) and all cancer patients (median 14.3 CTC U/3 mL, P<0.0001) were significantly higher than that of noncancer participants.
Figure 1.
FR+CTC levels. The box-and-whisker plot showing the median and IQR of FR+CTC levels in patients with lung cancers, colorectal cancers, prostate cancers, pancreatic cancers, lymphoma, and noncancer participants.
Abbreviation: FR+CTC, folate receptor-positive circulating tumor cell; IQR, interquartile range.
Diagnostic value of FR+CTCs
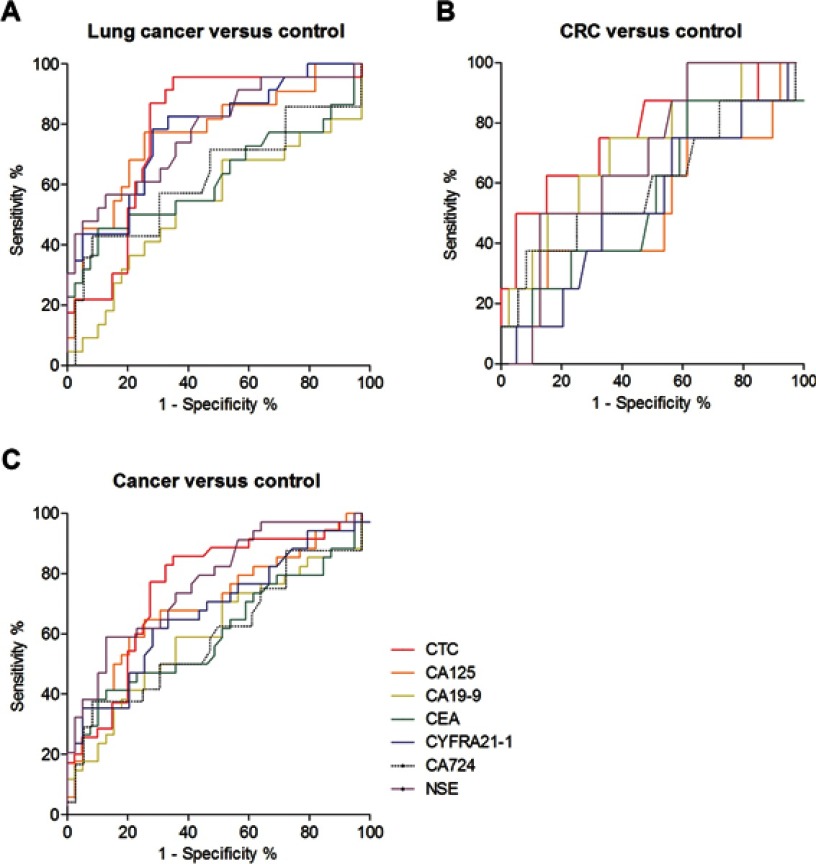
The results of ROC analysis are shown in Table 2. The AUC of FR+CTCs in differentiating lung cancers from noncancer participants was 0.7810 (95% CI: 0.6605–0.9015, Figure 2A). According to the Youden index, 10.0 CTC U/3 mL was the optimal cutoff threshold for lung cancer diagnosis, possessing a sensitivity of 95.7% and a specificity of 65.0%.
Table 2.
