Abstract
Cell lines represent an invaluable resource in modern science including basic and translational cancer research. Although there have been warnings over the past half century, the number of publications in the literature that erroneously used “non-existent” cell lines is still growing. For example, the Hep-2 cell line, first described in 1954 as laryngeal cancer cells, was reported as soon as in 1966 to be comprised of cervical adenocarcinoma cells derived via HeLa cell line contamination. Notably, the International Cell Line Authentication Committee (ICLAC) reported Hep-2 to be one among 488 misidentified cell lines, and one of 451 cell lines where no authentic stock is known. However, the number of laryngeal cancer research publications using the Hep-2 cell line has greatly increased over the past three decades. A comprehensive review of Hep-2 cell line misuse has been performed to identify the extent of the problem. 1,036 publications referenced in the MEDLINE database from 1954 to the first of January 2018 referred to the purported laryngeal origin of the Hep-2 cell line, with an increasing trend and with a peak of 93 publications in 2014. The rate of publications that focused on laryngeal cancer topics have increased over the past three decades to reach 80% in 2017. This increase was mainly driven by the remarkable productivity of Chinese researchers, of which English-language publications represented 76% of these articles in 2017. International collaborations and up-to-date national guidelines are needed.
Keywords: Cell line, HeLa, laryngeal neoplasms, cancer research, scientific misconduct
Introduction
The advent of cell line technology was a pivotal moment in the history of cancer research. Since its beginnings, however, cell line technology has faced two major and well-described methodological issues, namely cross-contamination and misidentification [1,2]. Cross-contamination is contamination of a cell line culture by another cell line [3]. Cell line misidentification occurs when a cell line is erroneously identified [2]. Quality assurance programs have been devised to reduce these issues at research facilities, and they are generally required for publication in the more highly regarded international academic journals [4]. Since 2010, The International Cell Line Authentication Committee (ICLAC) has developed and openly published a database of cross-contaminated and misidentified cell lines [5]. Its most recent update in December 2016 reported 488 misidentified cell lines of which 451 cell lines where no authentic stock is known to exist.
The Hep-2 cell line is a perfect example of this. Hep-2/Hep2 cells are invaluable for researchers engaged in the analysis of autoantibodies, and they are currently one of the most common substrates for antinuclear antibody detection by immunofluorescence [6,7]. The Hep-2 cell line was first specifically described by H.W. Toolan in 1954 [8]. A tumor specimen was obtained in 1951 from a patient presenting with a laryngeal carcinoma. However, Stan Gartler showed in 1966 and published in 1968 that the Hep-2 was in fact comprised of cervical adenocarcinoma cells, derived via HeLa cell line contamination [9]. This was definitely corroborated by Chen in 1988 [10]. The Hep-2 cell line was subsequently confirmed via PCR to be positive for human papillomavirus DNA sequences. Thus, fifty years after the first publication of the in fact never-existent Hep-2 cell line, and after worldwide spread of its non-existence in bioresource collections around the world, one would expect that the international research community would have ensured that this scientific misconduct would no longer present itself.
And yet, the problem of the “non-existent” cell lines problem has never been more endemic [11]. Indeed, it presently poses a significant threat to modern cancer research due to doubts and uncertainty regarding the reliability of the growing literature in basic and translational research. A review of the Hep-2 cell line example perfectly illustrates the quantitative and qualitative nature of this issue in translational research for laryngeal cancer.
Review of the literature regarding the Hep-2 cell line and laryngeal cancer
An increasing number of laryngeal research publications that used the Hep-2 cell line in vitro
The degree of the biomedical literature contamination was investigated in the MEDLINE database. From its first description in 1954 until the 1st of January 2018, the Hep-2/Hep2 cell line has been cited in 5,469 articles in MEDLINE. The number of Hep-2 cell line citations has increased quite linearly (Figure 1), although these citations comprise two very different groups of publications. The first group of articles refer to the Hep-2 cell line without stating their purported laryngeal origin. Since 1989 until present the number of publications in this group has ranged from approximately 100 to 150 publications per year. The second group of publications specifically refers to the purported laryngeal origin. It contains 1,036 articles published up to the 1st of January 2018, of which 794 articles are in English. The number has increased significantly since the publication by Chen in 1988: 8 articles reported the laryngeal origin out of 119 articles referring to the Hep-2 cell line in 1989 (6.7%); in 2017, it was 51 articles out of 160 (31.9%).
