Abstract
Circular RNAs (CircRNAs), the endogenous long noncoding RNAs, unlike linear RNAs, are structurally continuous, covalently closed loops without 5’ cap or 3’ polyadenylated tail. High-throughput RNA sequencing has enabled the discovery of several endogenous circRNAs in different species and tissues. The circRNAs mainly act as sponges to cytoplasmic microRNA, aid in protein translation, or interact with RNA-binding proteins to generate RNA-protein complexes which control transcription. Recently, circRNAs have been reported to participate in cancer pathogenesis, particularly tumor metastasis in humans, mainly due to their frequent aberrant expression in cancers. However, the detail molecular mechanism of circRNAs activity in tumor metastasis is still elusive. Some specifically expressed circRNAs can potentially be used as biomarkers and therapeutic targets for tumor treatment. Further understanding of the network interactions and regulation of circRNAs is paving the way for the identification of better therapeutic strategies in tumor metastasis. In this mini review, we have summarized the current state of research on functions and mechanisms of novel circRNAs that regulate tumorigenesis and have evaluated the relationship between dysregulation of circRNAs and tumor metastasis.
Keywords: Circular RNAs, tumor metastasis, back-splicing, epithelial-mesenchymal transition, exosomes, DNA demethylation
Introduction
A sub class of noncoding RNAs, the circular RNAs (CircRNAs), form a continuous, covalently closed loop linking the 3’ and 5’ ends generated by back-splicing [1]. CircRNAs were first discovered in viruses in 1976 [2] and were subsequently observed through an electron microscope in the monkey renal CV-1 cytoplasm [3]. However, circRNAs have remained an understudied and neglected junk-RNA until several years ago. The high-throughput RNA sequencing technique and bioinformatic tools have provided several millions of short RNA sequences, have enabled efficient measurement of circRNAs in various organisms [4]. These circRNAs have been recognized as a newly appreciated class of non-coding RNAs. In humans, the first circRNAs were discovered by Cocquerelle et al. in 1993 [5] localized in the cytoplasm. Due to the closed structure, circRNAs have been shown to be highly stable [6]. Because of the stability and specificity of circRNAs [7], they can act as highly accurate diagnostic biomarkers of human disease.
Cancer is an emerging public health problem globally and remains a major economic and social burden and a major cause of mortality [8]. A 2018 report states that worldwide, cancer accounted for more than 18.1 million new cases and 9.6 million deaths [9]. According to the GLOBOCAN 2018 estimate, in near future, almost one-half of cases and over one-half of the cancer deaths will occur in Asia. Additionally, tumor metastasis has a key part in the progression of cancer and has been striking the most in emerging economies. Most patients with cancer die as a result of cancer spreading to other organs. Accumulating evidence reveals that circRNAs, once thought to be a transcriptional error, play a prominent role in the cancer metastasis. While it is known the in the tissues from patients with tumor metastasis, circRNAs are highly differentially expressed, the research on the related mechanisms is still in nascent stage. In this review, we briefly summarize the latest research on circRNAs, including its functions and mechanism, with an emphasis on their role in tumor metastasis.
Classification and biogenesis of circRNAs
To offer greater insight into the functions of circRNAs, comprehending their structural features is essential. CircRNAs are generated by splicing events (Figure 1) during the maturation of the corresponding precursor mRNAs (pre-mRNA) subject to transcription by RNA polymerase II [10]. Based on the components from parental genes, circRNAs can be divided into three subclasses [11]: exonic circRNAs (ecircRNAs), circular intronic RNAs (ciRNAs) and exon-intron circRNAs (ElciRNAs).
Figure 1.
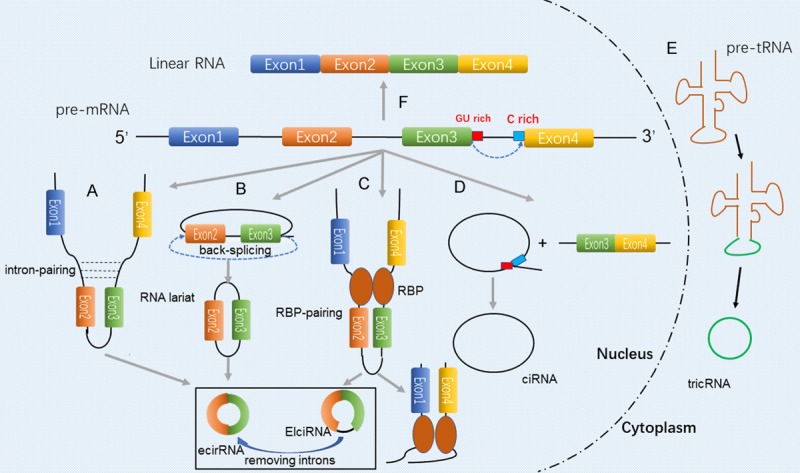
Biosynthesis of circRNAs. Various mechanisms are involved for generating different circRNAs types. A. Intron-pairing-driven circularization, involves the complementarity between the introns on either side of pre-mRNA. B. Lariat-driven circularization, involves formation of exonic circRNAs or EIciRNAs that does/does not involve intron removal with skipping of the exon along with RNA folding. C. Binding to the introns at specific sequences to influence circRNA biogenesis. D. CircRNA biogenesis involves the formation of lariat between conserved motifs at various combinations located upstream/downstream of introns. E. Formation of circular RNA (triRNA) by splicing of the pre-tRNA into two parts, with a 3’-5’ phosphodiester bond bound in one. F. Canonical pre-mRNA splicing and mRNA biogenesis.