Comparison of the diagnostic value of FR+CTC with tumor biomarkers
| Biomarkers | AUC | 95% CI | P-value | Cutoff threshold | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Lung cancer (n=23) versus noncancer (n=40) | ||||||
| FR+CTC | 0.7810 | 0.6605–0.9015 | 0.0002 | 10.0 | 95.7% | 65.0% |
| CA125 | 0.7716 | 0.6439–0.8992 | 0.0005 | 29.9 | 77.3% | 74.4% |
| CA199 | 0.5425 | 0.3821–0.7029 | 0.5836 | 17.9 | 54.6% | 64.1% |
| CEA | 0.6247 | 0.4626–0.7868 | 0.1081 | 7.3 | 45.5% | 89.7% |
| CYFRA21-1 | 0.7770 | 0.6566–0.8975 | 0.0003 | 4.8 | 78.3% | 71.8% |
| CA724 | 0.6280 | 0.4345–0.8214 | 0.1635 | 6.0 | 42.9% | 91.7% |
| NSE | 0.7770 | 0.6537–0.9003 | 0.0003 | 13.4 | 56.5% | 87.2% |
| Colorectal cancer (n=8) vs noncancer (n=40) | ||||||
| FR+CTC | 0.7641 | 0.5596–0.9685 | 0.0195 | 17.0 | 62.5% | 85.0% |
| CA125 | 0.5160 | 0.2775–0.7545 | 0.8875 | 56.4 | 37.5% | 84.6% |
| CA199 | 0.7179 | 0.5194–0.9165 | 0.0543 | 18.3 | 75.0% | 64.1% |
| CEA | 0.5593 | 0.3306–0.7880 | 0.6005 | 3.2 | 87.5% | 38.5% |
| CYFRA21-1 | 0.5369 | 0.3160–0.7577 | 0.7448 | 4.0 | 75.0% | 43.6% |
| CA724 | 0.6007 | 0.3556–0.8458 | 0.3776 | 5.8 | 37.5% | 91.7% |
| NSE | 0.6907 | 0.5155–0.8659 | 0.0922 | 9.5 | 100% | 38.5% |
| All cancers (n=34) vs noncancer (n=40) | ||||||
| FR+CTC | 0.7507 | 0.6367–0.8647 | 0.0002 | 10.0 | 85.7% | 65.0% |
| CA125 | 0.7036 | 0.5812–0.8260 | 0.0028 | 29.9 | 64.7% | 74.4% |
| CA199 | 0.6037 | 0.4708–0.7366 | 0.1284 | 17.9 | 58.8% | 64.1% |
| CEA | 0.6044 | 0.4698–0.7391 | 0.1257 | 6.3 | 41.2% | 87.2% |
| CYFRA21-1 | 0.6753 | 0.5501–0.8006 | 0.0102 | 4.8 | 61.8% | 71.8% |
| CA724 | 0.5978 | 0.4439–0.7517 | 0.2024 | 5.8 | 37.5% | 91.7% |
| NSE | 0.7711 | 0.6639–0.8783 | <0.0001 | 13.2 | 58.8% | 87.2% |
Notes: CYFRA21-1, CA724, and NSE tests had not performed for the lymphoma patient; thus, this patient was excluded from this analysis.
Abbreviation: AUC, area uner the curve; CI, confidence interval; FR+CTC, folate receptor-positive circulating tumor cell; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CYFRA21-1, cytokeratin 19 fragment antigen; NSE, neuron-specific enolase.
Figure 2.
Diagnostic value of FR+CTCs. ROC curve for FR+CTC, CA125, CA199, CEA, CYFRA21-1, CA724, and NSE in discriminating patients with noncancer participants from patients with (A) lung cancers; (B) colorectal cancers; and (C) all cancers.
Notes: Red line indicates FR+CTC; orange line indicates CA125; yellow line indicates CA19-9; green line indicates CEA; blue line indicates CYFRA21-1; dotted black line indicates CA724; purple line indicates NSE.
Abbreviations: CRC, colorectal cancer; FR+CTC, folate receptor-positive circulating tumor cell; ROC, receiver operating characteristic; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CYFRA21-1, cytokeratin 19 fragment antigen; NSE, neuron-specific enolase.
For colorectal cancer, the AUC for FR+CTCs was 0.7641 (95% CI: 0.5596–0.9685, Figure 2B). With 17.0 CTC U/3 mL as the cutoff threshold, the sensitivity and specificity were 62.5% and 85.0%, respectively.
When all cancer patients were considered in the analysis, the AUC for FR+CTCs was 0.7507 (95% CI: 0.6367–0.8647, Figure 2C). With 10.0 CTC U/3 mL as the cutoff threshold, the sensitivity and specificity were 85.7% and 65.0%, respectively.
