Abstract
MicroRNA-874 (miR-874) is downregulated and acts as a tumor suppressor gene in several human cancers. Its biological function and underlying molecular mechanism in rhabdomyosarcoma (RMS), however, remain unclear. In this study, we found that miR-874 expression was downregulated in human RMS tissue samples and cell lines through quantitative real-time polymerase chain reaction (qRT-PCR). Functional studies revealed that miR-874 overexpression in RMS cells remarkably inhibited proliferation, invasion, migration, and induced apoptosis. The results of luciferase activity assay, qRT-PCR and western blot analyses showed that miR-874 inhibited GEFT translation and suppressed GEFT expression by directly targeting the 3’-untranslated region (3’-UTR) of GEFT mRNA. GEFT expression was upregulated in RMS tissue samples and cell lines and was inversely correlated with miR-874 expression. Downregulation of GEFT has similar effects to miR-874 overexpression in RMS cells. Notably, GEFT restoration partially reversed the tumor-suppressive effects of miR-874. Our results indicated that miR-874 functions as a tumor suppressor in RMS and may suppress the growth and metastasis of RMS cells partially by targeting GEFT.
Keywords: Rhabdomyosarcoma, miR-874, GEFT
Introduction
Rhabdomyosarcoma (RMS) is one of the most common soft-tissue sarcomas and the third most common extracranial solid tumor among children [1]. It is a malignant cancer with a mesenchymal origin [2]. The two major subtypes of RMS are alveolar rhabdomyosarcoma (ARMS) and embryonal rhabdomyosarcoma (ERMS) showing different histological, genetic, and clinical features. During the last few decades, tumor resection, radiotherapy, and chemotherapy regimens are widely used to treat RMS. Despite advancements in strategies for the treatment of RMS, the survival rate of children with high-risk RMS remains low [3]. The development of RMS is a complex multistep process, and its molecular basis remains poorly understood. Therefore, the molecular mechanisms underlying the initiation and development of RMS must be uncovered to aid the identification of novel therapeutic targets and molecular diagnostic biomarkers for this malignancy.
MicroRNAs (miRNAs) are small noncoding RNAs that are 18-25 nucleotides in length. They have attracted considerable attention in cancer research given that they can potentially control roughly one-third of human messenger RNA (mRNA) expression [4,5]. They control the expression of protein-coding genes by inducing degradation or inhibiting translation through binding to the 3’-untranslated region (3’-UTR) of their target mRNAs [6-8]. Accumulating evidence indicates that miRNAs serve as tumor oncogenes or suppressors and participate in cell proliferation, apoptosis, invasion, migration, and differentiation [9-11]. Given these behaviors, the identification of novel microRNAs and their potential target genes has become a hotspot in research on human malignancies.
Numerous miRNAs are abnormally expressed in RMS; play essential roles in cancer cell growth, metastasis, and proliferation; and exert tumor-suppressive or oncogenic effects by regulating target genes. For example, Francesca Bersani et al. [12] demonstrated that miR-22 inhibited cell proliferation, invasiveness and promoted apoptosis by targeting TACC1 and RAB5B in RMS. J A Hanna et al. [13] showed that miR-206 was downregulated in RMS and relieves the differentiation arrest of fusion-negative RMS (FN-RMS) by inhibiting PAX7. Francesca Megiorni et al. [14] reported that miR-378a-3p caused significant changes in cell migration, apoptosis as well as cytoskeleton organization mainly by inhibiting IGF1R in RMS. This showed that exploring the mechanism and role of miRNAs in RMS is essential for the development of novel diagnostic and therapeutic strategies for this malignancy.
Recent studies have shown that microRNA-874 (miR-874) is downregulated and acts as a tumor-suppressor gene in several human malignancies, including gastric cancer [15,16], hepatocellular carcinoma [17], colorectal cancer [18], breast cancer [19], nonsmall-cell lung cancer [20], maxillary sinus squamous cell carcinoma [21], and osteosarcoma [22]. It also participates in cancer development and progression. Nevertheless, the potential role and mechanisms of miR-874 in the development of RMS are unclear. Therefore, we aimed to investigate and identify the functional importance and target genes of miR-874 in RMS. The results of this study will provide novel insights into the pathogenesis of RMS and will aid the development of new therapeutic strategies for this malignancy.
Materials and methods
Human tissue specimens
Human ERMS tissue samples (n = 10), human ARMS tissue samples (n = 10) and normal skeletal muscle tissue samples (n = 10) were collected from the First Affiliated Hospital of Shihezi University, China, and the First Affiliated Hospital of Xinjiang Medical University, China. Informed consent was obtained from all patients prior to surgery. Diagnosis was confirmed by pathologists. The study protocol and consent procedures were approved by the ethics committee of Shihezi University, China.
Cell culture
The ERMS cell line RD (purchased from the Cell Bank of the Chinese Academy of Sciences, China), ARMS cell line RH30 (purchased from Shanghai Fu Xiang Biotechnology Co., Ltd., China), human skeletal muscle cell line HSKMC (purchased from Be Na Biotechnology Co., Ltd., China), embryonic kidney cell line 293T (provided by the Key Laboratories for Xinjiang Endemic and Ethnic Diseases, School of Medicine, Shihezi University, China), and RD and RH30 cell lines stably transfected with guanine nucleotide exchange factor T (GEFT) or empty vector (EV) (provided by the Department of Pathology, School of Medicine, Shihezi University, China) were used in this study. All cell lines were routinely maintained in DMEM medium supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin-penicillin in a humidified incubator under 5% CO2 atmosphere at 37°C.
