Abstract
The present study aims to investigate retrospectively the efficacy and safety of sorafenib combined with radiofrequency ablation (RFA) to treat unresectable remnant large hepatocellular carcinoma (HCC) after transcatheter arterial chemoembolization (TACE). The 229 consecutive patients with unresectable remnant large HCC (diameter ≥ 5 cm) received RFA and sorafenib (RFA + Sor group, n = 102) or sorafenib (Sor group, n = 127) from January 2010 to January 2016. Complications and overall survival (OS) of the two groups were compared and subgroup analysis carried out. Survival curves were drawn using the Kaplan-Meier method. The RFA + Sor group had no additional serious adverse events. The average OS was 18.3 ± 1.6 months (95% confidence interval [CI]: 15.2-21.4) in the RFA + Sor group and 14.1 ± 1.1 months (95% CI: 11.8-16.3) in the Sor group, a difference the log-rank test indicated was significant (P = 0.03). The 1-, 2- and 3-year survival rates of the RFA + Sor group were 56.9%, 34.3%, and 11.7%, and those of the Sor group were 42.5%, 22.0%, and 5.5%, respectively. The between-group differences in 1- and 2-year survival rates were statistically significant, but not the difference in 3-year survival rates. Subgroup analysis showed that the RFA + Sor group achieved significantly more lifetime benefits than the Sor group in: patients with tumors 5-10 cm in diameter (hazard ratio [HR] 0.42, 95% CI 0.21-1.06 vs. HR 0.94, 95% CI 0.63-1.22); patients with an isolated tumor (HR 0.36, 95% CI 0.19-0.81 vs. HR 0.93, 95% CI 0.55-1.24); and patients with remnant lesion volume < 50% after TACE (HR 0.47, 95% CI 0.21-1.12 vs. HR 0.77, 95% CI 0.46-0.81). RFA with sorafenib is safe and effective for unresectable remnant large HCC, controlling tumor progression and prolonging survival better than sorafenib alone.
Keywords: Radiofrequency ablation, hepatocellular carcinoma
Introduction
Large hepatocellular carcinoma (HCC) is a hepatocellular carcinoma ≥ 5 cm in diameter [1]. Patients with large HCC are often diagnosed late, when tumor volume is already large and that of normal liver tissue is reduced; liver function is poor; and microvascular invasion and compression of adjacent organs is often present. Most of these patients are in the advanced stage, and the risk of surgical removal is relatively high [2,3]. Evidence-based research has confirmed that transcatheter arterial chemoembolization (TACE) and sorafenib can effectively control the growth of late-stage HCC, improve prognosis, and prolong survival [4]. However, most large HCCs are rich in blood supply, have mutated origin of the tumor blood supply arteries, and have poorly open small arteries [5-7]; therefore, complete embolization is difficult, even with superselective TACE. Even when the operator subjectively feels he/she has achieved complete embolization of the tumor, some residuals always remain, suggesting that TACE cannot always achieve complete tumor necrosis and postoperative remnant cancer is common [8]. In addition, TACE can easily form a hypoxic microenvironment after tumor embolization, leading to changes in hypoxia-related factors (e.g., vascular endothelial growth factor, microRNA-210), increasing the risk of tumor recurrence and metastasis [9-11]. Sorafenib, a multi-kinase inhibitor, inhibits tumor angiogenesis and tumor cell proliferation [12] and can theoretically make up for this deficiency. Many liver cancer treatment guidelines consider radiofrequency ablation (RFA) as effective as surgical resection to treat small HCC < 3 cm in diameter. It can be manipulated easily and can achieve complete coagulative necrosis [13], but because of the heat sink effect and the limited ablation range, this technique is still not suitable for large HCC [14]. In theory, RFA can be used as a complementary treatment for remnant cancer after TACE in large HCC, but it may increase the liver load. However, there are few reports of remnant cancer after TACE treatment of HCC, especially large HCC. Therefore, this study retrospectively analyzed our experience in treating remnant cancer after TACE for patients with large HCC, to compare the efficacy, safety, and prognostic factors of sorafenib with RFA and sorafenib alone.