Comparison of the diagnostic value between FR+CTC and tumor biomarkers
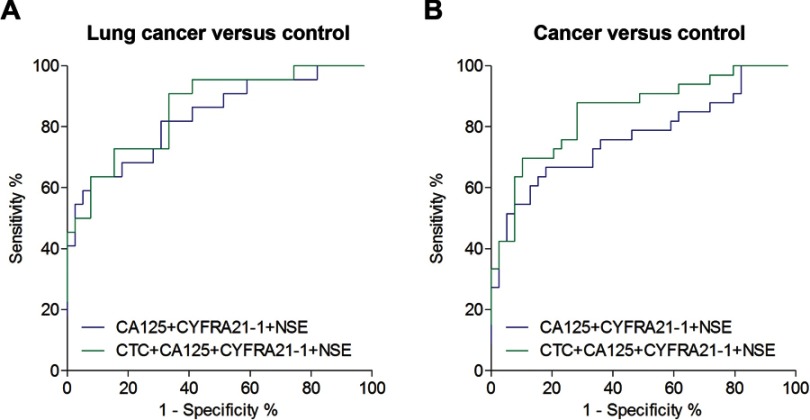
Compared to routine tumor biomarkers (CA125, CA199, CEA, CYFRA21-1, CA724, and NSE), the FR+CTC levels exhibited the best performance, in terms of AUC, in differentiating patients with lung cancer and colorectal cancer from noncancer participants (Table 2). Meanwhile, for differentiating cancers from noncancer participants, FR+CTC showed a similar AUC to NSE (Table 2). The diagnostic efficiency (in terms of AUC) further enhanced when FR+CTC was combined with CA125, CYFRA21-1, and NSE (Table 3, Figure 3). These results suggested that a comprehensive analysis of several biomarkers could significantly improve cancer diagnosis.
Table 3.
Diagnostic value of FR+CTC combined with established tumor biomarkers
| AUC | 95% CI | P-value | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Lung cancer (n=23) vs noncancer (n=40) | |||||
| CA125 + CYFRA21-1 + NSE | 0.8357 | 0.7266–0.9448 | <0.0001 | 63.6% | 92.3% |
| FR+CTC + CA125 + CYFRA21-1 + NSE | 0.8613 | 0.7652–0.9574 | <0.0001 | 90.9% | 66.7% |
| All cancers (n=34) vs noncancer (n=40) | |||||
| CA125 + CYFRA21-1 + NSE | 0.7692 | 0.6558–0.8827 | <0.0001 | 66.7% | 82.1% |
| FR+CTC + CA125 + CYFRA21-1 + NSE | 0.8485 | 0.7583–0.9387 | <0.0001 | 87.9% | 71.8% |
Notes: CYFRA21-1, CA724, and NSE tests had not performed for the lymphoma patient; thus, this patient was excluded from this analysis.
Abbreviation: FR+CTC, folate receptor-positive circulating tumor cell; AUC, area uner the curve; CI, confidence interval; CA, carbohydrate antigen; CYFRA21-1, cytokeratin 19 fragment antigen; NSE, neuron-specific enolase.
Figure 3.
Diagnostic value of FR+CTC combined with tumor marker. ROC curve for FR+CTC combined with tumor marker in discriminating noncancer participants from patients with (A) lung cancers and (B) all cancers. CYFRA21-1, CA724, and NSE tests had not performed for the lymphoma patient; thus, this patient was excluded from this analysis.
Abbreviation: FR+CTC, folate receptor-positive circulating tumor cell; CA, carbohydrate antigen; CYFRA21-1, cytokeratin 19 fragment antigen; NSE, neuron-specific enolase; ROC, receiver operating characteristic.
Clinical factors associated with FR+CTC levels
Comorbidities commonly found in the elderly population including diabetes, cardiovascular diseases (coronary heart disease, hypertension, heart failure, arrhythmia, and dyslipidemia), respiratory diseases (pulmonary infection, chronic obstructive pulmonary disease, asthma, and pulmonary interstitial fibrosis), and cerebral infarction and clinical factors including blood routine examination, BNP, and liver/kidney functions were not significantly associated with FR+CTC levels (P>0.05, Table 4), except for β2-microglobulin. Participants with a normal β2-microglobulin level showed a significantly lower FR+CTC level compared to those with β2-microglobulin level >3 mg/mL (P=0.0045). In addition, participants with diabetes and normal albumin level showed a trend of lower FR+CTC levels compared to those without diabetes and albumin level <35 g/L, but the differences were not statistically significant (P=0.0631 and 0.0645, respectively). In agreement with previous reports,11–14 metastatic cancer patients (n=19) also showed a trend of higher FR+CTC levels compared to nonmetastatic cancer patients (n=16). The difference was not statistically significant (15.1 vs 13.0 CTC U/3 mL, P=0.9868), probably due to the small sample size.
Table 4.