EcircRNAs contain only back-spliced exon sequences which remain predominantly in the cytoplasm and function as miRNAs sponge or RNA-binding protein sponge. Today, the term ‘circRNA’ is commonly applied to describe EcircRNAs, in which the downstream donor-exons splice to upstream acceptor-exons [12]. There are three hypotheses that steer the process of circularization during the formation of circRNAs during back-splicing. The first mechanism is that during the formation of ecircRNAs, a partial transcription of the pre-mRNA occurs, and the exon skips with the folding of the pre-mRNA, thus forming a lariat structure in which introns are contained along with the exons in the same segment [12]. The intron sequence is then removed to form ecircRNA followed by internal splicing in the lariat structure. Barrett et al. [13] found that through RNA-Seq and qPCR, exon skipping may induce the production of circRNAs. Another theory is that the ecircRNA is formed due to the presence of a reverse sequence on the introns on either side of the pre-mRNA [14,15]. These introns have complementarity to allow base pairing that mediates the formation of ecircRNA. Later, Jeck et al. [6] showed the presence of a tandem repeat of the ALU sequence in the looped sequence, a phenomenon which is consistent with the complementary pairing of the two introns. RBP pairing of circRNA circularization has also been reported [16]. Quaking (QKI) [17], Muscleblind (MBL) [14] and adenosine deaminase that act on RNA 1 (ADAR1) [18] participate in regulating the synthesis of circRNA. While QKI and MBL are capable of enhancing, ADAR1 suppresses the generation of ecircRNA and EIciRNA respectively. Hence, subject to conditions, RBPs may function to activate or inhibit circRNA formation. Although EcircRNAs are widely present in cells, the mechanism of their formation is still in the hypothetical stage, scientists need to further study related regulation and processing methods in their formation. Nevertheless, ciRNAs are diminutively formed by intron sequences based on a consensus motif which are composed of 11 nucleotide elements rich in C adjacent to the branchpoint site and a 7 nucleotide GU-rich element close to the 5’ splice site [19]. EIciRNAs comprise both exon and intron sequences, and in some circumstances, interact with U1 snRNP. Most ciRNAs and EIciRNAs which act as alternative splicing and gene transcription are located in the nucleus [20]. In brief, the biogenesis of ElciRNAs is still unclear on account of some specific component of introns that have been not spliced out. How these factors control the ElciRNAs circulation remains to be further investigated.
The biological functions of circRNAs
CircRNAs play a key role especially in regulatory activities of human physiology and pathology. Several biological functions of circRNAs have been demonstrated, such as miRNA sponges that affect downstream target genes of miRNAs and encoding proteins, regulate the transcription of parental genes, modulate alternative splicing, interact with RBPs, and other unknown functions (Figure 2). In this review, we attempt to provide an overview of the molecular mechanisms of circRNAs in regulating various processes of biology.
Figure 2.
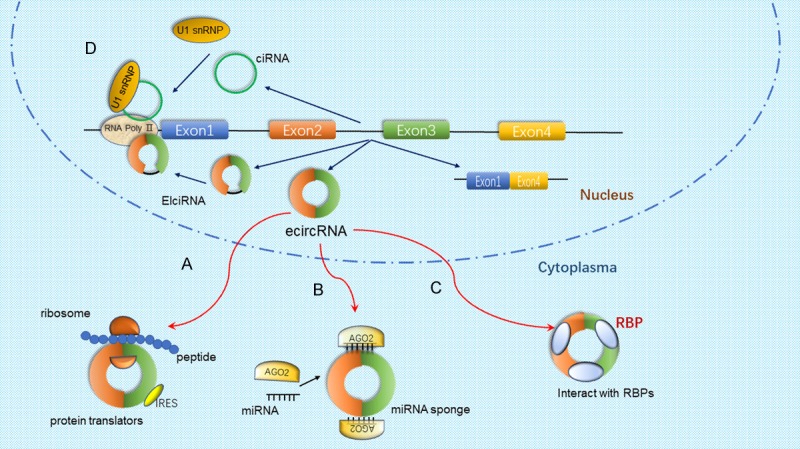
Multiple functions of circRNAs. A. A section of circRNAs are vitally involved in protein-coding. Encoding of proteins by circRNAs is also possible with an internal ribosome entry site (IRES). B. A majority of circRNAs function as microRNA (miRNA) sponges that interact with miRNA-Ago2 complexes to cause inhibition of miRNA functions. C. CircRNAs bind RBPs to form RNA-protein complexes to affect their functions and translocations. D. Transcriptional regulation. ElcircRNA and ciRNA can cause induction of transcription of parental genes by binding transcription complexes at the host promoters.
Competitive endogenous RNA or miRNA sponge
While a small number of ciRNAs cyclized by introns are predominantly localized in the nucleus, most of them are based in the cytosol. In tumor biology, the cirRNA functions like a sponge to regulate downstream target genes [21]. It has been found that most of ciRNAs carry at least one miRNA binding site. They can sequester and functionally inactivate miRNA by acting as a miRNA sponge, thereby regulating the downstream target gene expression inhibited by miRNA through the competitive endogenous RNAs (ceRNAs). The theory of competitive endogenous RNAs was proposed by Salmena et al. [22] in 2011. It was found that long non-coding RNAs, pseudogene RNAs, and circular RNAs contain a common site for binding to miRNAs. The competitive binding with miRNAs by bait or sponge allows these different types of RNA to form a network of mutual regulation that is vital for the cell. The strongest evidence for this ‘sponge’ activity came from studies of the circRNA ciRS-7. CiRS-7, a highly conserved, single-exon, also known as cerebellar degeneration-related protein 1 antisense transcript (CDR1as), which possesses more than seventy selectively conserved binding sites for miR-7. CiRS-7 can regulate miR-7 by adsorption, thereby reducing the inhibitory effect of miR-7 on its targets [21]. Overexpression of CiRS-7 can indirectly enhance the expression of epidermal growth factor receptor (EGFR) which is a downstream target of miR-7, thereby affecting cell growth, proliferation, differentiation and signal transduction [23]. Similarly, circITCH, which spans several exons of the E3 ubiquitinated protein ligase, derived from the parental gene ITCH, contains the binding site for miR-7, miR-17 and miR-214 [24]. Thus, the function of circRNAs as competitive endogenous RNA or miRNA sponge has been confirmed, but its specific biological mechanism in the development of tumors and its role in improving the tumor molecular targeted therapy by intervening in its pathway need further research.
Participation in protein translation
CircRNAs can function in encoding proteins. Since the advent of circRNA research, they have been considered as non-coding RNA, but advances in the field of high throughput RNA sequencing as well as computational tools, have led some scholars to discover that circRNA contain open reading frames that can be expressed as polypeptides or proteins [25]. CircRNAs that contain internal ribosome entry sites (IRES) can cause ribosome recruitment as well as translation initiation, while circRNAs lacking IRES do not encode proteins [26]. Zhou et al. [27] demonstrated for the first time that m6A modification is widespread in circRNAs. The methyl group which is added to the sixth N element of the A base of the RNA molecule modifies the circRNA, which can perform protein translation. Concurrently, Legnini et al. [28] hypothesized that circZNF609 may be involved in the process of muscle maturation, directly as a protein translation tool. Later, Yang et al. [29] observed that circFBXW7 can translate a novel protein that inhibits glioma, and is thus critically involved in comprehending circRNA functions and glioma development.