Materials and methods
Design
This retrospective study was approved by the hospital ethics committee, which waived patient informed consent. Inclusion criteria were: HCC ≥ 5 cm in diameter; remnant tumor tissue after TACE due to large tumor volume; Child-Pugh grade A or B; and platelets > 60 × 109/L, prothrombin time < 6 s, or values < 1.5 times the upper limit of normal for: serum alanine aminotransferase, aspartate aminotransferase, or creatinine. Exclusion criteria were: associated tumor thrombus in the portal or hepatic vein; new intrahepatic or extrahepatic metastases after TACE; rejection of sorafenib treatment; topical treatments other than RFA; and non-standardized sorafenib treatment. Data were collected from 1,437 consecutive patients with large HCC who underwent TACE between January 2010 and January 2016.
Devices
The therapeutic unit equipped with Siemens Miyabi Angio-CT system (Siemens Medical Solutions AG, Erlangen, Germany) and GE INNOVA 4100 IQ (GE Healthcare, Milwaukee, WI, USA). The RF therapy device was the RITA® RF System, Model 1500, with the RITA® RF ablation electrode needle (RITA Medical System, Mountain View, CA, USA).
Treatment methods
All patients with large HCCs underwent TACE for 2-4 weeks, then enhanced magnetic resonance imaging (MRI) (Figure 4C). The patient with remnant cancer or the patient’s guardian was informed of the estimated efficacy, potential risk, and cost of treatment of RFA with sorafenib versus sorafenib alone. The patient or guardian determined the method of treatment, which was initiated after informed consent was obtained.
Figure 4.
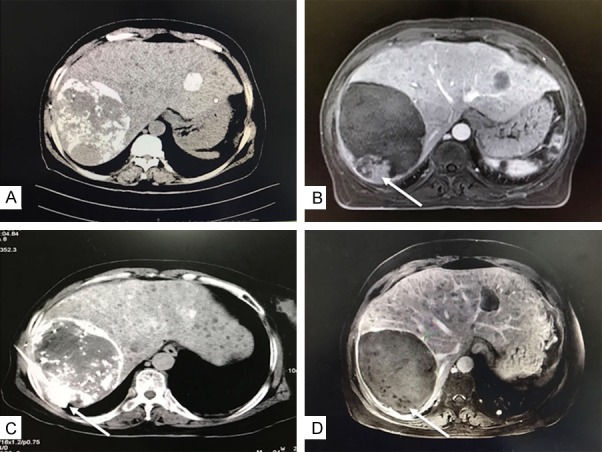
An older female patient showed large hepatocellular carcinoma with local remnants (arrow) after transcatheter arterial chemoembolization (TACE). A. The Computed Tomography scan showed lipiodol deposition in the tumor in one month after TACE. B. The magnetic resonance imaging (MRI) showed slightly uneven enhancement of the residual tumor during the arterial phase. C. Radiofrequency ablation (RFA) treatment (arrow) for the residual tumor and RFA combined with sorafenib therapy. D. The MRI showed complete remission (arrow) in the residual tumor.
Sorafenib treatment and management
The initial dose of sorafenib (Bayer Pharmaceuticals, Leverkusen, Germany) was 400 mg twice daily. The medication was suspended 1-2 days before RFA treatment and the initial oral dose resumed within one week after RFA according to the recovery of liver function. When intolerable adverse reactions occurred, the dose was reduced. Adverse reactions were evaluated according to the Common Terminology Criteria for Adverse Events 4.0 standard [15]. The dose was reduced according to the SHARP test [16]: first to 400 mg once a day, then to 400 mg once every other day. For a grade 3 or 4 toxic reaction, sorafenib was suspended. If toxicity was reduced to level ≤ 2 within 30 days, oral medication was resumed; otherwise, medication was stopped permanently. Patients took sorafenib for at least 3 months, until the lesion progressed or they died. Patients were followed through outpatient visits, telephone follow up, and the China Charity Federation APP.
Radiofrequency ablation
Two doctors with 10+ years’ experience performed RFA treatment for remnant cancer after TACE. After successful computed tomography-guided radiofrequency puncture, the 1500 RITA® radiofrequency ablation tumor treatment system was switched on, and the RFA treatment procedure was set. Parameters were adjusted according to the size, shape, and position of the remnant tumor. The range of ablation was defined to cover the whole tumor plus 0.5-1.0 cm. Multi-added, multi-needle overlapping ablation was carried out for patients with a relatively large volume of remnant tumor. At the end of ablation, the needle track was coagulated at a temperature of 70-90°C to reduce the risk of needle track bleeding and needle track implantation of tumor (Figure 4C).