FR+CTC levels according to clinical factors
| Clinical factors | Number. of participants (%) | CTC level, CTC U/3 mL median (IQR) |
P-value |
|---|---|---|---|
| Diabetes | 0.0631 | ||
| Present | 21 (28) | 9.1 (5.5–17.4) | |
| Absent | 54 (72) | 12.0 (9.3–17.2) | |
| Cardiovascular diseases | 0.7418 | ||
| Present | 64 (85) | 11.3 (8.7–17.1) | |
| Absent | 11 (15) | 13.0 (7.1–17.3) | |
| Respiratory diseases | 0.8361 | ||
| Present | 52 (69) | 11.4 (8.5–16.2) | |
| Absent | 23 (31) | 10.5 (8.6–18.4) | |
| Cerebral infarction | 0.7334 | ||
| Present | 34 (45) | 11.0 (8.6–17.5) | |
| Absent | 41 (55) | 11.8 (8.3–17.1) | |
| White blood cell count, ×109/L | 0.8927 | ||
| Low (<3.5) | 3 (4) | 12.2 (9.9–16.9) | |
| Normal (3.5–9.5) | 53 (71) | 11.1 (8.5–17.9) | |
| High (>9.5) | 19 (25) | 11.8 (7.7–16.2) | |
| Red blood cell count, ×109/L | 0.1798 | ||
| Low (<110) | 27 (36) | 14.7 (8.4–20.6) | |
| Normal (110–175) | 47 (63) | 10.5 (8.6–14.3) | |
| High (>175) | 1 (1) | 11.3 | |
| Platelet count, ×109/L | 0.7508 | ||
| Low (<125) | 9 (12) | 12.2 (8.5–18.0) | |
| Normal (125–350) | 59 (79) | 11.1 (8.4–16.2) | |
| High (>350) | 7 (9) | 11.4 (9.7–17.1) | |
| Total protein, g/L | 0.1923 | ||
| Low (<60) | 24 (33) | 12.4 (8.5–20.4) | |
| Normal (60–80) | 49 (66) | 10.7 (8.0–14.6) | |
| High (>80) | 1 (1) | 14.7 | |
| Albumin, g/L | 0.0645 | ||
| Low (<35) | 25 (33) | 12.0 (10.7–19.2) | |
| Normal (35–55) | 50 (67) | 9.9 (7.3–16.1) | |
| High (>55) | 0 | ||
| TBIL, μmol/L | 0.4466 | ||
| Low (<5) | 6 (8) | 10.7 (8.2–17.9) | |
| Normal (5–21) | 65 (87) | 11.8 (8.5–17.9) | |
| High (>21) | 4 (5) | 9.5 (4.8–11.4) | |
| DBIL, μmol/L | 0.9436 | ||
| Normal (<7) | 71 (95) | 11.3 (8.6–17.1) | |
| High (≥7) | 4 (5) | 10.8 (8.7–17.0) | |
| ALT, U/L | 0.1789 | ||
| Normal (<40) | 71 (95) | 11.1 (8.4–16.9) | |
| High (≥40) | 4 (5) | 15.7 (12.3–19.3) | |
| AST, U/L | |||
| Normal (<42) | 73 (97) | 11.3 (8.5–17.0) | |
| High (≥42) | 2 (3) | 15.8 (12.0–19.6) | |
| ALP, U/L | 0.4875 | ||
| Low (<40) | 1 (1) | 20.4 | |
| Normal (40–150) | 70 (95) | 11.2 (8.6–16.4) | |
| High (>150) | 3 (4) | 12.0 (5.3–17.3) | |
| GGT, U/L | 0.3884 | ||
| Normal (<52) | 60 (81) | 11.1 (8.7–15.9) | |
| High (≥52) | 14 (19) | 13.9 (8.1–19.1) | |
| BUN, mmol/L | 0.9433 | ||
| Low (<2.78) | 3 (4) | 12.2 (8.8–16.1) | |
| Normal (2.78–7.85) | 43 (57) | 11.3 (9.1–17.1) | |
| High (>7.85) | 29 (39) | 11.4 (6.9–18.0) | |
| SCr, μmol/L | 0.1592 | ||
| Low (<35) | 1 (1) | 28.1 | |
| Normal (35–106) | 62 (83) | 11.3 (9.1–16.4) | |
| High (>106) | 12 (16) | 9.8 (4.8–20.0) | |
| β2-microglobin, mg/mL | 0.0045 | ||
| Low (<1) | 0 | ||
| Normal (1–3) | 39 (59) | 9.3 (7.4–13.7) | |
| High (>3) | 27 (41) | 14.7 (10.7–18.7) | |
| BNP, pg/mL | 0.1605 | ||
| Normal (<100) | 14 (21) | 9.9 (6.9–16.2) | |
| High (≥100) | 53 (79) | 12.0 (9.1–17.2) |
Abbreviations: TBIL, total bilirubin; DBIL, direct bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; BUN, blood urea nitrogen; SCr, serum creatinine; BNP, B-type natriuretic peptide.