Regulation of transcription and alternative splicing
The expression of parental genes at the transcriptional level is regulated by circRNAs. Almost all circRNAs distributed in the cytoplasm are produced by exons. Intronic circRNAs (ciRNAs) originate from introns and cannot serve as sponges for miRNA on account of the presence of very few binding sites. Yet, maternal gene expression is augmented by ciRNAs through regulation of RNA polymerase II (RNA Pol II) [30]. Exon-intron circRNAs (EIciRNAs), as the name suggests involves both exons and introns, form a complex of EIciRNA-U1 snRNP via RNA-RNA binding to U1 small nuclear RNA (snRNA). This can regulate RNA pol II to boost parental gene expression [20]. An interaction between the RNA pol II complex and Ci-ankrd52 (originates from the second intron of the ankyrin repeat domain 52, ankrd52) enhances parental gene expression [31]. It is speculated that EcircRNA can modulate the cytoplasm, while ElciRNA and ciRNA may be involved in the regulation of parental genes in the nucleus. Furthermore, the cleavage efficiency of certain genes is inversely related to the levels of circRNA, suggesting that back spliced circRNAs can competitively regulate alternative splicing. The circMBL is produced by the second exon of the cleavage factor MBL, and that there are many MBL protein binding sites on the flanking intron, which can specifically bind to MBL protein [17]. The complex promotes the production of circMBL, thereby competitively inhibiting classical splicing.
Interaction with RBPs
RNA-binding proteins (RBPs) are capable of binding to circRNA [32]. Transcription of parental genes is augmented by RNA pol II-U1 snRNP complex binding which is facilitated by intron-exon interaction of circular RNA circEIF3J with U1 RNA [20]. The progression of the cell cycle is blocked by a complex of circ-Foxo3, kinase inhibitor protein (p21) and cell division protein kinase 2 (CDK2) to inhibit the latter [33]. AGO (miRNA effector) is ultimately degraded before its translation is inhibited or subjected to cleavage by binding to CDR1as [34]. The circ-Dnmt1 can promote the nuclear translation of P53 and AUF1 by interacting with them, resulting in cellular autophagy or reduction of target mRNA instability [35]. circMBL or circular form of the RNA splicing factor MBL is formed by binding to the exon 2 of its parental gene. This form lowers the overall levels of MBL and also the circular form, by binding to the factor [17]. Thus, the interactions between circRNAs and RBPs may link circRNAs to diverse biological processes.
Role in tumor metastasis
CircRNAs are involved in the regulation of tumor metastasis. The deregulation of circRNAs influences cell proliferation, epithelial-mesenchymal transition, apoptosis, angiogenesis, cell cycle, and thus, have the potential to serve as tumor-targeted sites for therapy against tumor metastasis. In this section, we will emphasize the function of various aberrantly expressed circRNAs in the development of tumor metastasis (Table 1).
Table 1.
CircRNAs involved in tumor metastasis: signaling pathway and function
| CircRNA | Cancer types | Expression in tumors | Signaling pathway | Function/clinical association | Ref. |
|---|---|---|---|---|---|
| circ_0067934 | Hepatocarcinoma | High | circ_0067934/miR-1324/FZD5/Wnt/β-catenin | Associates with the ability of these cells to divide, migrate and invade to function as a new target in therapy of HCC | [38] |
| circC3P1 | Hepatocarcinoma | Low | circC3P1/miR-4641/PCK1 | Inhibits the ability of these cells to divide, migrate and invade | [39] |
| circCdr1as | Hepatocarcinoma | High | circCdr1as/ miR-7/CCNE1/PIK3CD | Suppress the HCC cell proliferation and invasion | [40] |
| circ_102231 | Lung cancer | High | Not mentioned | Linked to poor overall survival, as well as the advanced TNM stage and metastasis to the lymph node | [41] |
| circ_100876 | Lung cancer | High | Not mentioned | Associated with the stage of tumor as well as metastasis to the lymph node | [42] |
| circPSMC3 | Gastric cancer | Low | CircPSMC3/miR-296-5p /PTEN | Suppress the proliferation and metastasis by sponging miR-296-5p with PTEN | [81] |
| circACAP2 | Colorectal cancer | High | circACAP2/miR-21-5p/Tiam1 | Vitally involved in progression of the tumor: Affects the ability of these cells to divide, migrate and invade | [46] |
| circCCDC66 | Colorectal cancer | High | Not mentioned | Promote tumor growth and metastasis | [47] |
| circ_001569 | Colorectal cancer | High | circ_001569/miR-7/E2F5,BAG4,FMNL2 | Positively regulates ability of tumor to divide and invade | [48] |
| circ_100876 | Esophageal carcinoma | High | Not mentioned | Contribute to proliferation and metastasis of esophageal squamous cell carcinoma | [49] |
| circ_0067934 | Esophageal carcinoma | High | Not mentioned | Promote the motility and migration and affect cell cycle status | [50] |
| circ_0006528 | Breast cancer | High | circ_0006528/miR-7-5p/Raf1/MEK/ERK | Promote breast cancer growth, invasion and migration | [51] |
| circ_0137287 | Thyroid carcinoma | Low | Not mentioned | Likely role in diagnosis of malignancy, metastasis to the lymph node and extrathyroidal extension | [52] |
| circ_0016347 | Osteosarcoma | High | Not mentioned | The ability of these cells to invade and metastasize was promoted | [53] |
| circNT5C2 | Osteosarcoma | High | circNT5C2/miR-448 | Act as an oncogene in the ability of these cells to divide and metastasize | [54] |
CircRNA and hepatocarcinoma
Among the ubiquitous and aggressive cancers across the globe, hepatocarcinoma [36] has a current rate of 10% patient survival over five years [37]. As the diagnosis in most of the cases was done when the cancer had metastasized, at advanced stages, there is an urgency to scour for newer markers to facilitate diagnosis at an earlier stage. Zhu et al. [38] showed that circ_0067934 promotes the invasion and metastasis of hepatocarcinoma cells by the miR-1324-inhibition and concomitant FZD5/Wnt/β-catenin signaling pathway activation. Likewise, Zhong et al. [39] also discovered that circC3P1 could promote PCK1 expression through sponging miR-4641 to dramatically inhibit the invasion and metastasis of hepatocarcinoma cells. Yu et al. [40] observed that there is an up-regulation of circCdr1as expression and down-regulation of miR-7 expression in hepatocarcinoma tissues in comparison to healthy neighboring tissues. In their study, circCdr1as-knockdown caused an augmentation of miR-7 that inhibited the ability of these cells to invade and metastasize. Hence, there is a negative association between the amounts of this circRNA and miR-7 in hepatocarcinoma tissues.