Follow up and response assessment
Patients were followed up from the start of oral administration of sorafenib until they either died or were lost to follow up. The follow up of surviving patients ended in August 2018. Adverse reactions, patients’ disease progression and/or time of death were recorded. After starting sorafenib, MRI examination was performed every 4-6 weeks, and the efficacy of tumor treatment was evaluated using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria [17]. Adverse reactions were graded and recorded using the National Cancer Institute Common Terminology Criteria, Version 4.0. The local objective response rate was calculated as the proportion of patients with complete remission (Figure 4D) and partial remission. Time to tumor progression was the time from beginning sorafenib use to the onset of progressive disease (PD). PD included local tumor progression and distant metastasis. Distant metastasis was defined as the emergence of new metastatic lesions in the liver region or in extrahepatic organs far from the original tumor lesion. Overall survival (OS) was the time from the start of sorafenib to the time of death or last follow up.
Statistical methods
Data analysis was performed using SPSS version 24.0 (IBM Corporation, Armonk, NY, USA). Rates, percentages, and baseline stratification were analyzed by chi-square tests. The normal distribution and homogeneity of variance were expressed by X ± S according to the independent-sample t-test. OS curves were drawn according to the Kaplan-Meier method and OS rates calculated using the log-rank test. The stratified Cox regression model was used to calculate the overall hazard ratio (HR) and 95% confidence interval (CI) for each subgroup, and GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) was used for subgroup analysis. Statistical significance was considered when P < 0.05.
Results
Patient baseline data
We collected data from 1,437 patients with large HCC who underwent TACE between January 2010 and January 2016. Among them, 1,113 patients had remnant cancer after TACE treatment. After excluding the 541 patients not treated with sorafenib, 106 with liver failure, 47 with portal vein tumor thrombus, 32 with extrahepatic metastases, 33 who underwent other local or systemic treatment, 8 who underwent surgical resection, 41 lost to follow up, and 76 treated with sorafenib irregularly, 229 patients were studied: 102 receiving RFA and sorafenib (RFA + Sor group) and 127 receiving sorafenib alone (Sor group) (Figure 1). The two groups had no significant differences in age, gender, tumor size, alpha-fetoprotein level, tumor staging, liver function grading, and physical fitness scores (Table 1).
Figure 1.
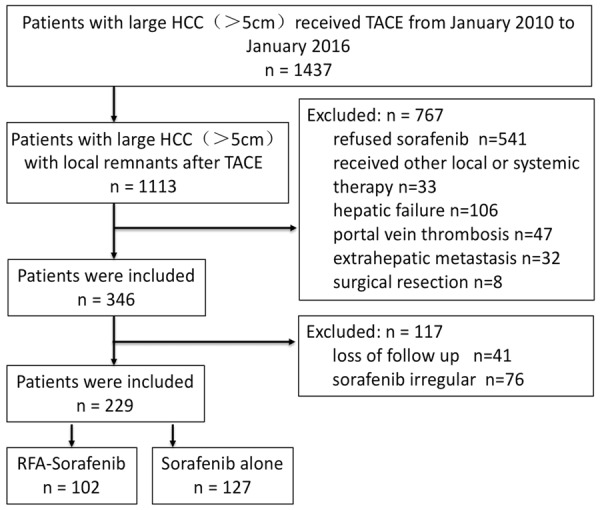
Flow chart of patients included in the study.
Table 1.