Cell transfection
The miR-874 mimic (miR-874) and corresponding negative control (NC) were purchased from Gene Pharma (Shanghai, China). The shRNAs for GEFT (sh-GEFT) and scrambled negative control (sh-control) were designed and synthesized by GeneChem (Shanghai, China). Transfection was performed with Lipofectamine 2000 (Life technologies, USA) in accordance with the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR) for miRNAs and mRNA
Total RNA was isolated from cultured cells or human samples by using miRNeasy Mini Kit (Qiagen, Germany) or miRNeasy FFPE Kit (Qiagen, Germany), respectively, in accordance with the manufacturer’s protocols. The total RNA samples were reverse transcribed into cDNA by using miScript II RT Kit (Qiagen, Germany). The qRT-PCR analysis was performed with miScript SYBR Green PCR Kit (Qiagen, Germany) in the 7500 Real-Time PCR System (Applied Biosystems, USA). The miRNA sequence-specific qRT-PCR primers for miR-874 were purchased from Qiagen. U6 was used as an internal control. The sequences of the forward and reverse U6 primers were 5’-GCTTCGGCAGCACATATACTAA-3’ and 5’-AACGCTTCACGAATTTGCGT-3’, respectively. The sequences of the forward and reverse GEFT mRNA primers were 5’-TAGGTACCACCATGCGGGGGGGGCACAAA-3’ and 5’-CGACCGGTGACAGCTCATCTTCATCCAG-3’, respectively. β-actin was used as an internal control. The sequences of the forward and reverse β-actin primers were 5’-TCATGAAGTGTGACGTGGACAT-3’ and 5’-CTCAGGAGGAGCAATGATCTTG-3’, respectively. The relative expression levels of miR-874 and GEFT were normalized to those of U6 and β-actin, respectively, through the 2-ΔΔCt method.
Cell proliferation and apoptosis assays
Cell proliferation rates were quantified by using Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) in accordance with the manufacturer’s protocol. In brief, different groups of cells were plated at the density of 4 × 103 cells/well in 96-well plates and cultured in the DMEM medium including 10% FBS. CCK-8 solution was added to each well at 0, 24, 48, and 72 h post plating, and absorbance was measured at 450 nm (OD450) after 120 min of incubation at 37°C.
Cell apoptosis was detected by using the Annexin V-APC/PI Apoptosis Detection Kit (KeyGEN, Chain) in accordance with the manufacturer’s instructions. The cell apoptosis assay was performed with PAS flow cytometry apparatus (PARTEC, Germany) at 48 h post transfection. This experiment was repeated thrice for each group.
Cell invasion and migration assays
Cell invasion and migration assays were performed using chambers with diameters of 6.5 mm (8 lm pore size, Costar, USA). Cells were resuspended in 0.2 ml serum-free medium at the density of 2 × 105 cells and seeded on top chambers with or without inserts coated with Matrigel (BD Biosciences, USA) for invasion and migration assays, respectively. Subsequently, 0.6 ml of 20% FBS-DMEM medium was added to the lower chambers. After 24 h of incubation, cells that have invaded or migrated through the membrane and cells on the lower surfaces of the membranes were fixed and stained. The fixed cells were photographed under microscopy (Olympus, Japan). Five fields were randomly selected from the photographs for cell counting.
Wound-healing assay
Cell migration was assessed through the classic in vitro wound-healing assay. Transfected cells (106/well) were cultured in six-well plates for 24 h. Linear wounds were created on confluent cell monolayers through scratching with a P10 pipet tip. Cells were washed thrice with PBS and then cultured in DMEM medium supplemented with 10% FBS. Cells were imaged at 0, 12, and 24 h after wounding. Individual cells were quantified as an average of at least five randomly selected fields for each experiment.
Luciferase reporter assay
The luciferase reporter assay was performed to determine the relationship between miR-874 and GEFT. The wild-type (Wt) 3’-UTR and the mutant (Mut) 3’-UTR were synthesized by GENECHEM (China). To perform the luciferase assay, 293T cells were seeded at the density of 3 × 104 cells/well in 24-well plates for 24 h and then transfected with 0.1 µL of GEFT-3’-UTR-Wt/Mut reporter plasmids and 100 nM of miR-874 or NC. In addition, 293T cells were transfected with 0.02 µL of Renilla luciferase expression plasmid as the reference control. Relative luciferase activity was quantified using the Dual-Luciferase Reporter Assay System (Promega, USA) at 48 h post transfection and normalized against Renilla luciferase activity.
Western blot analysis
Western blot was performed to quantify the protein expression levels of GEFT. Equal amounts of cell lysates were electrophoretically separated and transferred to a PVDF membrane. The membrane was blocked with 5% skimmed milk and then incubated with primary antibodies against GEFT (1:1000; Abcam, USA) and β-actin (1:1000; ZSGB-BIO, China) and the appropriate secondary antibodies. Proteins were visualized with a chemiluminescent detection system (Thermo, USA) and exposed to an autoradiography film (Kodak, China).
Statistical analysis
Data were expressed as the mean ± standard deviation (SD) of at least three separate experiments. Statistical analysis was performed with Statistical Package for Social Science (SPSS for Windows version 17.0., USA). Student’s t-test and one-way ANOVA were used to evaluate statistical significance. P < 0.05 was considered statistically significant.
Results
miR-874 expression was downregulated in RMS tissues and cell lines
We quantified miR-874 expression levels in RMS tissues and normal skeletal muscle tissues through qRT-PCR analysis to determine the potential role of miR-874 in RMS. As shown in Figure 1A, miR-874 expression in ERMS (n = 10) and ARMS tissues (n = 10) was lower than that in normal control tissues (n = 10). We also performed qRT-PCR analysis to compare miR-874 expression in the human skeletal muscle cell line with that in the human RMS cell lines. miR-874 expression was downregulated in RD and RH30 relative to that in HSKMC (Figure 1B). These results indicate that miR-874 likely plays a suppressive role in RMS.
Figure 1.