CircRNA and lung cancer
Globally lung cancer ranks first and second in cancer-related mortality of men and women, respectively. In his study, Zhong et al. [41] reported a significant upregulation of circ_102231 expression in lung cancer tissues, which associated with the advanced TNM stage, lymph node metastasis, and poor overall survival of lung cancer patients. Later, Yao et al. [42] revealed that the metastasis to lymph nodes as well as the non-small cell lung cancer stage is closely associated with an upregulation of circ_100876. Likewise, Luo et al. [43] showed a striking increase in circ_0000064 in lung cancer tissues that is associated with the TNM stage and metastasis to lymph nodes to hence may serve as a potential biomarker.
CircRNA and gastric cancer
Gastric cancer ranks second in mortality, often with low survival rate, low cure rate, high recurrence rate, and poor prognosis. Zhao et al. [44] found a negative association between E-cadherin and insulin-like growth factor 1 receptor (IGF1R). However, miR-7 could inhibit IGF1R and increased E-cadherin levels to partially reverse epithelial-mesenchymal transition, thereby inhibiting the ability of the cells to invade and metastasize. In summary, miR-7 causes inhibition in the ability of these cancer cells to divide, migrate and invade and promotes apoptosis of gastric cancer cells, while circR-7 acts as a miR-7 “sponge”, which targets to miR-7 and inhibits its biological function. Rong et al. [81] showed circPSMC3 could suppress the proliferation and metastasis of gastric cancer by sponging miR-296-5p to regulate Phosphatase and Tensin Homolog (PTEN). In this study, they found reduced circPSMC3 expression in gastric cancer and correlated with higher Tumor Node Metastasis (TNM) stage and shorter overall survival. They also demonstrated by measuring the number of lung metastasis, that overexpression of circPSMC3 could inhibit the metastasis of gastric cancer in mice model.
CircRNA and colorectal cancer
Close to 10% of all cancers are colorectal cancer cases that makes it among the ubiquitous tumors associated with high mortality [45]. The main reason for the low survival rate of colorectal cancer is the lack of effective tools for early diagnosis, a high risk of invasion and metastasis. He et al. [46] demonstrated that the up-regulation of circACAP2 could inhibit the expression of miR-21-5p, which further promote the transcription and translation of T lymphoma invasion and metastasis protein 1 (Tiam1). Hsiao et al. [47] found that circCCDC66-knockdown repressed the growth and metastasis of a tumor in mouse models which are indicative of a new aspect of circRNA in oncogenesis in terms of growth and metastasis. Circ_001569 is up-regulated in colorectal cancer, which promotes proliferation, invasion, and metastasis of cancer cells [48]. The up-regulation of circ_001569 directly inhibits miR-145 and indirectly regulates the functions of E2F5, BAG4 and FMNL2 which have been reported with the aggressiveness of several cancers. E2F5, BAG4 or FMNL2 expression was up-regulated in CRC tissues in the study [48].
CircRNA and esophageal carcinoma
Esophageal carcinoma is a deadly disease, ranking the sixth among all cancers leading to mortality. Despite advances in diagnosis and therapy, esophageal cancer still has a poor prognosis. Cao et al. [49] showed that up-regulation of circ_100876 expression leads to accelerating cell proliferation and metastasis by epithelial-mesenchymal transition progression in esophageal squamous cell carcinoma. Xia et al. [50] showed that circ_0067934 was significantly up-regulated in esophageal carcinoma tissues, and siRNA-mediated in vitro silencing of circ_0067934 confirmed that circ_0067934 could promote the proliferation and metastasis of esophageal cancer cells and affect its cell cycle progression.
CircRNA and breast cancer
Gao et al. [51] reported an increase in the expression of circ_0006528 in breast cancer patients (N = 97) in comparison to the neighboring healthy tissues. This increase showed a significant association with a poor prognosis as well as the stage of advanced tumor-node-metastasis (TNM). The involvement of the circ_0006528/miR-7-5p/Raf1 to activate the MAPK/ERK circuit was shown, that enhances the ability of these cells to divide, invade and migrate.
CircRNA and thyroid carcinoma
The past years have seen a nearly exponential rise in the cases of thyroid cancer. The unfavorable prognosis of thyroid carcinoma is significantly associated with several manifestations like distant metastases, lymph node, and advanced TNM stage. Lin et al. [52] were first to report the use of receiver operating characteristic curves to show that circ_0137287 has a likely role in the diagnosis of malignancy, metastasis to the lymph node and extrathyroidal extension.
CircRNA and osteosarcoma
Jin et al. [53] discovered that osteosarcoma metastasis involves circ_0016347 activity. Circ-0016347 acts as a natural miR-214 sponge that upregulates the amount of caspase-1 (its target later in the pathway) in these cells, and its knockdown causes a weakening of the property to metastasize. Liu et al. [54] validated an upregulation of circNT5C2 in 52 pairs of osteosarcoma tissue and cell lines. Thus, circNT5C2 was shown as a potential and new target for therapeutic intervention in this cancer through sponging miR-448 to suppress tumor proliferation and invasion.
Multiple mechanisms of circRNAS
Studies have hypothesized, with emerging evidence that there are three routes involved in the metastasis of cancer, including regulation of epithelial-mesenchymal transition, coordination of DNA methylation and demethylation and mediation by exosomes (Table 2, Figure 3).
Table 2.