Baseline characteristics of study patients
| Parameter | RFA + Sorafenib group | Sorafenib group | χ2/t | P-Value |
|---|---|---|---|---|
| Age (years) | 55.7 ± 11.2 | 56.1 ± 9.6 | 0.290 | 0.771 |
| Sex | 0.183 | 0.668 | ||
| Male | 88 | 107 | ||
| Female | 14 | 20 | ||
| Hepatitis virus | 0.107 | 0.947 | ||
| HBV | 89 | 109 | ||
| HCV | 6 | 8 | ||
| Negative | 7 | 10 | ||
| Platelet count (× 109/L) | 131.4 ± 28.5 | 127.1 ± 32.2 | 1.056 | 0.291 |
| Serum AST (IU/L) | 41.7 ± 10.3 | 42.3 ± 11. 2 | 0.417 | 0.676 |
| Serum ALT (IU/L) | 36.4 ± 8.1 | 35.1 ± 10.7 | 1.015 | 0.311 |
| Total bilirubin (μmol/L) | 12.5 ± 11.7 | 11.8 ± 12.3 | 0.437 | 0.662 |
| Maximum diameter of primary tumor (cm) | 8.8 ± 2.7 | 8.3 ± 3.0 | 1.310 | 0.191 |
| 0.002 | 0.958 | |||
| 5~10 cm | 71 | 88 | ||
| ≥ 10 cm | 31 | 39 | ||
| AFP level (ng/L) | 0.002 | 0.957 | ||
| ≥ 400 | 63 | 78 | ||
| < 400 | 39 | 49 | ||
| No. of tumors | 0.906 | 0.635 | ||
| ≥ 3 | 32 | 33 | ||
| 2 | 20 | 29 | ||
| 1 | 50 | 65 | ||
| Child-Pugh class | 0.493 | 0.482 | ||
| A | 90 | 108 | ||
| B | 12 | 19 | ||
| Local remnants after TACE | 0.627 | 0.428 | ||
| < 50% | 74 | 86 | ||
| ≥ 50% | 28 | 41 | ||
| ECOG performance | 0.344 | 0.557 | ||
| 0 | 85 | 102 | ||
| 1 | 17 | 25 |
Note: AFP = a-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus.
Safety and side effects
Patients in the RFA + Sor group received RFA treatment at least once, and the mean duration of sorafenib treatment was 7.3 ± 1.4 months (range 2-45 months), while the mean duration of sorafenib treatment was 6.9 ± 1.8 months (range 1-51 months) in the Sor group (P = 0.06). At the end of the follow-up period, a total of 220 patients died but no treatment-related deaths occurred. The main causes of death were liver function decompensations (including hepatic encephalopathy), hepatorenal syndrome, gastrointestinal bleeding, and tumor progression. The main RFA-related complications were hepatic subcapsular hemorrhage in 5 cases (5.0%), biliary tumor in 2 cases (2.0%), and pleural effusion in 1 case (1.0%). All patients had different degrees of toxicity and side effects after taking sorafenib, such as skin reactions of the hands and feet, decreased appetite, fatigue, and diarrhea. The RFA + Sor group had 65 cases (63.7%) and the Sor group had 75 cases (59.1%) of severe adverse reactions (grade 3/4 toxicity), an insignificant difference in the incidence of these side effects (P = 0.559). For these patients, we reduced the dose, suspended the use of sorafenib, and carried out symptomatic treatment.
Efficacy analysis
During the follow-up period, 171 patients died of tumor progression. The data of local efficacy are shown in Table 2. In the RFA + Sor group, according to the mRECIST criteria, complete remission of the target lesion (large lesion) was achieved in 32 patients (31.4%), partial remission in 59 patients (57.8%), and stable disease in 11 patients (10.8%). The disease control rate (DCR = complete remission + partial remission + stable disease) was 100% in the RFA + Sor group, but 65.3% in the Sor group (P = 0.006). In the RFA + Sor group, complete remission of the overall lesion was achieved in 13 patients (12.7%), partial remission in 47 patients (46.1%), and stable disease in 7 patients (6.85); the DCR was 65.7% (67/102), while the DCR in the Sor group was 41.7% (53/127) (P < 0.001). The Kaplan-Meier survival analysis curve is shown in Figure 2. The mean survival time of patients in the RFA + Sor group was 18.3 ± 1.6 months (95% CI: 15.2-21.4) and that in the sorafenib group was 14.1 ± 1.1 months (95% CI: 11.8-16.3); the median OS of patients in the RFA + Sor group was 14.0 months (95% CI: 10.7-17.3) and that in the sorafenib group was 9.0 months (95% CI: 6.8-11.2). The log-rank test showed a significant difference in survival time between the two groups (P = 0.03). The 1-, 2- and 3-year survival rates of the RFA + Sor group were 56.9%, 34.3%, 11.7%; those of the sorafenib group were 42.5%, 22.0%, 5.5%, respectively. Between the two groups, the differences in 1- and 2-year survival rates were statistically significant, but not the difference in 3-year survival rates.
Table 2.