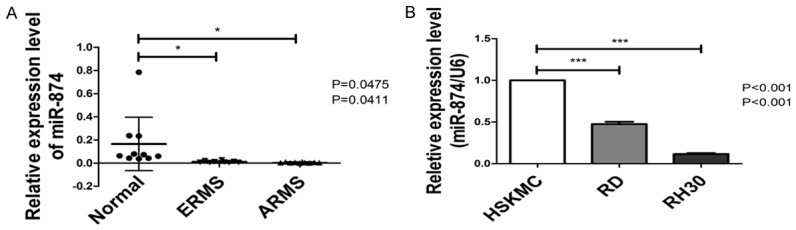
miR-874 is downregulated in RMS tissues and cell lines. A. The relative expression levels of miR-874 in ERMS (n = 10), ARMS (n = 10) and normal skeletal muscle tissues (n = 10) were determined through qRT-PCR analysis. B. The relative expression levels of miR-874 in RMS (RD and RH30) and human skeletal muscle (HSKMC) cell lines were determined through qRT-PCR analysis. *P < 0.05, ***P < 0.001.
miR-874 inhibited cell proliferation and induced cell apoptosis in RMS cells
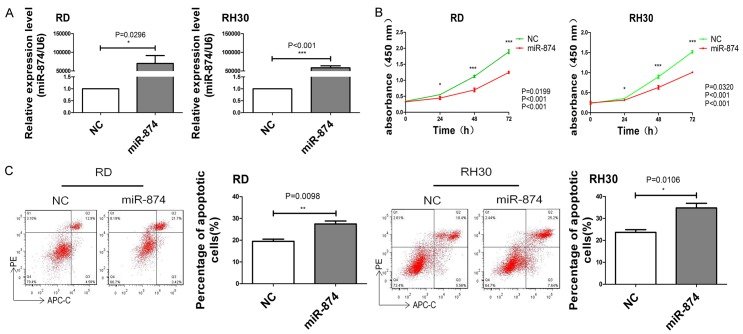
To investigate the biological role of miR-874 in the development of RMS, we transfected miR-874 or NC into two cell lines (RD and RH30) with low miR-874 expression. The cells were then cultured for 48 h. As shown in Figure 2A, RD and RH30 cells transfected with miR-874 had higher intracellular miR-874 levels than those transfected with the NC. Meanwhile, we determined the proliferation and apoptosis rates of RD and RH30 cells transfected with miR-874 or NC. Growth curves constructed on the basis of CCK8 results revealed that miR-874 overexpression decreased cell proliferation rates (Figure 2B). Flow cytometry results showed that the apoptosis rates of miR-874-transfected cells increased (Figure 2C). These data imply that miR-874 could inhibit the growth of RMS cells in vitro by inhibiting cell proliferation and inducing cell apoptosis.
Figure 2.
miRNA-874 inhibits cell proliferation and induces apoptosis in RMS. A. The expression levels of miR-874 in RD and RH30 cells transfected with miR-874 or NC were analyzed through qRT-PCR. B. The proliferative abilities of RD and RH30 cells transfected with miR-874 or NC were determined through CCK8 assay. C. The apoptosis rates of RD and RH30 cells transfected with miR-874 or NC were determined through flow cytometry. *P < 0.05, **P < 0.01, ***P < 0.001.
miR-874 inhibited the invasion and migration of RMS cells
To further investigate the effect of miR-874 on the invasive and migratory capabilities of RMS cells, we transfected miR-874 or NC into RD and RH30 cell lines and performed invasion and migration assays with a Transwell system. The data revealed that miR-874 overexpression inhibited the invasion (Figure 3A) and migration (Figure 3B) of RD and RH30 cells. Additionally, the results of wound-healing assays showed that the migration of miR-874-overexpressing cells was dramatically inhibited relative to that of control cells (Figure 3C).
Figure 3.
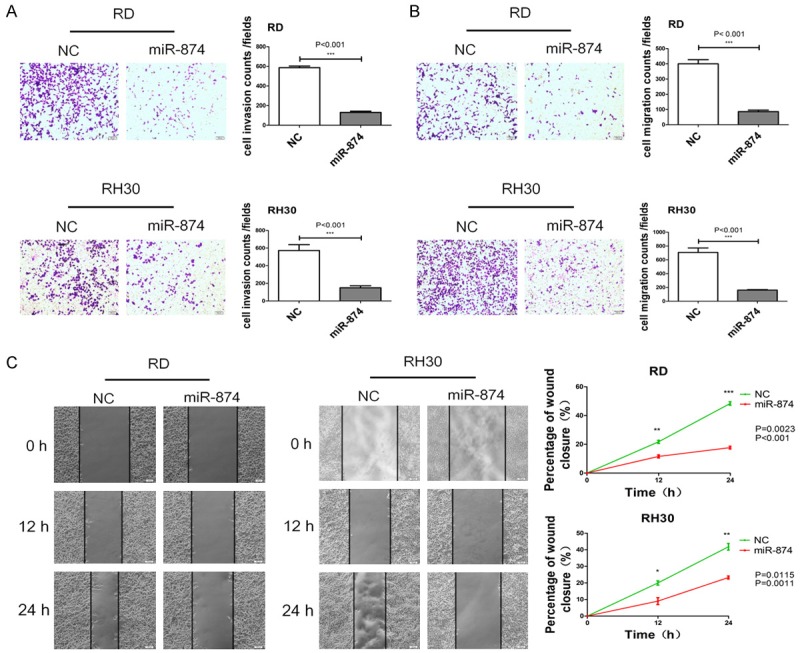
miRNA-874 inhibits cell invasion and migrationin in RMS. A. The invasive capabilities of RD and RH30 cells transfected with miR-874 or NC were determined with a Transwell system. B. The migratory capabilities of RD and RH30 cells transfected with miR-874 or NC were determined with a Transwell system. C. The migratory capabilities of RD and RH30 cells transfected with miR-874 or NC were determined through the wound-healing assay. *P < 0.05, **P < 0.01, ***P < 0.001.