Next, Pearson’s correlation analysis was carried out to further examine the correlation between those clinical factors with FR+CTC level. Results demonstrated that none of the above-mentioned factors were correlated with the FR+CTC level (P>0.05), except for cancer (Pearson’s coefficient=0.431, P<0.001, Table 5). In addition, total protein level and β2-microglobulin level showed a slight correlation with FR+CTC level, but the correlations were not statistically significant (P=0.080 and 0.071, respectively).
Table 5.
The association of FR+CTC level with different clinical factors
| Clinical factors | Number of (tested) participants | Pearson’s coefficient | P-value |
|---|---|---|---|
| Cancers | 35 with cancer 40 without cancer |
0.431 | <0.001 |
| Diabetes | 21 with diabetes 54 without diabetes |
−0.148 | 0.205 |
| Cardiovascular diseases | 64 with cardiovascular disease 11 without cardiovascular disease |
−0.046 | 0.696 |
| Respiratory diseases | 52 with respiratory disease 23 without respiratory disease |
−0.056 | 0.634 |
| Cerebral infarction | 34 with cerebral infarction 41 without cerebral infarction |
−0.046 | 0.694 |
| White blood cell count | 75 | −0.031 | 0.792 |
| Red blood cell count | 75 | −0.081 | 0.491 |
| Platelet count | 75 | −0.041 | 0.728 |
| Total protein | 74 | −0.205 | 0.080 |
| Albumin | 75 | −0.106 | 0.364 |
| TBIL | 75 | −0.031 | 0.790 |
| DBIL | 75 | 0.113 | 0.333 |
| ALT | 75 | 0.132 | 0.260 |
| AST | 75 | 0.153 | 0.189 |
| ALP | 74 | −0.054 | 0.647 |
| GGT | 74 | 0.102 | 0.389 |
| BUN | 75 | 0.007 | 0.955 |
| SCr | 75 | −0.082 | 0.486 |
| β2-microglobin | 66 | 0.224 | 0.071 |
| BNP | 67 | 0.108 | 0.386 |
Abbreviations: TBIL, total bilirubin; DBIL, direct bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, γ-glutamyl transpeptidase; BUN, blood urea nitrogen; SCr, serum creatinine; BNP, B-type natriuretic peptide.
Discussion
In the current study, the FR+CTC level was slightly higher compared to previous studies.11–16 The median FR+CTC levels in cancer patients and noncancer participants were 14.3 and 9.2 CTC U/3 mL, respectively. A possible explanation is that long-term folate deficiency in the elderly population leads to the overexpression of FR in cancer tissues, which subsequently prompts the increase in FR+CTC levels.19,20 In addition, the decrease in CD45 expression in leukocytes of the elderly population may cause the incomplete depletion of leukocytes during the negative enrichment process,21 resulting in a relative increase in FR+CTC levels due to nonspecific binding of the ligand–oligonucleotide conjugates to leukocytes. Nevertheless, the diagnostic efficiency was not affected in the elderly population. In ROC analysis, the AUC of FR+CTC was the highest compared to other tumor biomarkers in the diagnosis of lung cancer and colorectal cancer. Similar to the previous studies,11,13 combining FR+CTC with established tumor biomarkers further improved the diagnostic efficiency in terms of AUC. We also observed a higher FR+CTC level in colorectal cancers (median=18.8 CTC U/3 mL) compared to other cancer types. This is in concordance with two previous studies using microfluidic techniques to isolate CTCs in colorectal cancers.22,23
According to a study in Spain, the mortality rate of very old patients (≥90 years) is double that of the younger elderly.24 Cancer is one of the major complications in the elderly population that increased the mortality rate (odds ratio: 1.60). Although elderly patients bear higher risk in surgical treatment, a significant portion of them show a long-term survival.2 Earlier detection of cancer can benefit elderly patients from early treatment of localized cancer compared to advanced stages.25 Moreover, the elderly population has a higher prevalence of other diseases, approximately 80% of them having three or more chronic conditions.26 Although the performance status of these patients is poor, detection of cancer could help manage such patients through introducing palliative care as required and assist in clinical decision-making.1
Comorbidity was reported to associate with increased overall mortality in elderly cancer patients, especially for colorectal and lung cancer which was found to have an increased comorbidity burden.27 To explore the influence of these comorbidities to the FR+CTC enumeration, we compared the FR+CTC levels of patients with certain comorbidity to that without comorbidity. The correlation of FR+CTC levels with clinical factors such as blood routine examination, BNP, and liver/kidney functions was also analyzed. Our results demonstrated that the FR+CTC levels were not associated with these comorbidities and clinical factors (P>0.05 according to Pearson’s correlation analysis). Hence, the diagnostic efficiency of FR+CTC would not be affected by the presence of comorbidities or other clinical abnormalities in the elderly population.