Figure 1.
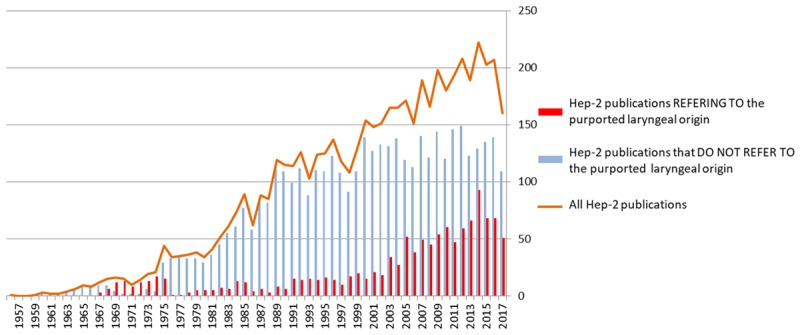
The annual number of articles in the MEDLINE database referring to the Hep-2 cell line with or without stating its historically assumed laryngeal carcinoma origin.
However, studies published that used Hep-2 cell line must be distinguished between studies where the larynx was the specific target, as opposed to studies for which it was not the case. In the first case, the non-laryngeal nature of the Hep-2 cells cannot be addressed by making changes to the text of the publication, since the model was wrong from the start. Conversely, some studies have referred to a presumed laryngeal origin without having specifically targeted the larynx in the study. In an article describing this kind of study, a straightforward correction of the text could easily be achieved by simply removing the citation about the laryngeal origin, without detracting from the merits or the relevance of the paper’s findings. A careful review of the 794 articles that were published in English in the MEDLINE database until the 1st of January 2018 indicates that the increasing number over the past thirty years has mainly been due to articles referring to the laryngeal origin of the Hep-2 cell line. In 1989 there were no such articles, in 2000 this was the case for 4 out of 13 (30.8%), in 2010 it was the case for 28 out of 47 (59.6%), and in 2017 it was the case for 40 out of 50 (80%) (Figure 2).
Figure 2.
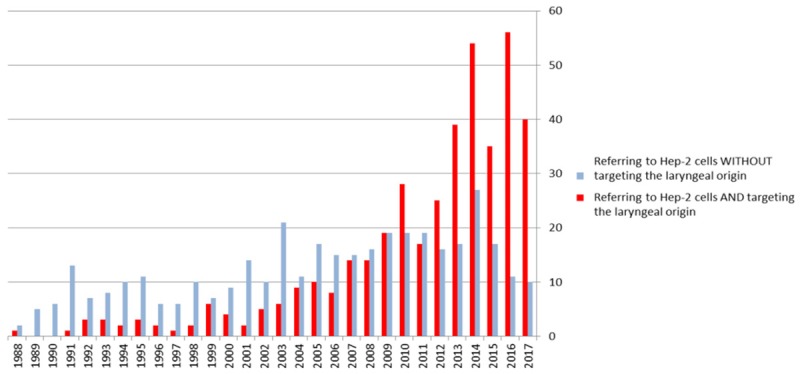
The number of articles in the MEDLINE database referring to the Hep-2 cell line and its historically assumed laryngeal carcinoma origin, according to whether or not the laryngeal nature of the cells was directly relevant to the findings.
Focus on cancer research
Since Chen’s publication in 1988 until the 1st of January 2018, neoplasm nature of the cell line has increasingly been the reason for using the “laryngeal” Hep-2 cell line: Thus, this was the case for 2 out of 5 publications in 1989 that were in in English (40%), 8 out of 13 (61.5%) in 2000, 36 out of 47 (76.6%) in 2010, and 43 out of 50 (86%) in 2017.