CircRNA related mechanisms in tumor metastasis
| CircRNA | Cancer types | Regulation | Signaling pathway | Mechanisms | Function/clinical association | Ref. |
|---|---|---|---|---|---|---|
| circPRMT5 | Urothelial carcinoma of the Bladder | Up | circPRMT5/miR-30c | EMT | Promoting UCB cell’s EMT and aggressiveness; Serve as an exploitable therapeutic target | [59] |
| circMTO1 | Bladder cancer | Down | circMTO1/miR-221 | EMT | Negatively regulate the E-cadherin/N-cadherin; Inhibits EMT as well as metastasis | [60] |
| circ_0023642 | Gastric cancer | Up | Not mentioned | EMT | Promote the EMT signaling pathway; Serve as a metastasis activator and molecular therapeutic target | [61] |
| circSMAD2 | Hepatocellular carcinoma | Down | circSMAD2/miR-629 | EMT | Impedes ability of HCC cells to migrate and invade as well as EMT | [62] |
| circAMOTL1L | Prostate cancer | Down | P53/RBM25/circAMOTL1L/miR193a-5p/Pcdha | EMT | Suppress ability of cells to migrate and invade via lowering of E-cadherin and increasing vimentin | [63] |
| circANKS1B | Breast cancer. | Up | circANKS1B/ miR-148a/152-3p/USF1 | EMT | Invasion and metastasis are promoted by EMT via TGF-β1 induction: however, nothing is observed for growth of breast cancer | [64] |
| circ_0061140 | Ovarian Cancer | Up | circ_0061140/miR-370/FOXM1 | EMT | Competes with endogenous RNA of miR-370 which allows for growth of cells and metastasis | [65] |
| circMYLK | Bladder cancer | Up | circMYLK/miR-29a/VEGFA/VEGFR2 | EMT | Contribute to EMT and the development of bladder cancer via activating VEGFA/VEGFR2 | [66] |
| circFECR1 | Breast cancers | Up | circFECR1/DNMT1/TET1 | DNA demethylation | Interact with the FLI1 promoter; regulate metastasis by coordinating DNA methylation and demethylation | [72] |
| circIARS | Pancreatic cancer | Up | circIARS/miR-122/ZO-1 | Mediated by exosome | Regulates the permeability of endothelial monolayer to augment the invasion and metastasis | [79] |
| circ-DB | Hepatocellular carcinoma | Up | circ-DB/ miR-34a/ USP7/Cyclin A2 | Mediated by adipose-derived exosome | Promote the tumorigenesis of HCC | [80] |
Figure 3.
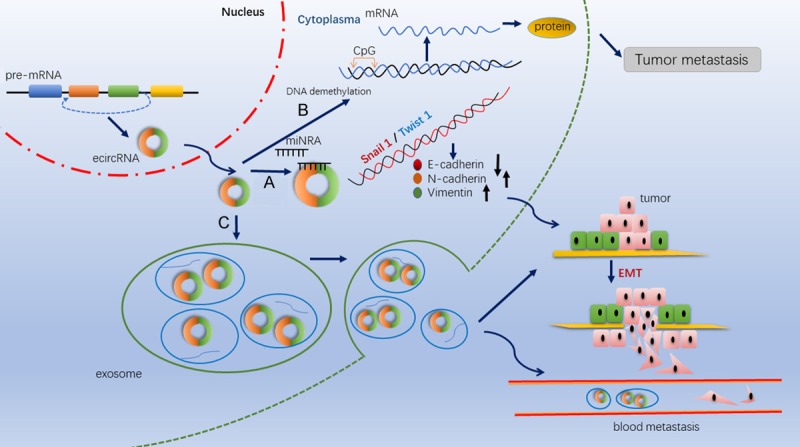
Mechanism of circRNAs activity in tumor metastasis. A. CircRNAs function as miRNA sponges to promote the EMT signaling pathway via lowering of E-cadherin and increasing N-cadherin and vimentin. B. CircRNAs could recruit demethylase and induce DNA demethylation in the CpG islands to promote tumor cell metastasis. C. The presence of circRNAs in exosomes might promote tumor metastasis by promoting cancer cell’s EMT.
Control of EMT
Epithelial-mesenchymal transition (EMT) involves the loss of intercellular adhesion and polarity that exacerbates the process of invasion and metastasis of many cell types [55]. EMT facilitates tissue remodeling and is rendered as a prerequisite in tumor metastasis and infiltration [56]. It has recently been proposed that there are distinct and intermediate states in this pathway rather than being a binary process, from primordially epithelial to completely mesenchymal states, involving several hybrid or transitional states [57]. The different subpopulations of EMT are associated with plasticity of cells, the ability to invade and metastasize. Several factors are involved in the regulation of EMT such as vimentin, with an important role, E-cadherin, N-cadherin and non-coding RNAs [58].
CircRNAs potentially contribute to tumor metastasis by activating the EMT pathway. Chen et al. [59] found through a circRNA microarray that circ PRMT5 was overexpressed in urothelial carcinoma of the bladder (UCB) in comparison to surrounding healthy normal tissue. circPRMT5-knockdown of in a UCB model showed an increase in E-cadherin which acts as the epithelial marker, whereas N-cadherin and vimentin were lowered, which act as the mesenchymal markers in Western blot analysis. They further identified that circPRMT5 could enhance UCB cell’s EMT by sponging miR-30c which significantly correlated with tumor metastasis. Instead, Li et al. [60] revealed that EMT regulated negatively by circMTO1, which competed with miR-221 and metastasis in bladder cancer cells. Zhou et al. [61] showed that circRNA_0023642 could regulate the EMT signaling pathway to promote the ability of gastric cancer to proliferate, migrate and invade. Similarly, the expression of vimentin snail and N-cadherin increased, whereas that of E-cadherin decreased. Zhang et al. [62] reported of miR-29 being targeted by circSMAD2 in hepatocellular carcinoma cells that inhibited EMT as well as the ability of these cells to migrate. Furthermore, they also confirmed that these inhibitory effects of circSMAD2 could be reversed through cotransfection of miR-629 mimics.
Yang et al. [63] showed that circAMOTL1L regulated metastatic progression of prostate cancer by modulating the expression level of vimentin snail, N-cadherin, and E-cadherin via the circAMOTL1L-miR-193a-5p-Pcdha pathway. Targeting this novel regulatory axis can yield a putative strategy in the therapy of aggressive prostate cancer. Furthermore, Zeng et al. [64] discovered that circANKS1B, traced back to exons 5 to 8 of ANKS1B, inducing EMT by activating the TGF-B1 signaling pathway, thereby promoting the invasion and metastasis of breast cancer and providing a new strategy for the treatment of breast cancer metastasis. Chen et al. [65] confirmed that circ_0061140 functioned as a ceRNA of miR-370 which boosted ovarian cancer metastasis. These findings have been linked to circ_0061140-miR-370-FOXM1 regulatory axis along with to EMT in the metastasis of this cancer. Zhong et al. [66] showed that an upregulated circMYLK in bladder cancer can induce EMT and activate VEGFA/VEGFR2 signaling pathway that promotes the metastasis and angiogenesis of bladder cancer. They also showed that circMYLK knockdown induced apoptosis and decreased cell proliferation. These findings suggest that circRNA-miRNA-target genes signal axis might be a potential molecular target for the treatment of tumor metastasis.