GEFT was directly targeted by miR-874
To investigate the exact molecular mechanism underlying the effects of miR-874 in the development of RMS, we first predicted the potential target genes of miR-874 by using the TargetScan, miRanda, and miRDB algorithms. GEFT, a Rho family guanine nucleotide exchange factor, attracted our attention because all three algorithms predicted that it is targeted by miR-874 (Figure 4A). To investigate whether miR-874 directly target GEFT, we performed luciferase reporter assay with a vector encoding the complete sequence of the 3’-UTR of GEFT mRNA or a vector encoding the Mut 3’-UTR of GEFT mRNA to change the predicted miR-874 target site. We found that miR-874 inhibited luciferase activity in cells transfected with the Wt 3’-UTR of GEFT mRNA but not in cells transfected with the Mut 3’-UTR of GEFT mRNA (Figure 4B).
Figure 4.
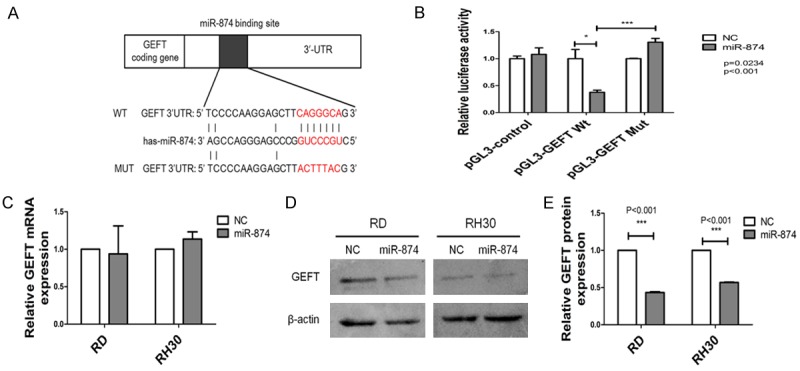
GEFT is the direct target of miR-874 in RMS cells. A. Predicted miR-874 binding sites in the 3’-UTR of the GEFT mRNA sequence and their induced mutations. B. 293T cells cotransfected with miR-874 or NC and luciferase reporter plasmid (Wt/Mut 3’-UTR GEFT) were subjected to the luciferase reporter assay. C. The expression levels of GEFT in RD and RH30 cells transfected with miR-874 or NC were analyzed through qRT-PCR. D, E. The expression levels of GEFT in RD and RH30 cells transfected with miR-874 or NC were analyzed through western blot analyses. *P < 0.05, ***P < 0.001.
We investigated the effect of miR-874 on GEFT expression in RD and RH30 cells through miR-874 overexpression experiments. We transfected miR-874 or NC into two cell lines (RD and RH30) with low miR-874 expression. The cells were then cultured for 48 h. As shown in Figure, the restoration of miR-874 expression reduced GEFT protein expression in RD and RH30 cells (Figure 4D, 4E) but did not affect GEFT mRNA expression (Figure 4C). These results indicate that miR-874 can inhibit GEFT translation and suppress GEFT expression by directly targeting the 3’-UTR of GEFT mRNA.
GEFT expression was upregulated in RMS tissues and cell lines
Given that GEFT was identified as the target of miR-874, we employed qRT-PCR and western blot analyses to determine whether reductions in miR-874 expression were correlated with increased levels of GEFT expression in RMS. We found that the expression level of GEFT mRNA and protein in RMS tissues and cell lines were higher than that in normal tissues and cell lines (Figure 5A-F) and was inversely correlated with the expression level of miR-874 in RMS tissues (Figure 5G: r = 0.4473, P < 0.05; Figure 5H: r = 0.5324, P < 0.05).
Figure 5.
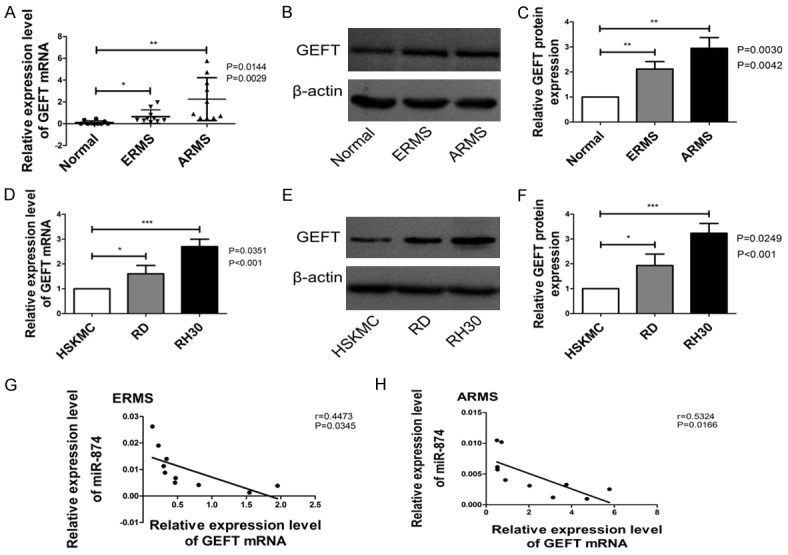
GEFT expression was upregulated in RMS tissues and cell lines. A. GEFT mRNA levels in ERMS (n = 10), ARMS (n = 10) and normal skeletal muscle tissues (n = 10) were quantified through qRT-PCR analyses. B, C. GEFT protein levels in ERMS, ARMS and normal skeletal muscle tissues were quantified through western blot analysis. D. GEFT mRNA levels in RMS (RD and RH30) and human skeletal muscle (HSKMC) cell lines were quantified through qRT-PCR analyses. E, F. GEFT protein levels in RMS (RD and RH30) and human skeletal muscle (HSKMC) cell lines were quantified through western blot analysis. G. The correlation between miR-874 and GEFT in ERMS (n = 10) was analyzed through Spearman’s correlation analysis. H. The correlation between miR-874 and GEFT in ARMS (n = 10) was analyzed through Spearman’s correlation analysis. *P < 0.05, **P < 0.01, ***P < 0.001.
