Abstract
Physical activity is associated with diminished risk of several cancers, and preclinical studies suggest exercise training may alter tumor cell growth in certain tissue(s) (e.g., adipose). From moderate-intensity exercise-trained rats versus sedentary controls, we hypothesized 1) there will be a decreased prostate cancer cell viability and migration in vitro and, within the prostate, a reduced 5α-reductase 2 (5αR2) and increased caspase-3 expression, and 2) that exercise training in tumor-bearing (TB) animals will demonstrate a reduced tumor cell viability in prostate-conditioned media. Serum and prostate were harvested from sedentary or exercise-trained (treadmill running, 10-11 weeks) immune-competent (Copenhagen; n = 20) and -deficient (Nude; n = 18) rats. AT-1 and PC-3 prostate cancer cells were grown in one or more of the following: serum-supplemented media (SSM), SSM from TB rats (SSM-TB), prostate-conditioned media (PCM) or PCM from TB rats (PCM-TB) for 24-96 h under normoxic (18.6% O2) or hypoxic (5% O2) conditions. Under normoxic condition, there was a decreased AT-1 cell viability in SSM and PCM from the exercise-trained (ET) immune-competent rats, but no difference in PC-3 cell viability in SSM and PCM from ET Nude rats versus the sedentary (SED) group, or in SSM-TB from ET-TB Nude rats versus the SED-TB group. However, there was a decreased PC-3 cell viability in the PCM-TB of the ET-TB group versus SED-TB group. PC-3 cell viability in all conditioned media types was not altered between groups with hypoxia. In the prostate, exercise training did not alter 5αR2 expression levels, but increased caspase-3 expression levels. In conclusion, prior exercise status reduced prostate cancer cell viability in the serum and prostate of trained rats but did not modify several other key prostate tumor cell growth characteristics (e.g., migration, cell cycle except in S phase of PC-3 cells in PCM-TB). Importantly, once the tumor was established, exercise training reduced tumor cell viability in the surrounding prostate, which may help explain the reduced severity of the disease in patients that exercise.
Keywords: Exercise, prostate cancer, growth characteristics, 5α-reductase 2, caspase-3, normoxia, hypoxia
Introduction
In males, prostate cancer is the most commonly diagnosed cancer in the US and second leading cause of cancer death [1], with tumor aggressiveness being negatively related to patient prognosis and survivorship [2]. Although emerging research suggests that exercise training may alter the tumor microenvironment to facilitate conventional treatment [3], the effects of exercise training on prostate cancer survival and progression are equivocal [4-8]. In general, men who are physically inactive have an increased risk for prostate cancer relative to physically active men [9] (see [10] for exception), suggesting the adoption of a sedentary lifestyle as a potential risk factor for prostate cancer [9]. However, once diagnosed with prostate cancer, men that engage in exercise training do have a lower rate of progression [6] and increased survivorship of the disease [7]. This suggests exercise training may have both a preventative and mitigating influence upon the development of prostate cancer or its impact once the disease is established [6,7,9].
Exercise training-induced systemic changes in serum and local alterations in certain tissue(s) (e.g., adipose) may alter or arrest tumor cell growth with certain cancers. For example, exercise training inhibited serum-stimulated LNCaP prostate cancer cell growth in vitro [11], and delayed tumor formation in mice when LNCaP [12] and MCF7 breast [13] cancer cells were pre-incubated with exercise-conditioned serum, prior to subcutaneous injection (i.e., ectopic model). Further, in male rats fed a high-fat diet, exercise training mitigated the adipose-dependent proliferative effects of MCF7 cells in vitro [14]. Thus, it is possible that exercise training impacts systemic blood composition (i.e., serum) as well as the local prostate environment, to diminish cancer cell viability, proliferation and tumorigenesis. In a normal prostate, the initial stages of prostate cancer progression depend, in part, upon androgens that can increase cell proliferation as well as inhibit apoptosis [15]. Testosterone, the primary circulating androgen in males, is converted by isoenzymes of the 5α-reductase family into the more potent dihydrotestosterone (DHT), which can stimulate prostate tumor development and progression [16]. Specifically, 5α-reductase 2 (5αR2) is found predominantly in the prostate and catalyzes the conversion of testosterone to DHT [17]. It is yet to be determined if exercise training modulates prostate 5αR2 expression. On the other hand, caspases play a significant role in apoptotic cell death, with caspase-3 being the prominent executioner caspase [18]. In humans, caspase-3 expression was decreased in prostate cancer compared to benign prostatic hyperplasia [19]. Therefore, caspase-3 expression and induction may serve as an important marker for tumor progression, as well as a locus of therapeutic manipulation (e.g., via exercise training) by promoting programmed cell death.