China’s contribution
As we saw earlier, 242 non-English articles are available in the MEDLINE database that were published between 1954 and the 1st of January 2018 and that referred to the laryngeal origin of the Hep-2 cell line. Of these articles, 201 (83.1%) were in Chinese, by researchers from either the People’s Republic of China or from the Republic of China (Taiwan). After a peak in 2005, with 23 Chinese articles, the number has been decreasing steadily over the years, and only one Chinese article was published in 2017. The trend is the opposite, however, in English-language publications (Figure 3). While 1 out of 13 (7.7%) of the English-language articles were from China in 2000, there were 25 out of 47 (52.2%) in 2010, and 38 out of 50 (76%) in 2017.
Figure 3.
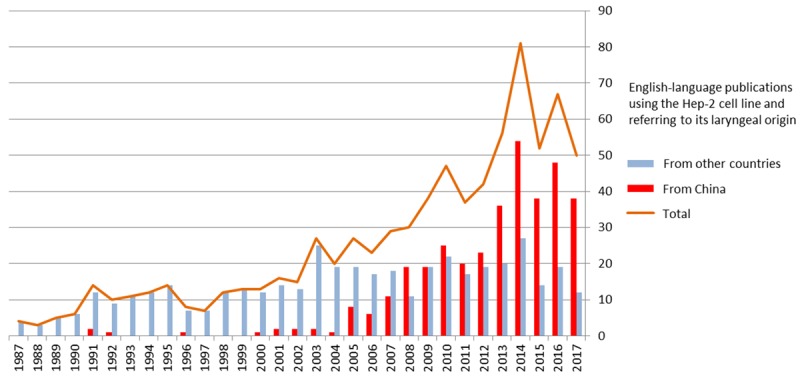
The number of English-language articles in the MEDLINE database citing the Hep-2 cell line and its historically assumed laryngeal carcinoma origin, according to the country of the corresponding author(s).
Among the 38 English-language articles from China in 2017, 30 reported the bioresource collection from which the Hep-2 cell line had been obtained. In 19 out of 30, the Hep-2 cell line was reported to have been obtained from various Chinese bioresource collections. In the remaining 11 studies, the Hep-2 cell line was reported to have been obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA).
Discussion
The findings presented here indicate that the number of laryngeal cancer research publications using the “never-existent” Hep-2 cell line has increased greatly in the past three decades. This increase was mainly driven by the remarkable productivity of Chinese researchers. It is therefore quite reasonable to question whether the scientific literature on cancer can in fact still be trusted given the quite considerable extent of this problem in basic and translational research.
The cell line misidentification issue has already been reported as a global problem in science, with approximately 20% of all cell lines being incorrectly labelled [12-14]. The extent of misidentification has in fact recently been shown to affect 73.2% of the cell lines established by Chinese researchers, accounting for 40.6% of all misidentifications [15]. HeLa cells are the most infamous culprits of cell line cross-contamination and misidentification [1,16,17]. More than fifty years ago, at the Second Decennial Review Conference on Cell, Tissue, and Organ Culture in 1966, Stan Gartler already reported that 18 human cell lines, including the Hep-2 cell line, were in fact all HeLa cells. Walter Nelson-Rees further developed techniques for the authentication of cell lines, and he reported evidence of widespread contamination of cell lines by HeLa cells in research facilities and bioresource collections [18,19]. He published updated lists of cross-contaminated and misidentified cell lines to raise awareness of the problem in the scientific community [20-22]. In the 2010’s, an international group of scientists were finally able to achieve coordination of the American Type Culture Collection with a large number of major cell banks worldwide to form the International Cell Line Authentication Committee (ICLAC) [5,14,23-26]. The ICLAC provides resources relating to the use of human cell lines in research: an updated register of misidentified cell lines, advice to scientists about incorporating authentication into everyday culture practice, cell line checklists for manuscripts and grant applications, a guide for human cell line authentication, and other forms of support in this regard. Unfortunately, the major Chinese bioresource collections are not yet partner organizations of the ICLAC. Therefore, despite all of the above described efforts, cell line misidentification is still increasing in the cancer research literature.