Downregulation of GEFT has similar effects to miR-874 overexpression in RMS cells
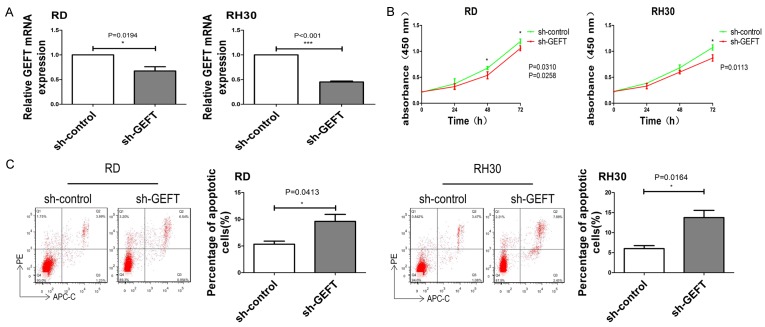
To investigate the biological functions of GEFT in RMS cells, we knocked down its expression in RD and RH30 cell lines using shRNA. The expression levels of GEFT were repressed in sh-GEFT transfectants in comparison with sh-control transfectants (Figure 6A). Growth curves constructed on the basis of CCK8 results revealed that GEFT inhibition decreased cell proliferation rates (Figure 6B). Flow cytometry results showed that the apoptosis rates of sh-GEFT-transfected cells increased (Figure 6C). Silencing GEFT inhibited the invasion (Figure 7A) and migration (Figure 7B) of RD and RH30 cells. Additionally, the results of wound-healing assays showed that the migration of GEFT down-regulating cells was dramatically inhibited relative to that of control cells (Figure 7C). These findings suggest that downregulation of GEFT mimicked the inhibitory effects of miR-874 overexpression in RD and RH30 cell lines and indicated that GEFT is a functional target of miR-874.
Figure 6.
Downregulation of GEFT inhibits cell proliferation and induces apoptosis in RMS. A. The expression levels of GEFT in RD and RH30 cells transfected with sh-GEFT or sh-control were analyzed through qRT-PCR. B. The proliferative abilities of RD and RH30 cells transfected with sh-GEFT or sh-control were determined through CCK8 assay. C. The apoptosis rates of RD and RH30 cells transfected with sh-GEFT or sh-control were determined through flow cytometry. *P < 0.05, ***P < 0.001.
Figure 7.
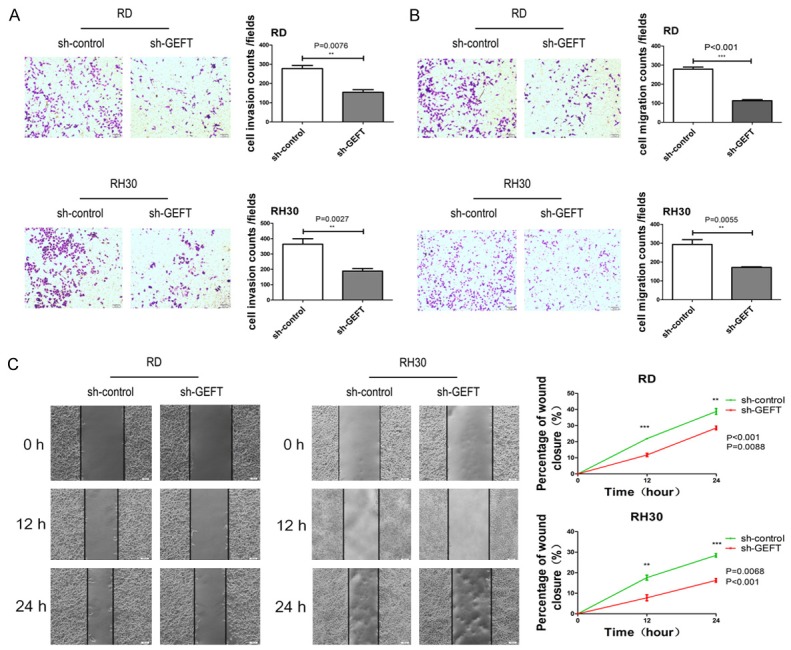
Downregulation of GEFT inhibits cell invasion and migrationin in RMS. A. The invasive capabilities of RD and RH30 cells transfected with sh-GEFT or sh-control were determined with a Transwell system. B. The migratory capabilities of RD and RH30 cells transfected with sh-GEFT or sh-control were determined with a Transwell system. C. The migratory capabilities of RD and RH30 cells transfected with sh-GEFT or sh-control were determined through the wound-healing assay. **P < 0.01, ***P < 0.001.
Restoration of GEFT reversed the effects of miR-874 on RMS cells
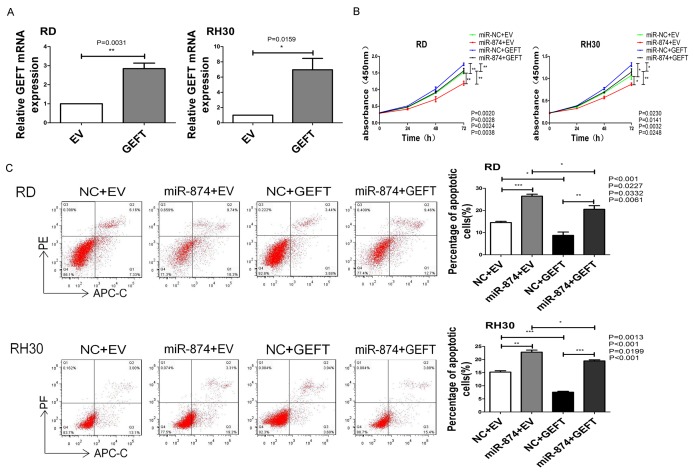
We further studied the involvement of GEFT in the function of miR-874 in RMS cells. The expression levels of GEFT in RD and RH30 cell lines stably transfected with GEFT or EV were analyzed through qRT-PCR. The results showed that GEFT expression was increased in RD and RH30 cell lines stably transfected with GEFT (Figure 8A). Then, we subjected RD and RH30 cells that were stably transfected with GEFT or EV after transfection with miR-874 or NC to CCK8, flow cytometry, wound-healing, cell invasion, and migration assays. The inhibitory effects of miR-874 on cell proliferation were rescued by the coexpression of GEFT in RD and RH30 cells (Figure 8B). The upregulation of GEFT markedly reduced the elevation of cell apoptosis rates by miR-874 (Figure 8C). GEFT overexpression rescued the suppressive effects of miR-874 overexpression on the invasive (Figure 9A) and migratory (Figure 9B, 9C) capabilities of cells. Therefore, GEFT restoration reversed the tumor-suppressive effects of miR-874 on RMS cells. These data suggest that miR-874 may partially exert its tumor-suppressive effect by targeting GEFT.