There were three specific purposes of this series of investigations, including 1) examining the effects of moderate-intensity exercise training on serum-supplemented media (SSM) and prostate-conditioned media (PCM) on prostate cancer cell growth characteristics in vitro. We hypothesized that SSM and PCM from moderate-intensity exercise-trained (ET) rats will decrease prostate cancer cell viability versus sedentary (SED) counterparts. 2) Quantifying prostate 5αR2 and caspase-3 protein expression after exercise training. Further, given that data suggests a slower progression of prostate cancer in patients that engage in exercise training [6], 3) to investigate the effects of exercise training on prostate cancer cell viability from the prostate after a tumor is established. Specifically, we hypothesized that exercise training will result in a diminished cancer cell viability in PCM from prostate tissue surrounding an established tumor.
A key facet of malignancy is the ability of cancer cells to metastasize via the invasion of local tissue(s) and blood vessels [20]. Cell proliferation and metabolism are influenced by low oxygen tension (hypoxia). Solid tumors contain regions with hypoxia as a result of rapid oxygen depletion, insufficient vascularization, and suboptimal tumor blood flow to the growing tumor nodules [21]. Thus, establishing a hypoxic environment for cell culture is vital to mimicking the physiologic conditions that exist in the core of tumors. Therefore, in vitro experiments were repeated in a hypoxic environment to recapitulate expected in vivo conditions [22-24].
Materials and methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at Kansas State University and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council Committee, Washington, D. C., rev. 2011). A total of 20 male Copenhagen rats (COP/CrCrl; Charles River, Wilmington, MA) and 18 male Nude rats (Crl: NIH-Foxn1rnu; Charles River) were investigated at ~6-7 months of age. The rats were housed at 23°C and maintained on a 12:12-h light-dark cycle and provided standardized laboratory rat chow and water ad libitum.
Study 1: Effects of aerobic exercise training on prostate cancer cell growth characteristics in serum or prostate-conditioned media from healthy rats
Exercise training
Copenhagen (immune-competent) and Nude (immune-deficient) rats (~3-4 mo old) were randomly assigned to an ET (Copenhagen, n = 10; Nude, n = 6) or SED control (Copenhagen, n = 10; Nude, n = 4) groups. Rats were exercise-trained by habituating them to treadmill exercise, during which both Copenhagen and Nude rats walked on a motor-driven treadmill at 15 m/min (0° incline), 5 min/day for 3 days. After the habituation period, the incline was raised to 15° for the duration of the training period while the 15 m/min speed was maintained for Copenhagen rats. From preliminary studies, the initial exercise capacity was lower in the Nude versus Copenhagen rats; therefore, the incline was raised to 6° for the duration of the training period while the 15 m/min speed was increased to 25 m/min for Nude rats. Given the considerable differences in exercise capacity and training responses in murine models [25], this was not surprising. Importantly, a training effect was demonstrated from both ET groups (see “Results”). During the first 5 weeks of training, the time of exercise training was increased by 10 min/week, until 60-min duration was reached by the 6th week. All animals continued to exercise 5 days/week for 60 min/day for the remainder of the 10- to 11-week training period. This training program corresponded to a moderate-intensity exercise training program, as previously described [26]. Sedentary counterparts were confined to normal cage activity during this 10- to 11-week period. All animals subjected to exercise training were euthanized 24 h after the last exercise bout to avoid potential effects of acute exercise on reported measures. Sedentary animals were euthanized at a corresponding time and age. After collection of serum and prostate tissue (see below) and euthanasia, the red portion of the gastrocnemius, soleus muscle, and heart were immediately excised, weighed, flash-frozen in liquid nitrogen, and stored at -80°C for future analysis of citrate synthase activity within locomotory muscles. Training efficacy was confirmed when an ET group demonstrated at least one of the following versus SED counterparts: increased heart:body weight (BW) ratio or elevated citrate synthase activity of the soleus or red portion of the gastrocnemius muscle.