Outcomes of tumor response after treatment
| Outcome | RFA + Sorafenib group | Sorafenib group | P-Value |
|---|---|---|---|
| Tumor response in target lesions | |||
| CR | 31.4% (32/102) | 3.1% (4/127) | < 0.001 |
| PR | 57.8% (59/102) | 21.2% (27/127) | < 0.001 |
| SD | 10.8% (11/102) | 40.9% (52/127) | < 0.001 |
| DCR | 100% | 65.3% (83/127) | 0.006 |
| Tumor response in general lesions | |||
| CR | 12.7% (13/102) | 0.8% (1/127) | < 0.001 |
| PR | 46.1% (47/102) | 11.8% (15/127) | < 0.001 |
| SD | 6.8% (7/102) | 29.1% (37/127) | < 0.001 |
| DCR | 65.7% (67/102) | 41.7% (53/127) | < 0.001 |
| OS (mo) | 18.3 ± 1.6 | 14.1 ± 1.1 | 0.03 |
| mOS (mo)* | 14.0 (10.7, 17.2) | 9.0 (6.8, 11.2) | 0.03 |
| Survival rate (%) | |||
| 1 year | 56.9% (58/102) | 42.5% (54/127) | 0.031 |
| 2 year | 34.3% (35/102) | 22.0% (28/127) | 0.048 |
| 3 year | 11.7% (12/102) | 5.5% (7/127) | 0.088 |
Note: Objective Response Rate (ORR), Disease Control Rate (DCR), OS = overall survival, DCR = CR + PR + SD;
Data in parentheses are the 95% confidence interval.
Figure 2.
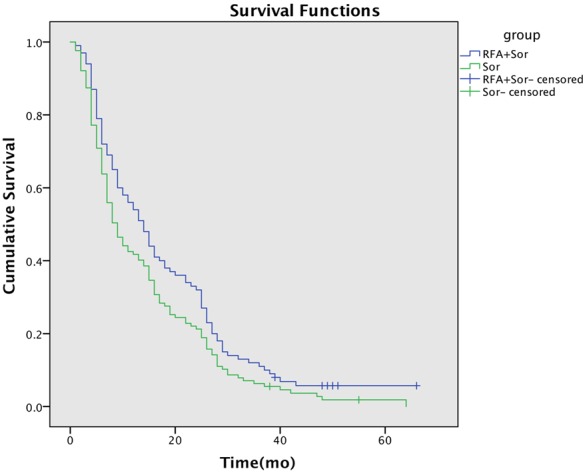
Kaplan-Meier curves show overall survival in the radiofrequency ablation with sorafenib (RFA + sorafenib) and sorafenib groups.
Subgroup analysis
The stratified Cox regression model was used to calculate the HR and 95% CI for each subgroup. The forest plots for subgroup analysis (Figure 3) showed that the RFA + Sor group had significant OS benefits for all 18 subgroups. Compared with the Sor group, survival benefits were most significant in the following RFA + Sor subgroups: patients with a tumor diameter of 5-10 cm (HR 0.42, 95% CI 0.21-1.06 vs. HR 0.94, 95% CI 0.63-1.22), patients with an isolated tumor (HR 0.36, 95% CI 0.19-0.81 vs. HR 0.93, 95% CI 0.55-1.24), and patients with remnant lesion volume < 50% after TACE treatment (HR 0.47, 95% CI 0.21-1.12 vs. HR 0.77, 95% CI 0.46-0.81).
Figure 3.
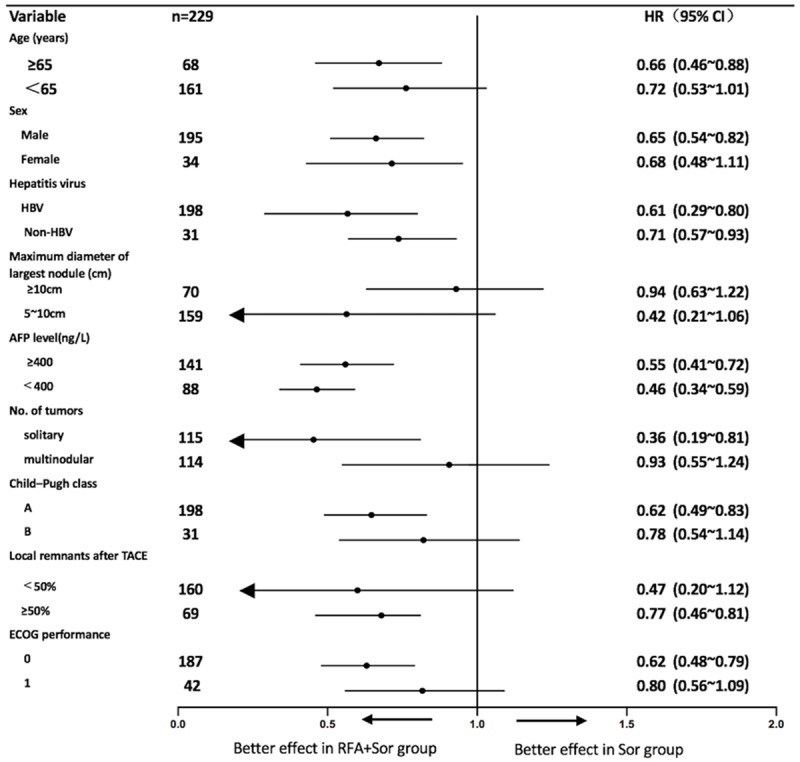
Subgroup analysis: overall survival of the corresponding subgroup of patients.