Coordinating DNA methylation and demethylation
The critical role of DNA methylation in controlling the structure of chromosomes and regulating expression of genes has been established [67]. This process involves the catalysis by enzyme DNA methyltransferase (DNMT) that adds a methyl group to cytosine-phosphate-guanine (CpG) and non-CpG dinucleotide sites [68]. Over the last decade, it has been further confirmed that aberrant DNA methylation closely participates in the tumorigenesis via covalent modification of the genetic code [69]. All the steps of tumor formation involve alterations across the genome as well as methylation at distinct locations [70]. There is hardly any report that highlights the probability of methylation in loci that are responsible for circRNAs origin, until several months ago. Then, Ferreira et al. [71] found that there is silencing of circRNA in human cancer due to excessive methylation at CpG islands of their promoters; this point out at the involvement of loss of circRNA due to methylation. Meanwhile, Chen et al. [72] demonstrated on that FECR1 circRNA, is composed of Friend leukemia virus integration 1 (FLI1) exons, belongs to the family of ETS transcription factors and enhances breast cancer metastasis through DNA methylating and demethylating enzymes.
The role of exosomes
Exosomes are a type of nano-vesicles of 50-140 nm in size, evolved by multivesicular endosomes (MVEs) [73]. They are involved in intercellular communication through transporting abundant bioactive substances, including microRNAs, LncRNAs, mRNA and protein protected by a lipid bilayer [74,75]. Therefore, there is a close association between exosomes and immunity, tumor metastasis, chemotherapy resistance and angiogenesis of tumors through different molecular mechanisms. Hakulinen et al. [76] have confirmed that Membrane type 1 matrix metalloproteinase (MT1-MMP) released by exosomes of melanoma and fibrosarcoma can degrade collagen and promote cancer invasion and metastasis. Moreover, exosomes derived from highly invasive Hs578Ts(i) triple-negative breast cancer cells (TNBT) can significantly increase the proliferation, migration and invasion capacity of all recipient cell lines [77]. Recent studies have shown that circRNAs are stable in exosomes [78]. Whether these exosome-delivered circRNAs associate with tumor metastasis by exosomes was unclear for the past few years. Li et al. [79] revealed that circular RNA_IARS located in exosomes of pancreatic cancer cells is able to promote tumor metastasis by regulating endothelial monolayer permeability. The expression levels of circular RNA_IARS in plasma exosomes of 85 pancreatic ductal adenocarcinoma (PDAC) was up-regulated which correlated with the tumor-node-metastasis stage, as well as postoperative survival time. Zhang et al. [80] also reported that deubiquitination of hepatocellular carcinoma (HCC) could be regulated by adipocytes that synthesize exosome circRNA_DB and promote cell proliferation and tumor metastasis by suppressing miR-34a, leading to the activation of the USP7/Cyclin A2 signaling pathway. This finding provided an insight into a comprehensive understanding of the association between exosome circRNA and tumor metastasis. Therefore, exosomal circRNA is highly anticipated as future therapeutic targets for tumor metastasis for clinical applications.
Conclusion and future perspectives
In the past decade, circRNAs were regarded as non-functional. Advances in the realms of genomics, especially in high-throughput RNA sequencing, have proved that circRNAs act as a “sponge” for miRNAs. Due to the special loop structure, circRNAs are highly stable and widely distributed in various tissues and organs. The expression of circRNAs is spatially and temporally different, which can regulate the proliferation and metastasis of cancer cells. Therefore, circRNAs are expected to be used as biomarkers for early diagnosis of tumors. CircRNAs act as a stable and efficient miRNA inhibitors, miRNA sponge, and may become a novel method to replace gene knockout, which can simultaneously inhibit the expression of other paralogous miRNAs.
Tumor metastasis is a crucial factor which accounts for high mortality of patients with cancer. The main therapeutic purpose for cancer patients is to block tumor metastasis and enhance the survival rate. At present, the clinical judgment of whether tumor metastasis has occurred is mainly confirmed by imaging tests and pathological biopsy, which are not robust enough, leading to a poor understanding of tumor metastasis mechanism. The tumor environment, including the cell status, blood supplement, cell-extracellular matrix are altered before the tumor cells reach the metastatic site, thereby adapting to the malignant clonal proliferation of the metastatic cell. Additionally, the genetic instability is thought to be the driving force for tumor metastasis. Although it is still a hypothesis, most experts agree with this statement. Concurrently, the current interpretation of the mechanism of tumor metastasis is not comprehensive and needs to be explored from a new perspective.
Recent research has shown that there is a circRNA network that functions as a regulator of tumor metastasis. This review is a summary of circRNAs implicated in modulating the metastatic potential of tumor and seeks to illuminate the mechanisms, including control of epithelial-mesenchymal transition, regulation of DNA methylation mediated by exosomes. Meanwhile, the involvement of circRNAs in the metastasis of common malignancies are also briefly discussed to facilitate the study in tumorigenesis.
To summarize, circRNAs are closely associated with tumor metastasis. Continued research on the role of circRNAs in tumor metastasis will have a deeper understanding of the transfer mechanism of the cells. CircRNAs can be used as independent prognostic factors and are expected to develop into a diagnostic factor for tumor metastasis. Further evaluation of the role of circRNAs metastasis will lay the foundation for the final identification of the tumor metastasis mechanism.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of Ningbo (No. 2016A610158; No. 2014A610226; No. 2018A610373), the Zhejiang Medical and Health Project (No. 2018ZH025; No. 2019KY155) and the Scientific benefit for people Project of Ningbo (No. 2014C51001).
Disclosure of conflict of interest
None.