Notably, some clinical factors indeed showed a slight correlation with the FR+CTC level. The level of β2-microglobin showed weak positive correlation with the FR+CTC level. β2-microglobulin level was reported to be increased in various types of cancers, possibly due to the active synthesis and increased cell breakdown of the tumor cells.28,29 As the presence of cancer is strongly associated with the FR+CTC level, β2-microglobulin level is indirectly associated with the FR+CTC level. For the level of albumin and total protein, a weak negative correlation with the FR+CTC level was observed. This could be explained by the poor nutrition status of cancer patients. Indeed, this is in agreement with a previous study from Samanta et al which suggested that cancer patients had a significantly lower level of albumin and total protein.30
To the best of our knowledge, there is yet no research reporting the expression pattern of FR+CTC in elderly cancer patients. The ligand-targeted PCR-based detection of FR+CTC as a tumor marker is a fairly innovative way to diagnose cancer in the elderly population. To further confirm the clinical significance of this novel method, additional, larger studies are required.
Conclusion
In conclusion, FR+CTC showed a satisfactory overall performance in this exploratory study and could be used as a promising noninvasive diagnostic method for elderly cancer patients. Its high sensitivity makes it an effective tool to be applied in elderly cancer screening.
Acknowledgments
This work was supported by the transverse project of the China–Japan Friendship Hospital. We thank GenoSaber Biotech Co. Ltd. for providing support.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Martín-Lesende I, Recalde E, Viyiane-Wunderling P, et al. Mortality in a cohort of complex patients with chronic illnesses and multimorbidity: a descriptive longitudinal study. BMC Palliat Care. 2016;15:42. doi: 10.1186/s12904-016-0111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolton WD, Rice DC, Correa AM, et al. Influence of age on choice of therapy and surgical outcomes in patients with nonsmall cell lung cancer. Am Surg. 2009;75:598–603. [PubMed] [Google Scholar]
- 3.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosseini H, Obradović MM, Hoffmann M, et al. Early dissemination seeds metastasis in breast cancer. Nature. 2016;540:552–558. doi: 10.1038/nature20785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014 [DOI] [PubMed] [Google Scholar]
- 6.Mateo J, Gerlinger M, Rodrigues DN, de Bono JS. The promise of circulating tumor cell analysis in cancer management. Genome Biol. 2014;15:448. doi: 10.1186/s13059-014-0448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas A, Maltzman J, Hassan R. Farletuzumab in lung cancer. Lung Cancer. 2013;80:15–18. doi: 10.1016/j.lungcan.2012.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W, Kularatne SA, Kalli KR, et al. Quantitation of circulating tumor cells inblood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int J Cancer. 2008;123:1968–1973. doi: 10.1002/ijc.23717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy JA, Haneline LS, Srour EF, et al. Expression and functional characterization of the beta-isoform of the folate receptor on CD34(+) cells. Blood. 1999;93:3940–3948. [PubMed] [Google Scholar]
- 10.Nunez MI, Behrens C, Woods DM, et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol. 2012;7:833–840. doi: 10.1097/JTO.0b013e31824de09c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Zhou F, Li X, et al. Folate receptor-positive circulating tumor cell detected by LT-PCR based method as a diagnostic biomarker for non-small cell lung cancer. J Thorac Oncol. 2015;10:1163–1171. doi: 10.1097/JTO.0000000000000606 [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Chen Z, Dong J, et al. Receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol. 2013;6:697–702. doi: 10.1593/tlo.13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Wu C, Qiao L, et al. Clinical significance of folate receptor-positive circulating tumor cells detected by ligand-targeted polymerase chain reaction in lung cancer. J Cancer. 