Discussion
Tumor size is reported to be an important risk factor for TACE outcomes [18,19]. Chung et al. [20] reported that 35 cases with < 4 cm tumors had an extrahepatic blood supply rate of < 3%; when the tumor diameter was > 6 cm, this proportion increased to 63%. Large HCC has a multi-arterial blood supply, including the portal vein, requiring a high level of expertise in embolization [5-7,21]. Therefore, TACE can produce necrosis in only 30%-50% of tumors, with only 2% of large HCCs showing complete necrosis [22]. Indeed, most patients with large HCC have resistance to multiple TACE treatments [23,24] and the remnant lesion after TACE can seriously affect the treatment efficacy and prognosis [25]. Therefore, the remnant cancer must be treated.
Our study concluded that RFA combined with sorafenib is a safe and effective treatment for remnant large HCC after TACE. When the tumor diameter is < 3 cm, the complete ablation rate of RFA can reach 90% [26], but the efficacy of RFA decreases as tumor size increases, and RFA alone is not suitable for the initial treatment of large HCCs [27]. We tried to use RFA to ablate the remnant cancer after TACE in large HCCs. In this process, there will inevitably be omissions in the three-dimensional space, with the result of incomplete treatment. Combining RFA with sorafenib may result in more thorough treatment and prolonged patient survival. In our study, the objective remission rates of the target lesions in the RFA + Sor group and the Sor group were 100% and 65.3%, respectively. The outcome of the RFA + Sor treatment was significantly better than that of sorafenib alone for the target lesions, and the objective remission rate of the RFA + Sor group was also significantly better than that of the Sor group (65.7% vs. 41.7%) (P < 0.001). In the RFA + Sor group, significant necrosis of the remnant cancer occurred, and the complete remission rate was slightly lower than that of the thermal ablation of large HCCs (69-81.8%) reported by Li et al. [27] Safety results showed that only a few patients in the RFA + Sor group had RFA-related complications, with similar rates of overall drug-related grade 3/4 adverse reactions in the two groups, a result similar to those reported by Zhu et al. [28] and Kan et al. [29] RFA did not increase the number or severity of adverse reactions to sorafenib and no new safety-related issues arose.
In the present study, the modified OS of the RFA + Sor group was 18.3 ± 1.6 months and that of the Sor group was 14.1 ± 1.1 months, indicating a significantly prolonged survival in the RFA + Sor group. However, although the 1- and 2-year survival rates of the RFA + Sor group were significantly better than those of the Sor group, the 3-year survival rates were not significantly different (P > 0.05). We consider that the poor long-term survival of those with large HCC may be related to the biological characteristics of large HCC, making it more likely to progress [30]. In addition, one purpose of the present study was to characterize the population suitable for the use of RFA and sorafenib to treat remnant cancer after TACE in large HCC. Analysis of patient subgroups showed that RFA + Sor could achieve a more significant survival benefit for patients with a tumor diameter of 5-10 cm, those with an isolated tumor, and those with remnant lesion volume < 50% after TACE treatment. We speculate that these patients in particular will receive the best benefits. The possible reason is that neovascular invasion and extrahepatic dissemination occur more easily in large HCC patients with larger-volume lesions, multiple lesions, and poor outcome of TACE [5-7,21,31,32], and the long-term clinical outcomes of these patients are not easily improved.
Limitations include the fact that this study is a retrospective exploration of the treatment of large HCC, the grade of the results of the efficacy analysis is relatively low, and the cost-benefit of the combination therapy should be further evaluated.