References
- 1.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 4.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7:155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 6.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Memczak S, Papavasileiou P, Peters O, Rajewsky N. Identification and characterization of circular RNAs as a new class of putative biomarkers in human blood. PLoS One. 2015;10:e0141214. doi: 10.1371/journal.pone.0141214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuire S. World cancer report 2014. Geneva, switzerland: world health organization, international agency for research on cancer, WHO Press, 2015. Adv Nutr. 2016;7:418–9. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 10.Lasda E, Parker R. Circular RNAs: diversity of form and function. RNA. 2014;20:1829–1842. doi: 10.1261/rna.047126.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development. 2016;143:1838–1847. doi: 10.1242/dev.128074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett SP, Wang PL, Salzman J. Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife. 2015;4:e07540. doi: 10.7554/eLife.07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, Hanan M, Behm M, Bartok O, Ashwal-Fluss R, Herzog M, Schreyer L, Papavasileiou P, Lvanov A, Öhman M, Refojo D, Kadener S, Rajewsky N. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL, Yang L. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, Rajewsky N. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 21.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 22.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weng W, Wei Q, Toden S, Yoshida K, Nagasaka T, Fujiwara T, Cai S, Qin H, Ma Y, Goel A. Circular RNA ciRS-7-a promising prognostic biomarker and a potential therapeutic target in colorectal cancer. Clin Cancer Res. 2017;23:3918–3928. doi: 10.1158/1078-0432.CCR-16-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F, Zhang L, Li W, Deng J, Zheng J, An M, Lu J, Zhou Y. Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β-catenin pathway. Oncotarget. 2015;6:6001–6013. doi: 10.18632/oncotarget.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S. Translation of circRNAs. Mol Cell. 2017;66:9–21. e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CC, Xiao X, Wang Z. Extensive translation of circular RNAs driven by N 6-methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Molinie B, Daneshvar K, Pondick JV, Wang J, Van Wittenberghe N, Xing Y, Giallourakis CC, Mullen AC. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20:2262–2276. doi: 10.1016/j.celrep.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade M, Laneve P, Rajewsky N, Bozzoni I. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37. e29. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F, Huang N, Yang X, Zhao K, Zhou H, Huang S, Xie B, Zhang N. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110:304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Liu T, Wang X, He A. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. 2017;16:58. doi: 10.1186/s12943-017-0630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17:205–11. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 32.Salzman J. Circular RNA expression: its potential regulation and function. Trends Genet. 2016;32:309–316. doi: 10.1016/j.tig.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ, Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du WW, Yang W, Li X, Awan FM, Yang Z, Fang L, Lyu J, Li F, Peng C, Krylov SN, Xie Y, Zhang Y, He C, Wu N, Zhang C, Sdiri M, Dong J, Ma J, Gao C, Hibberd S, Yang BB. A circular RNA circ-DNMT1 enhances breast cancer progression by activating autophagy. Oncogene. 2018;37:5829–5842. doi: 10.1038/s41388-018-0369-y. [DOI] [PubMed] [Google Scholar]
- 36.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends - an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 37.Lee SS, Shin HS, Kim HJ, Lee SJ, Lee HS, Hyun KH, Kim YH, Kwon BW, Han JH, Choi H, Kim BH, Lee JH, Kang HY, Shin HD, Song IH. Analysis of prognostic factors and 5-year survival rate in patients with hepatocellular carcinoma: a single-center experience. Korean J Hepatol. 2012;18:48–55. doi: 10.3350/kjhep.2012.18.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Q, Lu G, Luo Z, Gui F, Wu J, Zhang D, Ni Y. CircRNA circ_0067934 promotes tumor growth and metastasis in hepatocellular carcinoma through regulation of miR-1324/FZD5/Wnt/β-catenin axis. Biochem Biophys Res Commun. 2018;497:626–632. doi: 10.1016/j.bbrc.2018.02.119. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Gong X, Sun L, Zhou Q, Lu B, Zhu L. The circular RNA Cdr1as act as an oncogene in hepatocellular carcinoma through targeting miR-7 expression. PLoS One. 2016;11:e0158347. doi: 10.1371/journal.pone.0158347. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Zhong L, Wang Y, Cheng Y, Wang W, Lu B, Zhu L, Ma Y. Circular RNA circC3P1 suppresses hepatocellular carcinoma growth and metastasis through miR-4641/PCK1 pathway. Biochem Biophys Res Commun. 2018;499:1044–1049. doi: 10.1016/j.bbrc.2018.03.221. [DOI] [PubMed] [Google Scholar]
- 41.Zong L, Sun Q, Zhang H, Chen Z, Deng Y, Li D, Zhang L. Increased expression of circRNA_102231 in lung cancer and its clinical significance. Biomed Pharmacother. 2018;102:639–644. doi: 10.1016/j.biopha.2018.03.084. [DOI] [PubMed] [Google Scholar]
- 42.Luo YH, Zhu XZ, Huang KW, Zhang Q, Fan YX, Yan PW, Wen J. Emerging roles of circular RNA hsa_circ_0000064 in the proliferation and metastasis of lung cancer. Biomed Pharmacother. 2017;96:892–898. doi: 10.1016/j.biopha.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Yao JT, Zhao SH, Liu QP, Lv MQ, Zhou DX, Liao ZJ, Nan KJ. Over-expression of CircRNA_100876 in non-small cell lung cancer and its prognostic value. Pathol Res Pract. 2017;213:453–456. doi: 10.1016/j.prp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X, Dou W, He L, Liang S, Tie J, Liu C, Li T, Lu Y, Mo P, Shi Y, Wu K, Nie Y, Fan D. MicroRNA-7 functions as an anti-metastatic microRNA in gastric cancer by targeting insulin-like growth factor-1 receptor. Oncogene. 2013;32:1363–1372. doi: 10.1038/onc.2012.156. [DOI] [PubMed] [Google Scholar]
- 45.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 46.He JH, Li YG, Han ZP, Zhou JB, Chen WM, Lv YB, He ML, Zuo JD, Zheng L. The CircRNA-ACAP2/Hsa-miR-21-5p/Tiam1 regulatory feedback circuit affects the proliferation, migration, and invasion of colon cancer SW480 cells. Cell Physiol Biochem. 2018;49:1539–1550. doi: 10.1159/000493457. [DOI] [PubMed] [Google Scholar]