Figure 8.
GEFT restoration partially reversed the effects of miR-874-regulated tumor cell proliferation and induced apoptosis on RMS. A. The expression levels of GEFT in RD and RH30 cell lines stably transfected with GEFT or EV were analyzed through qRT-PCR. B. The proliferative capabilities of RD and RH30 cells stably transfected with GEFT or EV after transfection with miR-874 or NC were determined through CCK8 assay. C. The apoptosis rates of RD and RH30 cells stably transfected with GEFT or EV after transfection with miR-874 or NC were determined through flow cytometry assay. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 9.
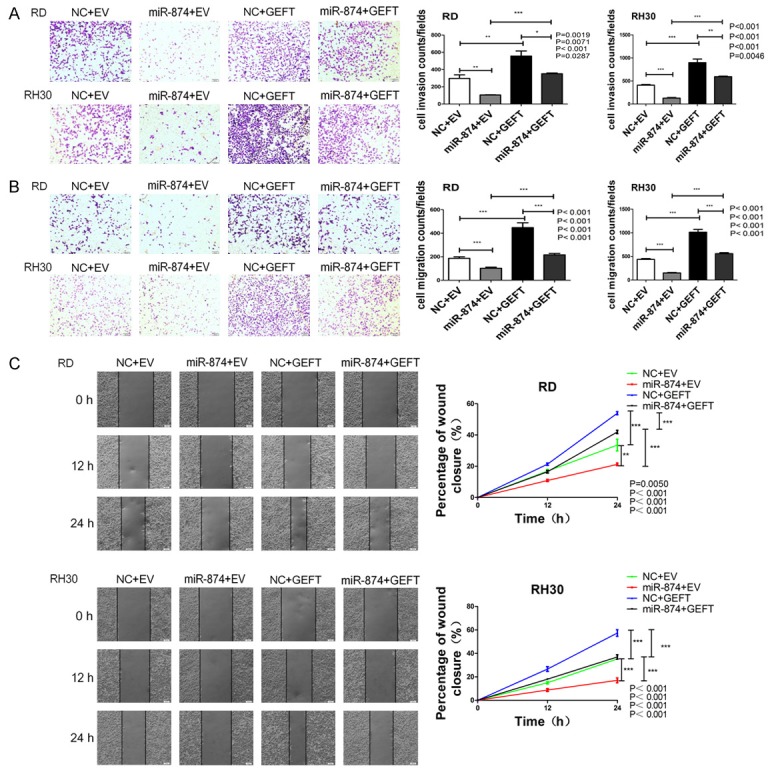
GEFT restoration partially reversed the effects of miR-874-regulated tumor cell invasion and migration on RMS. A. The invasive capabilities of RD and RH30 cells stably transfected with GEFT or EV after transfection with miR-874 or NC were determined with a Transwell system. B. The migratory capabilities of RD and RH30 cells stably transfected with GEFT or EV after transfection with miR-874 or NC were determined with a Transwell system. C. The migratory capabilities of RD and RH30 cells stably transfected with GEFT or EV after transfection with miR-874 or NC were determined through the wound-healing assay. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, we focused on the functional significance of miR-874. miR-874 is located on chromosome 5q31.2, a fragile site in the human genome that is often deleted in cancers [23,24]. It participates in cancer development and progression and functions as a tumor suppressor. Recently investigations revealed that miR-874 is downregulated in human malignancies, and furthermore, tumor suppressive functions of miR-874 in these cancer cells support that miR-874 is a tumor suppressor [15-22]. In addition, miR-874 overexpression could inhibit cancer progression and development through the regulation of target genes. For example, Zhao et al. [18] demonstrated that miR-874 functions as a tumor suppressor in human colorectal cancer by targeting STAT3. Jiang et al. [16] reported that miR-874 inhibits the growth, migration, invasion, and tumorigenicity of GC cells by targeting AQP3. Song et al. [25] showed that miR-874 drastically alters cancer cell proliferation and invasion patterns by inhibiting HCA587/MAGE-C2. These studies provide evidence for the potential role of miR-874 as a tumor suppressor in various cancers.
Nevertheless, the biological function of miR-874 in RMS remains unclear. Here, our qRT-PCR results showed that miR-874 expression was downregulated in human RMS tissues and cell lines. Furthermore, we demonstrated that the overexpression of miR-874 in RMS cell lines decreased the rates of cell proliferation, invasion, migration, and antiapoptosis. Notably, we found that miR-874 directly targeted the 3’-UTR of GEFT mRNA to inhibit GEFT translation and suppress GEFT expression, and GEFT overexpression partially attenuated the tumor-suppressive effects of miR-874 on RMS cells. These data indicate that miR-874 may function as a tumor suppressor in RMS, and miR-874 may partially exert its suppressive effects on the growth and metastasis of RMS cells by targeting GEFT.