However, scientific mistakes such as this issue regarding cell line may be just part of a global problem of increasingly reported instances of scientific misconducts, and several ethics failures have been shown to have arisen on several occasions [27]. The investigation reported in this article was initiated at the beginning of 2017 after the review of a manuscript written by a team of Chinese researchers who studied the association of miR-448 and AEG1 expression in laryngeal cancer. Unfortunately, the laryngeal carcinoma cell line used in this study was the Hep-2 cell line. Without addressing this issue, the same manuscript aside from addition of another name to the list of authors, was submitted to a different journal just a couple of months later that resulted in it recently being published. Reports of scientific misconduct are, unfortunately, quite common. The never ending publish or perish curse certainly remains the reason for the constant pressure on academics to publish, as long as competition among researchers for a limited number of grants and positions continues to be the dominant model [28]. Furthermore, it is striking that the increasing involvement of Chinese researchers in cell line misidentification occurred simultaneously with the monetary reward system for science in China, whereby Chinese research institutions adopted cash-per-publication reward policies for researchers [29,30]. The effect of replacing recognition by peers with cash earnings as the reward for scientific productivity has been shown to further foster an emphasis on the quantity of publications and to promote short-term evaluation of research quality. It certainly amounts to additional pressure to publish all of the work that has been performed, potentially blinding researchers to secondary ethical aspects [31].
Although scientific misconduct is a global problem, it appears to be particularly sensitive in China [32-37]. China has a more dynamic but also a shorter history of modern scientific endeavor than in Western countries. In the past three decades, China has become a major contributor to science and technology, and it is predicted to outspend the United States in terms of science by 2020 [38]. However, China still needs to establish a national comprehensive ethical system in science. Most of Chinese researchers are nowadays aware of the problem of scientific misconduct, and they regard academic integrity violations as regrettable yet common occurences [39]. To address this issue and to protect the academic integrity of the vast majority of Chinese researchers, in May of 2018 the Chinese authorities released a document establishing the framework for a scientific integrity mechanism based on national guidelines. This is undoubtedly very good news, for the international scientific community, as well as for scientists of the foreseeable world leader of scientific research. An important step should be tackling the issue of the “non-existent” cell lines. Participation of the Chinese bioresource collections as partners of the ICLAC in the near future will be required to help disseminate the resources for proper use of human cell lines in scientific research.
Finally, one of the most disturbing aspects of this issue is the astonishing number of international journals that continue to publish misleading data. The best example, as far as we are concerned, is the Academic journal which was referred to earlier, which recently asked us to review another manuscript with exactly the same Hep-2 cell line issue, despite previous letters about the need to address the failure of their editorial process in this regard. In 2002, John R. Masters already reported that some journal editors used peer review as a shield to deflect their responsibility, while authors and reviewers generally appeared to be unaware of the problem [1]. Sixteen years later, the proportion of scientific journals that request cell line authentication quality controls as a pre-requisite for publication is still remarkably low. The number of erroneous articles published in 2018 is already high. Clearly, things will not change until we do.
Acknowledgements
The author is very grateful to Dr. Hervé Maisonneuve for his advices.
Disclosure of conflict of interest
None.