The current study analyzed a difficult problem in the treatment of large HCC. We believe RFA combined with sorafenib is safe and effective in improving the local control of HCC, reducing the liver cancer remnant, and increasing postoperative tumor necrosis. The conclusions of this study should be further verified by subsequent multi-center large-scale randomized clinical studies.
Disclosure of conflict of interest
None.
References
- 1.Sherman M. Hepatocellular carcinoma. Gastroenterologist. 2016;20:703–720. [Google Scholar]
- 2.Bertino G, Di Carlo I, Ardiri A, Calvagno GS, Demma S, Malaguarnera G, Bertino N, Malaguarnera M, Toro A, Malaguarnera M. Systemic therapies in hepatocellular carcinoma: present and future. Future Oncol. 2016;9:1533–48. doi: 10.2217/fon.13.171. [DOI] [PubMed] [Google Scholar]
- 3.Pascual S, Herrera I, Irurzun J. New advances in hepatocellular carcinoma. World J Hepatol. 2016;8:421–438. doi: 10.4254/wjh.v8.i9.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JW, Koh YH, Kim HB, Kim HY, An S, Choi JI, Woo SM, Nam BH. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56:1336–42. doi: 10.1016/j.jhep.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Giuliante F, De Rose AM, Guerra V, Ardito F, Nuzzo G, Carr BI. Clinical characteristics and survival of european patients with resectable large hepatocellular carcinomas. J Gastrointest Cancer. 2013;44:329–35. doi: 10.1007/s12029-013-9523-5. [DOI] [PubMed] [Google Scholar]
- 6.Yang JD, Mohamed EA, Aziz AO, Shousha HI, Hashem MB, Nabeel MM, Abdelmaksoud AH, Elbaz TM, Afihene MY, Duduyemi BM, Ayawin JP, Gyedu A, Lohouès-Kouacou MJ, Ndam AW, Moustafa EF, Hassany SM, Moussa AM, Ugiagbe RA, Omuemu CE, Anthony R, Palmer D, Nyanga AF, Malu AO, Obekpa S, Abdo AE, Siddig AI, Mudawi HM, Okonkwo U, Kooffreh-Ada M, Awuku YA, Nartey YA, Abbew ET, Awuku NA, Otegbayo JA, Akande KO, Desalegn HM, Omonisi AE, Ajayi AO, Okeke EN, Duguru MJ, Davwar PM, Okorie MC, Mustapha S, Debes JD, Ocama P, Lesi OA, Odeghe E, Bello R, Onyekwere C, Ekere F, Igetei R, Mah’moud MA, Addissie B, Ali HM, Gores GJ, Topazian MD, Roberts LR Africa Network for Gastrointestinal and Liver Diseases. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa liver cancer consortium. Lancet Gastroenterol Hepatol. 2017;2:103–111. doi: 10.1016/S2468-1253(16)30161-3. [DOI] [PubMed] [Google Scholar]
- 7.Liu XY, Xu JF. Liver resection for young patients with large hepatocellular carcinoma: a single center experience from China. World J Surg Oncol. 2014;12:175. doi: 10.1186/1477-7819-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan H, Liu F, Li X, Guan Y, Wang M. Transcatheter arterial chemoembolization combined with simultaneous DynaCT-guided radiofrequency ablation in the treatment of solitary large hepatocellular carcinoma. Radiol Med. 2019;124:1–7. doi: 10.1007/s11547-018-0932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh PS, Chan AC, Cheung TT, Chok KS, Dai WC, Poon RT, Lo CM. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford) 2016;18:72–8. doi: 10.1016/j.hpb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erridge S, Pucher PH, Markar SR, Malietzis G, Athanasiou T, Darzi A, Sodergren MH, Jiao LR. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433–1442. doi: 10.1002/bjs.10597. [DOI] [PubMed] [Google Scholar]
- 11.Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;2:10327–35. doi: 10.3748/wjg.v21.i36.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raoul JL, Kudo M, Finn RS, Edeline J, Reig M, Galle PR. Systemic therapy for intermediate and advanced hepatocellular carcinoma: sorafenib and beyond. Cancer Treat Rev. 2018;68:16–24. doi: 10.1016/j.ctrv.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Camp ER, Esnaola NF, Curley SA. Hepatocellular Carcinoma. New York, NY: Springer; 2011. Radiofrequency ablation for hepatocellular carcinoma[M] pp. 261–273. [Google Scholar]