Serum, prostate collection and PCM
Twenty-four hours after the last bout of exercise, the rats were anesthetized by isoflurane (5%/O2 balance). A midline abdominal incision from pubis to xiphoid was made to the abdominal cavity. The abdominal aorta was exposed below the renal vessels [27], punctured, and arterial blood was collected with a 5-ml syringe and placed in serum separator tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). Subsequently, the rat was euthanized by removal of the heart under a deep plane of anesthesia. The collected arterial blood was allowed to clot at room temperature for 30 min, centrifuged at 2000 g for 10 min (4°C), then the serum was extracted and stored at -20°C for future analysis. The prostate was isolated from the urinary bladder, excised and weighed. Under aseptic conditions, one half of each prostate from Copenhagen rats and the whole prostate from Nude rats was used for preparing PCM, as modified from Theriau et al [14]. Briefly, the prostate was washed in phosphate buffered saline (PBS) and minced into ~5- to 10-mg pieces. The prostate pieces from Copenhagen rats were then added to a 15-ml vented conical tube, containing a solution of 10 ml RPMI-1640 media (GE Healthcare Life Sciences, Marlborough, MA) supplemented with 10% fetal bovine serum (FBS; RMBIO, Missoula, MT), 2 mM L-glutamine (Fisher Scientific, Hampton, NH), 1% PenStrep (100 U/ml Penicillin and 100 µg/ml Streptomycin; Thermo Fisher Scientific, Waltham, MA), 100 mM sodium pyruvate (Thermo Fisher Scientific) and 0.025 mM dexamethasone (Cayman Chemical, Ann Arbor, MI). The prostate pieces from Nude rats were added to a 15-ml vented conical tube, containing a solution of 10 ml RPMI-1640 media (ATCC, Manassas, VA) supplemented with 10% FBS, 2 mM L-glutamine and 1% PenStrep, and incubated for 24 h at 37°C with 5% CO2. After 24 h incubation, the PCM was centrifuged at 1000 g for 15 min (37°C), filtered with a 0.22-µm sterile filter, collected and stored at -80°C until use. The PCM was later diluted 5 times with RPMI-1640 media (without FBS) for the studies detailed below, as a highly concentrated conditioned media is detrimental to cell growth [28]. This resulted in a final FBS concentration in PCM of 2%. The other half of each prostate from Copenhagen rats was fixed in 10% neutral buffered formalin for 48 h and then transferred to 70% ethanol, until processing in the Kansas State University Veterinary Diagnostic Laboratory as described below (see “Immunohistochemical Analyses”).
Cancer cell culture
AT-1 (Dunning R-3327; rat dorsal prostate adenocarcinoma) and PC-3 human prostate adenocarcinoma cells were purchased from American Type Culture Collection (ATCC) and cultured following provider’s instructions. Briefly, AT-1 cells were cultured in RPMI-1640 media (GE Healthcare Life Sciences) supplemented with 10% FBS (RMBIO), 2 mM L-glutamine (Fisher Scientific), 1% PenStrep (100 U/ml Penicillin and 100 µg/ml Streptomycin; Thermo Fisher Scientific), 100 mM sodium pyruvate (Thermo Fisher Scientific) and 0.025 mM dexamethasone (Cayman Chemical), and incubated at 37°C with 5% CO2. PC-3 cells were cultured in RPMI-1640 media (ATCC) supplemented with 10% FBS and 2 mM L-glutamine, and incubated at 37°C with 5% CO2.