References
- 1.Masters J. False cell lines. Int J Cancer. 2002;99:154. doi: 10.1002/ijc.10315. [DOI] [PubMed] [Google Scholar]
- 2.Canny G. Cell line contamination and misidentification. Biol Reprod. 2013;89:76. doi: 10.1095/biolreprod.113.113423. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama S. HeLa cell lines. Methods Enzymol. 1987;151:38–50. doi: 10.1016/s0076-6879(87)51007-2. [DOI] [PubMed] [Google Scholar]
- 4.Geraghty RJ, Capes-Davis A, Davis JM, Downward J, Freshney RI, Knezevic I, Lovell-Badge R, Masters JR, Meredith J, Stacey GN, Thraves P, Vias M Cancer Research UK. Guidelines for the use of cell lines in biomedical research. Br J Cancer. 2014;111:1021–1046. doi: 10.1038/bjc.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capes-Davis A, Theodosopoulos G, Atkin I, Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA, Reddel RR, Freshney RI. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int J Cancer. 2010;127:1–8. doi: 10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 6.Buchner C, Bryant C, Eslami A, Lakos G. Anti-nuclear antibody screening using HEp-2 cells. J Vis Exp. 2014:e51211. doi: 10.3791/51211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daves M, Blecken J, Matthias T, Frey A, Perkmann V, Dall Acqua A, Joos A, Platzgummer S. New automated indirect immunofluorescent antinuclear antibody testing compares well with established manual immunofluorescent screening and titration for antinuclear antibody on HEp-2 cells. Immunol Res. 2017;65:370–374. doi: 10.1007/s12026-016-8874-y. [DOI] [PubMed] [Google Scholar]
- 8.Toolan HW. Transplantable human neoplasms maintained in cortisone-treated laboratory animals: H.S. No. 1; H.Ep. No. 1; H.Ep. No. 2; H.Ep. No. 3; and H.Emb.Rh. No. 1. Cancer Res. 1954;14:660–666. [PubMed] [Google Scholar]
- 9.Gartler SM. Apparent Hela cell contamination of human heteroploid cell lines. Nature. 1968;217:750–751. doi: 10.1038/217750a0. [DOI] [PubMed] [Google Scholar]
- 10.Chen TR. Re-evaluation of HeLa, HeLa S3, and HEp-2 karyotypes. Cytogenet Cell Genet. 1988;48:19–24. doi: 10.1159/000132579. [DOI] [PubMed] [Google Scholar]
- 11.Horbach S, Halffman W. The ghosts of HeLa: how cell line misidentification contaminates the scientific literature. PLoS One. 2017;12:e0186281. doi: 10.1371/journal.pone.0186281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markovic O, Markovic N. Cell cross-contamination in cell cultures: the silent and neglected danger. In Vitro Cell Dev Biol Anim. 1998;34:1–8. doi: 10.1007/s11626-998-0040-y. [DOI] [PubMed] [Google Scholar]
- 13.MacLeod RA, Dirks WG, Matsuo Y, Kaufmann M, Milch H, Drexler HG. Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int J Cancer. 1999;83:555–563. doi: 10.1002/(sici)1097-0215(19991112)83:4<555::aid-ijc19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Nardone RM. Eradication of cross-contaminated cell lines: a call for action. Cell Biol Toxicol. 2007;23:367–372. doi: 10.1007/s10565-007-9019-9. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Liu Y, Zheng C, Shen C. Investigation of cross-contamination and misidentification of 278 widely used tumor cell lines. PLoS One. 2017;12:e0170384. doi: 10.1371/journal.pone.0170384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold M. A conspiracy of cells. State University of New York Press; 1986. [Google Scholar]
- 17.Lucey BP, Nelson-Rees WA, Hutchins GM. Henrietta Lacks, HeLa cells, and cell culture contamination. Arch Pathol Lab Med. 2009;133:1463–1467. doi: 10.5858/133.9.1463. [DOI] [PubMed] [Google Scholar]
- 18.Nelson-Rees WA, Flandermeyer RR, Hawthorne PK. Distinctive banded marker chromosomes of human tumor cell lines. Int J Cancer. 1975;16:74–82. doi: 10.1002/ijc.2910160109. [DOI] [PubMed] [Google Scholar]