2017;8:104–110. doi: 10.7150/jca.16856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou JT, Ben SQ, Yang GH, et al. Quantification of rare circulating tumor cells in non-small cell lung cancer by ligand-targeted PCR. PLoS One. 2013;8:e80458. doi: 10.1371/journal.pone.0080458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi F, Liu Y, Zhao R, et al. Quantitation of rare circulating tumor cells by folate receptor α ligand-targeted PCR in bladder transitional cell carcinoma and its potential diagnostic significance. Tumor Biol. 2014;35:7217–7223. doi: 10.1007/s13277-014-1894-0 [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Zhao J, Jiang T, et al. Predictive and prognostic value of folate receptor-positive circulating tumor cells in small cell lung cancer patients treated with first-line chemotherapy. Oncotarget. 2017;8:49044–49052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Wang J, Tacha DE, et al. Folate receptor α associated with triple-negative breast cancer and poor prognosis. Arch Pathol Lab Med. 2014;138:890–895. doi: 10.5858/arpa.2013-0455-OA [DOI] [PubMed] [Google Scholar]
- 19.Reynolds EH. Folic acid, ageing, depression, and dementia. BMJ. 2002;324:1512–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu WY, Alliegro MA, Melera PW. The rate of folate receptor alpha (FR alpha) synthesis in folate depleted CHL cells is regulated by a translational mechanism sensitive to media folate levels, while stable overexpression of its mRNA is mediated by gene amplification and an increase in transcript half-life. J Cell Biochem. 2001;81:205–219. [DOI] [PubMed] [Google Scholar]
- 21.Kudlacek S, Jahandideh-Kazempour S, Graninger W, Willvonseder R, Pietschmann P. Differential expression of various T cell surface markers in young and elderly subjects. Immunobiology. 1995;192:198–204. doi: 10.1016/S0171-2985(11)80097-5 [DOI] [PubMed] [Google Scholar]
- 22.Tsai WS, Chen JS, Shao HJ, et al. Circulating tumor cell count correlates with colorectal neoplasm progression and is a prognostic marker for distant metastasis in non-metastatic patients. Sci Rep. 2016;6:24517. doi: 10.1038/srep24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh BY, Kim J, Lee WY, Kim HC. A new size-based platform for circulating tumor cell detection in colorectal cancer patients. Clin Colorectal Cancer. 2017;16:214–219. doi: 10.1016/j.clcc.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 24.Barba R, Martínez JM, Zapatero A, et al. Mortality and complications in very old patients (90+) admitted to departments of internal medicine in Spain. Eur J Intern Med. 2011;22:49–52. doi: 10.1016/j.ejim.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 25.Kotwal AA, Schonberg MA. Cancer screening in the elderly: a review of breast, colorectal, lung, and prostate cancer. Cancer J. 2017;23:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorica F, Stefanelli A, Fisichella R, et al. Comorbidity assessment and radiotherapy in elderly cancer patients. Eur Rev Med Pharmacol Sci. 2012;16:1605–1606. [PubMed] [Google Scholar]
- 27.Jørgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;106:1353–1360. doi: 10.1038/bjc.2012.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiou SJ, Chen CH. Decipher β2-microglobin: gain- or loss-of-function (a mini-review). Med Sci Monit Basic Res. 2013;19:271–273. doi: 10.12659/MSMBR.889457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silvia CR, Vasudevan DM, Prabhu KS. Alteration of serum beta 2-microglobulin in oral carcinoma. Indian J Clin Biochem. 2002;17:104–107. doi: 10.1007/BF02867981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samanta S, Sharma A, Das B, et al. Significance of total protein, albumin, globulin, serum effusion albumin gradient and LDH in the differential diagnosis of pleural effusion secondary to tuberculosis and cancer. J Clin Diagn Res. 2016;10:BC14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]