- 47.Hsiao KY, Lin YC, Gupta SK, Chang N, Yen L, Sun HS, Tsai SJ. Non-coding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77:23399–2350. doi: 10.1158/0008-5472.CAN-16-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y, Yang S, Zeng Z, Liao W, Ding YQ, Liang L. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 2016;7:26680–26691. doi: 10.18632/oncotarget.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao S, Chen G, Yan L, Li L, Huang X. Contribution of dysregulated circrna_100876 to proliferation and metastasis of esophageal squamous cell carcinoma. Onco Targets Ther. 2018;11:7385–7394. doi: 10.2147/OTT.S177524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia W, Qiu M, Chen R, Wang S, Leng X, Wang J, Xu Y, Hu J, Dong G, Xu L, Yin R. Circular RNA has_circ_0067934 is upregulated in esophageal squamous cell carcinoma and promoted proliferation. Sci Rep. 2016;6:35576. doi: 10.1038/srep35576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao D, Qi X, Zhang X, Fang K, Guo Z, Li L. hsa_circRNA_0006528 as a competing endogenous RNA promotes human breast cancer progression by sponging miR-7-5p and activating the MAPK/ERK signaling pathway. Mol Carcinog. 2019;58:554–564. doi: 10.1002/mc.22950. [DOI] [PubMed] [Google Scholar]
- 52.Lan X, Cao J, Xu J, Chen C, Zheng C, Wang J, Zhu X, Zhu X, Ge M. Decreased expression of hsa_circ_0137287 predicts aggressive clinicopathologic characteristics in papillary thyroid carcinoma. J Clin Lab Anal. 2018;32:e22573. doi: 10.1002/jcla.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin H, Jin X, Zhang H, Wang W. Circular RNA hsa-circ-0016347 promotes proliferation, invasion and metastasis of osteosarcoma cells. Oncotarget. 2017;8:25571–25581. doi: 10.18632/oncotarget.16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Zhong Y, Li J, Shan A. Circular RNA circ-NT5C2 acts as an oncogene in osteosarcoma proliferation and metastasis through targeting miR-448. Oncotarget. 2017;8:114829–114838. doi: 10.18632/oncotarget.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 56.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 57.Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S, De Clercq S, Minguijón E, Balsat C, Sokolow Y, Dubois C, De Cock F, Scozzaro S, Sopena F, Lanas A, D’Haene N, Salmon I, Marine JC, Voet T, Sotiropoulou PA, Blanpain C. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–468. doi: 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- 58.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Chen RX, Wei WS, Li YH, Feng ZH, Tan L, Chen JW, Yuan GJ, Chen SL, Guo SJ, Xiao KH, Liu ZW, Luo JH, Zhou FJ, Xie D. PRMT5 circular RNA promotes metastasis of urothelial carcinoma of the bladder through sponging miR-30c to induce epithelial-mesenchymal transition. Clin Cancer Res. 2018;24:6319–6330. doi: 10.1158/1078-0432.CCR-18-1270. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Wan B, Liu L, Zhou L, Zeng Q. Circular RNA circMTO1 suppresses bladder cancer metastasis by sponging miR-221 and inhibiting epithelial-to-mesenchymal transition. Biochem Biophys Res Commun. 2019;508:991–996. doi: 10.1016/j.bbrc.2018.12.046. [DOI] [PubMed] [Google Scholar]
- 61.Zhou LH, Yang YC, Zhang RY, Wang P, Pang MH, Liang LQ. CircRNA_0023642 promotes migration and invasion of gastric cancer cells by regulating EMT. Eur Rev Med Pharmacol Sci. 2018;22:2297–2303. doi: 10.26355/eurrev_201804_14818. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Luo P, Jing W, Zhou H, Liang C, Tu J. circSMAD2 inhibits the epithelial-mesenchymal transition by targeting miR-629 in hepatocellular carcinoma. Onco Targets Ther. 2018;11:2853–2863. doi: 10.2147/OTT.S158008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Z, Qu CB, Zhang Y, Zhang WF, Wang DD, Gao CC, Ma L, Chen JS, Liu KL, Zheng B, Zhang XH, Zhang ML, Wang XL, Wen JK, Li W. Dysregulation of p53-RBM25-mediated circAMOTL1L biogenesis contributes to prostate cancer progression through the circAMOTL1L-miR-193a-5p-Pcdha pathway. Oncogene. 2019;38:2516–2532. doi: 10.1038/s41388-018-0602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeng K, He B, Yang BB, Xu T, Chen X, Xu M, Liu X, Sun H, Pan Y, Wang S. The pro-metastasis effect of circANKS1B in breast cancer. Mol Cancer. 2018;17:160. doi: 10.1186/s12943-018-0914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Q, Zhang J, He Y, Wang Y. hsa_circ_0061140 knockdown reverses FOXM1-mediated cell growth and metastasis in ovarian cancer through miR-370 sponge activity. Mol Ther Nucleic Acids. 2018;13:55–63. doi: 10.1016/j.omtn.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong Z, Huang M, Lv M, He Y, Duan C, Zhang L, Chen J. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017;403:305–317. doi: 10.1016/j.canlet.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Grewal SI, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301:798–802. doi: 10.1126/science.1086887. [DOI] [PubMed] [Google Scholar]
- 68.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 69.Esteller M, Fraga MF, Guo M, Garcia-Foncillas J, Hedenfalk I, Godwin AK, Trojan J, Vaurs-Barrière C, Bignon YJ, Ramus S, Benitez J, Caldes T, Akiyama Y, Yuasa Y, Launonen V, Canal MJ, Rodriguez R, Capella G, Peinado MA, Borg A, Aaltonen LA, Ponder BA, Baylin SB, Herman JG. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Human Mol Genet. 2001;10:3001–3007. doi: 10.1093/hmg/10.26.3001. [DOI] [PubMed] [Google Scholar]
- 70.Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 71.Ferreira HJ, Davalos V, de Moura MC, Soler M, Perez-Salvia M, Bueno-Costa A, Setien F, Moran S, Villanueva A, Esteller M. Circular RNA CpG island hypermethylation-associated silencing in human cancer. Oncotarget. 2018;9:29208–29219. doi: 10.18632/oncotarget.25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen N, Zhao G, Yan X, Lv Z, Yin H, Zhang S, Song W, Li X, Li L, Du Z, Jia L, Zhou L, Li W, Hoffman AR, Hu JF, Cui J. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018;19:218. doi: 10.1186/s13059-018-1594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 74.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, Yoshizaki T, Pagano JS, Shackelford J. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33:4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105:1211–1218. doi: 10.1002/jcb.21923. [DOI] [PubMed] [Google Scholar]
- 77.O’Brien K, Rani S, Corcoran C, Wallace R, Hughes L, Friel AM, McDonnell S, Crown J, Radomski MW, O’Driscoll L. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer. 2013;49:1845–1859. doi: 10.1016/j.ejca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, Chen D, Gu J, He X, Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Li Z, Jiang P, Peng M, Zhang X, Chen K, Liu H, Bi H, Liu X, Li X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J Exp Clin Cancer Res. 2018;37:177. doi: 10.1186/s13046-018-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, Fan Q, Li J, Ning T, Tian F, Li H, Sun W, Ying G, Ba Y. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene. 2018 doi: 10.1038/s41388-018-0619-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Rong D, Lu C, Zhang B, Fu K, Zhao S, Tang W, Cao H. CircPSMC3 suppresses the proliferation and metastasis of gastric cancer by acting as a competitive endogenous RNA through sponging miR-296-5p. Mol Cancer. 2019;18:25. doi: 10.1186/s12943-019-0958-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