- 19.Nelson-Rees WA, Flandermeyer RR. HeLa cultures defined. Science. 1976;191:96–98. doi: 10.1126/science.1246601. [DOI] [PubMed] [Google Scholar]
- 20.Nelson-Rees WA, Flandermeyer RR. Inter- and intraspecies contamination of human breast tumor cell lines HBC and BrCa5 and other cell cultures. Science. 1977;195:1343–1344. doi: 10.1126/science.557237. [DOI] [PubMed] [Google Scholar]
- 21.Nelson-Rees WA. The identification and monitoring of cell line specificity. Prog Clin Biol Res. 1978;26:25–79. [PubMed] [Google Scholar]
- 22.Nelson-Rees WA, Daniels DW, Flandermeyer RR. Cross-contamination of cells in culture. Science. 1981;212:446–452. doi: 10.1126/science.6451928. [DOI] [PubMed] [Google Scholar]
- 23.Masters JR. False cell lines: the problem and a solution. Cytotechnology. 2002;39:69–74. doi: 10.1023/A:1022908930937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardone RM. Curbing rampant cross-contamination and misidentification of cell lines. Biotechniques. 2008;45:221–227. doi: 10.2144/000112925. [DOI] [PubMed] [Google Scholar]
- 25.American Type Culture Collection Standards Development Organization Workgroup ASN-0002. Cell line misidentification: the beginning of the end. Nat Rev Cancer. 2010;10:441–448. doi: 10.1038/nrc2852. [DOI] [PubMed] [Google Scholar]
- 26.Masters JR. Cell-line authentication: end the scandal of false cell lines. Nature. 2012;492:186. doi: 10.1038/492186a. [DOI] [PubMed] [Google Scholar]
- 27.Gao Q, Wang XY, Zhou J, Fan J. Cell line misidentification: the case of the chang liver cell line. Hepatology. 2011;54:1894–1895. doi: 10.1002/hep.24475. [DOI] [PubMed] [Google Scholar]
- 28.Publish or perish. Nature. 2015;521:259. doi: 10.1038/521259a. [DOI] [PubMed] [Google Scholar]
- 29.Qiu J. Publish or perish in China. Nature. 2010;463:142–143. doi: 10.1038/463142a. [DOI] [PubMed] [Google Scholar]
- 30.Ding Y. Chinese academy of sciences. In China, publish or perish is becoming the new reality. Science. 2001;291:1477–1479. doi: 10.1126/science.291.5508.1477. [DOI] [PubMed] [Google Scholar]
- 31.Jafarey A. Deceit and fraud in medical research - publish or perish culture is to blame. Int J Surg. 2006;4:132. doi: 10.1016/j.ijsu.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Finding fraud in China. Nature. 2006;441:549–550. doi: 10.1038/441549b. [DOI] [PubMed] [Google Scholar]
- 33.Jia H. Frequent cases force China to face up to scientific fraud. Nat Med. 2006;12:867. doi: 10.1038/nm0806-867a. [DOI] [PubMed] [Google Scholar]
- 34.Scientific fraud: action needed in China. Lancet. 2010;375:94. doi: 10.1016/S0140-6736(10)60030-X. [DOI] [PubMed] [Google Scholar]
- 35.Zeng W, Resnik D. Research integrity in China: problems and prospects. Dev World Bioeth. 2010;10:164–171. doi: 10.1111/j.1471-8847.2009.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W. Research integrity in China. Science. 2013;342:1019. doi: 10.1126/science.1247700. [DOI] [PubMed] [Google Scholar]
- 37.Normile D. China cracks down on fraud. Science. 2017;357:435. doi: 10.1126/science.357.6350.435. [DOI] [PubMed] [Google Scholar]
- 38.Xie Y, Zhang C, Lai Q. China’s rise as a major contributor to science and technology. Proc Natl Acad Sci U S A. 2014;111:9437–9442. doi: 10.1073/pnas.1407709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao QJ, Zhang YY, Fan YC, Zheng MH, Bai Y, Eslick GD, He XX, Zhang SB, Xia HH, He H. Perceptions of Chinese biomedical researchers towards academic misconduct: a comparison between 2015 and 2010. Sci Eng Ethics. 2018;24:629–645. doi: 10.1007/s11948-017-9913-3. [DOI] [PubMed] [Google Scholar]