GEFT, also known as ARHGEF25 or p63RhoGEF, is located on chromosome 12q13.3, which is highly expressed in excitable tissue, such as muscle, heart, and brain tissues. GEFT activates Rho GTPases by accelerating GDP/GTP exchange [26], regulates cell processes, and plays essential roles in the genes of important function during the processes of skeletal muscle regeneration and myogenic differentiation. Endogenous GEFT expression is transcriptionally upregulated during myogenic differentiation and downregulated during adipogenic differentiation [27]. Exogenous GEFT expression promotes myogenesis of cells by activating Rho A, Rac1, and Cdc42, while a dominant-negative mutant of GEFT strongly inhibits this process, suggesting GEFT expression is very necessary for myogenesis to occur [27]. In current study showed that GEFT overexpression is associated with the advanced clinical stage, lymph node metastasis, and distant metastasis of RMS [28,29]. We demonstrated that silencing GEFT inhibits the proliferative, migratory, invasive, and antiapoptotic capabilities of RMS cell lines. These findings indicate that GEFT overexpression may function as an oncogene in RMS. The mechanism underlying GEFT regulation in RMS, however, remains undefined. Here, our luciferase activity assay, qRT-PCR, and western blot assay results confirmed that the 3’-UTR of GEFT mRNA is the direct target of miR-874 in RMS cells. In addition, GEFT expression was upregulated in human RMS tissues and was inversely correlated with miR-874 expression. We used RMS cell lines to confirm that miR-874 inhibits cell proliferation, invasion, and migration and induces apoptosis by targeting GEFT.
Notably, we performed miR-874 overexpression experiments to investigate the effect of miR-874 on GEFT expression in RMS cell lines. The restoration of miR-874 expression decreased GEFT protein expression in RD and RH30 cells. Nevertheless, GEFT mRNA expression in RD and RH30 cells transfected with miR-874 negligibly differed from that in control cells. This phenomenon piqued our interest in the mechanism underlying the inhibitory effects of miRNAs on protein synthesis, because it is reported in a large number of documents that miRNAs usually inhibit mRNA expression and protein expression. Pillai et al. [30] showed that in plants, miRNAs generally show almost perfect complementarity to target sequences positioned in either 3’-UTR or coding regions of target mRNAs, and the perfect base pairing triggers mRNA degradation; in animals, with rare exceptions, miRNAs regulate target gene expression by base pairing imperfectly to the 3’-UTR of target mRNAs, and the role of efficient repression is either achieved by inhibiting translation or by causing mRNA degradation that are initiated by deadenylation and decapping of the mRNA. Chen et al. [31] reported that arsenic induces SATB2 overexpression by inhibiting miR-31 expression, which suppresses SATB2 translation, since SATB2 mRNA levels remain the same but protein levels decrease. Wang et al. [19] demonstrated that miR-874 suppressed CDK9 protein expression without affecting its mRNA expression, indicating that miR-874 can directly target the 3’-UTR of CDK9 mRNA, resulting CDK9 translation inhibition and suppressing its expression. Therefore, miR-874 suppresses GEFT protein expression through translational repression by targeting the 3’-UTR of GEFT mRNA in RMS.
In summary, the current study provided evidence that miR-874 reduces the proliferative, invasive, migratory, and antiapoptotic capabilities of RMS cells by directly targeting GEFT. This behavior indicates that miR-874 has a potential role as a tumor suppressor in RMS and may be applied in the treatment of this malignancy.
Conclusions
miR-874 functions as a tumor suppressor in RMS and may suppress the growth and metastasis of RMS cells through translational repression by targeting the 3’-UTR of GEFT mRNA.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81660441 and 81460404).
Disclosure of conflict of interest
None.
Abbreviations
- ARMS
alveolar rhabdomyosarcoma
- CCK8
Cell Counting Kit-8
- ERMS
embryonal rhabdomyosarcoma
- EV
empty vector
- FBS
fetal bovine serum
- mRNA
messenger RNA
- miRNAs
microRNAs
- miR-874
microRNA-874
- Mut
mutant
- NC
negative control
- qRT-PCR
quantitative real-time polymerase chain reaction
- RMS
rhabdomyosarcoma
- Wt
wild-type
- 3’-UTR
3’-untranslated region
References
- 1.Kramer S, Meadows A, Jarrett P, Evans A. Incidence of childhood cancer: experience of a decade in a population-based registry. J Natl Cancer Inst. 1983;70:49–55. [PubMed] [Google Scholar]
- 2.Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4:34–44. [PubMed] [Google Scholar]
- 3.Malempati S, Hawkins D. Rhabdomyosarcoma: review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer. 2012;59:5–10. doi: 10.1002/pbc.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esquela-Kerscher A, Slack F. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 5.Filipowicz W, Bhattacharyya S, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 6.Fabian M, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 7.Guo H, Ingolia N, Weissman J, Bartel D. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Getz G, Miska E, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert B, Mak R, Ferrando A, Downing J, Jacks T, Horvitz H, Golub T. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Qiu C, Zhang H, Wang J, Cui Q, Yin Y. Human microRNA oncogenes and tumor suppressors show significantly different biological patterns: from functions to targets. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J, Ahmad A, Sarkar F. The role of microRNAs in breast cancer migration, invasion and metastasis. Int J Mol Sci. 2012;13:13414–13437. doi: 10.3390/ijms131013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Zhang J, Wang H, Zhao J, Xu C, Du Y, Luo X, Zheng F, Liu R, Zhang H, Ma D. miRNA-135a promotes breast cancer cell migration and invasion by targeting HOXA10. BMC Cancer. 2012;12:111. doi: 10.1186/1471-2407-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bersani F, Lingua M, Morena D, Foglizzo V, Miretti S, Lanzetti L, Carrà G, Morotti A, Ala U, Provero P, Chiarle R, Singer S, Ladanyi M, Tuschl T, Ponzetto C, Taulli R. Deep sequencing reveals a novel miR-22 regulatory network with therapeutic potential in rhabdomyosarcoma. Cancer Res. 2016;76:6095–6106. doi: 10.1158/0008-5472.CAN-16-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna J, Garcia M, Go J, Finkelstein D, Kodali K, Pagala V, Wang X, Peng J, Hatley M. PAX7 is a required target for microRNA-206-induced differentiation of fusion-negative rhabdomyosarcoma. Cell Death Dis. 2016;7:e2256. doi: 10.1038/cddis.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Megiorni F, Cialfi S, McDowell H, Felsani A, Camero S, Guffanti A, Pizer B, Clerico A, De Grazia A, Pizzuti A, Moles A, Dominici C. Deep Sequencing the microRNA profile in rhabdomyosarcoma reveals down-regulation of miR-378 family members. BMC Cancer. 2014;14:880. doi: 10.1186/1471-2407-14-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Tang J, Zhi X, Xie K, Wang W, Li Z, Zhu Y, Yang L, Xu H, Xu Z. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget. 2015;6:1605–1617. doi: 10.18632/oncotarget.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang B, Li Z, Zhang W, Wang H, Zhi X, Feng J, Chen Z, Zhu Y, Yang L, Xu H, Xu Z. miR-874 Inhibits cell proliferation, migration and invasion through targeting aquaporin-3 in gastric cancer. J Gastroenterol. 2014;49:1011–1025. doi: 10.1007/s00535-013-0851-9. [DOI] [PubMed] [Google Scholar]
- 17.Leong K, Cheng C, Wong C, Ng I, Kwong Y, Tse E. miR-874-3p is down-regulated in hepatocellular carcinoma and negatively regulates PIN1 expression. Oncotarget. 2017;8:11343–11355. doi: 10.18632/oncotarget.14526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Dong A. MiR-874 inhibits cell growth and induces apoptosis by targeting STAT3 in human colorectal cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:269–277. [PubMed] [Google Scholar]
- 19.Wang L, Gao W, Hu F, Xu Z, Wang F. MicroRNA-874 inhibits cell proliferation and induces apoptosis in human breast cancer by targeting CDK9. FEBS Lett. 2014;588:4527–4535. doi: 10.1016/j.febslet.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Kesanakurti D, Maddirela D, Chittivelu S, Rao J, Chetty C. Suppression of tumor cell invasiveness and in vivo tumor growth by microRNA-874 in non-small cell lung cancer. Biochem Biophys Res Commun. 2013;434:627–633. doi: 10.1016/j.bbrc.2013.03.132. [DOI] [PubMed] [Google Scholar]
- 21.Nohata N, Hanazawa T, Kikkawa N, Sakurai D, Fujimura L, Chiyomaru T, Kawakami K, Yoshino H, Enokida H, Nakagawa M, Katayama A, Harabuchi Y, Okamoto Y, Seki N. Tumour suppressive microRNA-874 regulates novel cancer networks in maxillary sinus squamous cell carcinoma. Br J Cancer. 2011;105:833–841. doi: 10.1038/bjc.2011.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong D, Gong Y, Zhang D, Bao H, Gu G. miR-874 suppresses the proliferation and metastasis of osteosarcoma by targeting E2F3. Tumour Biol. 2016;37:6447–6455. doi: 10.1007/s13277-015-4527-3. [DOI] [PubMed] [Google Scholar]
- 23.Fundia A, Gorla N, Bonduel M, Azpilicueta O, Lejarraga H, Muriel F, Larripa I. Increased expression of 5q31 fragile site in a Bloom syndrome family. Hum Genet. 1992;89:569–572. doi: 10.1007/BF00219187. [DOI] [PubMed] [Google Scholar]
- 24.Thorland E, Myers S, Gostout B, Smith D. Common fragile sites are preferential targets for HPV16 integrations in cervical tumors. Oncogene. 2003;22:1225–1237. doi: 10.1038/sj.onc.1206170. [DOI] [PubMed] [Google Scholar]
- 25.Song X, Song W, Wang Y, Wang J, Li Y, Qian X, Pang X, Zhang Y, Yin Y. MicroRNA-874 functions as a tumor suppressor by targeting cancer/testis antigen HCA587/MAGE-C2. J Cancer. 2016;7:656–663. doi: 10.7150/jca.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stam J, Collard J. The DH protein family, exchange factors for Rho-like GTPases. Prog Mol Subcell Biol. 1999;22:51–83. doi: 10.1007/978-3-642-58591-3_4. [DOI] [PubMed] [Google Scholar]
- 27.Bryan B, Mitchell D, Zhao L, Ma W, Stafford L, Teng B, Liu M. Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT. Mol Cell Biol. 2005;25:11089–11101. doi: 10.1128/MCB.25.24.11089-11101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Li D, Jiang J, Hu J, Zhang W, Chen Y, Cui X, Qi Y, Zou H, Zhang W, Li F. Analysis of molecular cytogenetic alteration in rhabdomyosarcoma by array comparative genomic hybridization. PLoS One. 2014;9:e94924. doi: 10.1371/journal.pone.0094924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun C, Liu C, Li S, Li H, Wang Y, Xie Y, Li B, Cui X, Chen Y, Zhang W, Li F. Overexpression of GEFT, a Rho family guanine nucleotide exchange factor, predicts poor prognosis in patients with rhabdomyosarcoma. Int J Clin Exp Pathol. 2014;7:1606–1615. [PMC free article] [PubMed] [Google Scholar]
- 30.Pillai R, Bhattacharyya S, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Li J, Sun H, Wu F, Zhu Y, Kluz T, Jordan A, DesMarais T, Zhang X, Murphy A, Costa M. Role of miR-31 and SATB2 in arsenic-induced malignant BEAS-2B cell transformation. Mol Carcinog. 2018;57:968–977. doi: 10.1002/mc.22817. [DOI] [PMC free article] [PubMed] [Google Scholar]