- 14.Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758–65. doi: 10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 15.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP, Bruner DW, Cleeland CS, Sloan JA, Chilukuri R, Baumgartner P, Denicoff A, St Germain D, O’Mara AM, Chen A, Kelaghan J, Bennett AV, Sit L, Rogak L, Barz A, Paul DB, Schrag D. Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase 3 studies. J Hepatol. 2017;67:999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Kantarci M, Pirimoglu B. Radiological response to the locoregional treatment in hepatocellular carcinoma: RECIST, mRECIST, and others. J Gastrointest Cancer. 2017 doi: 10.1007/s12029-017-9969-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012;39:503–509. doi: 10.1053/j.seminoncol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Su JY. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:7–17. doi: 10.3350/cmh.2016.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HC, Chung JW, Lee W, Jae HJ, Park JH. Recognizing extrahepatic collateral vessels that supply hepatocellular carcinoma to avoid complications of transcatheter arterial chemoembolization. Radiographics. 2005;25(Suppl 1):S25–39. doi: 10.1148/rg.25si055508. [DOI] [PubMed] [Google Scholar]
- 21.Di Costanzo GG, Tortora R. Intermediate hepatocellular carcinoma: how to choose the best treatment modality? World J Hepatol. 2015;7:1184–1191. doi: 10.4254/wjh.v7.i9.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YB, Lee DH, Cho Y, Yu SJ, Lee JH, Yoon JH, Lee HS, Kim HC, Yi NJ, Lee KW, Suh KS, Chung JW, Kim YJ. Comparison of transarterial chemoembolization and hepatic resection for large solitary hepatocellular carcinoma: a propensity score analysis. J Vasc Interv Radiol. 2015;26:651–9. doi: 10.1016/j.jvir.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Sacco R, Mismas V, Marceglia S, Romano A, Giacomelli L, Bertini M, Federici G, Metrangolo S, Parisi G, Tumino E, Bresci G, Corti A, Tredici M, Piccinno M, Giorgi L, Bartolozzi C, Bargellini I. Transarterial radioembolization for hepatocellular carcinoma: an update and perspectives. World J Gastroenterol. 2015;21:6518–25. doi: 10.3748/wjg.v21.i21.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, Yamakado K, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T Liver Cancer Study Group of Japan. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523–9. doi: 10.1080/02841850801958890. [DOI] [PubMed] [Google Scholar]
- 26.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20–5. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 27.Fan WZ, Yang JY, Lü MD, Xie XY, Yin XY, Huang YH, Kuang M, Li HP, Xu HX, Li JP. Abstract no. 39: transcatheter arterial chemoembolization (TACE) combined percutaneous thermal ablation in large hepatocellular carcinoma: clinical observation of the efficacy and predictors of prognostic factors. Zhonghua Yi Xue Za Zhi. 2011;91:2190–4. [PubMed] [Google Scholar]
- 28.Zhu K, Huang J, Lai L, Huang W, Cai M, Zhou J, Guo Y, Chen J. Medium or large hepatocellular carcinoma: sorafenib combined with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;288:300–307. doi: 10.1148/radiol.2018172028. [DOI] [PubMed] [Google Scholar]
- 29.Kan X, Jing Y, Wan QY, Pan JC, Han M, Yang Y, Zhu M, Wang Q, Liu KH. Sorafenib combined with percutaneous radiofrequency ablation for the treatment of medium-sized hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2015;19:247–255. [PubMed] [Google Scholar]
- 30.Giuliante F, De Rose AM, Guerra V, Ardito F, Nuzzo G, Carr BI. Clinical characteristics and survival of European patients with resectable large hepatocellular carcinomas. J Gastrointest Cancer. 2013;44:329–35. doi: 10.1007/s12029-013-9523-5. [DOI] [PubMed] [Google Scholar]
- 31.Lai EC, Ng IO, Ng MM, Lok AS, Tam PC, Fan ST, Choi TK, Wong J. Long-term results of resection for large hepatocellular carcinoma: a multivariate analysis of clinicopathological features. Hepatology. 2010;11:815–818. doi: 10.1002/hep.1840110516. [DOI] [PubMed] [Google Scholar]
- 32.Lim C, Mise Y, Sakamoto Y, Yamamoto S, Shindoh J, Ishizawa T, Aoki T, Hasegawa K, Sugawara Y, Makuuchi M, Kokudo N. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World J Surg. 2014;38:2910–8. doi: 10.1007/s00268-014-2704-y. [DOI] [PubMed] [Google Scholar]