Establishment of optimal growth media for serum
Previously, serum with supplemental fetal calf serum [12], and without supplemental FBS [11], has been used to determine characteristics of tumor cell survival. Therefore, prior to the determination of cell viability, cell migration and cell cycle phases, we first optimized the growth media for the serum-supplemented media from the SED rats. Ten thousand AT-1 cells were seeded in 96-well tissue culture plates with RPMI-1640 media supplemented with 5% serum from Copenhagen SED rats, with or without FBS, for 24, 48 and 96 h. Viable cell number was quantified using the CellTiter 96 Non-Radioactive Cell Proliferation Assay (MTT; Promega Corporation, Madison, WI) with a UV/Vis spectrophotometer (accuSkan GO; Fisher Scientific) at 570 nm. Results presented are from two replicates (rat group x media type) of the experiments. Five percent serum from SED rats with 5% FBS significantly increased viable cell number at 48 and 96 h incubation, compared to 5% serum from SED rats without FBS (Figure 1). Subsequently, this protocol (i.e., 5% serum with 5% FBS), referred to as serum-supplemented media (SSM), was applied for cell viability, cell migration, and cell cycle studies for group comparisons.
Figure 1.
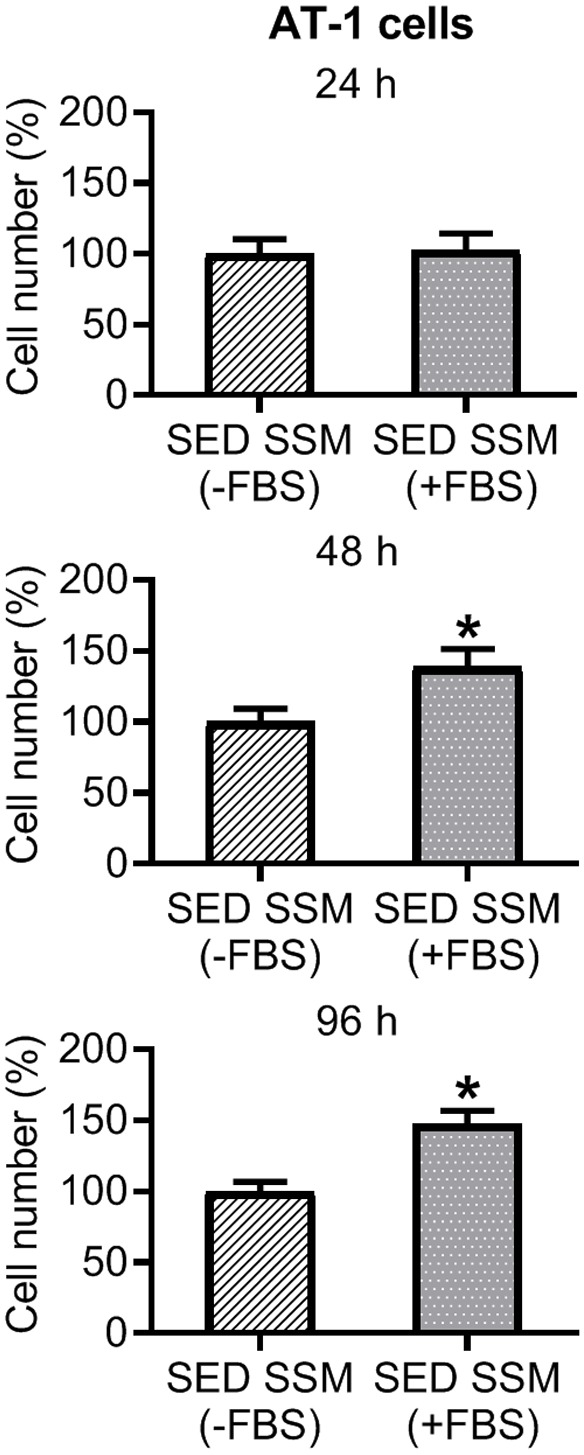
Effect of 24, 48 and 96 h incubation with serum-supplemented media (SSM) from Copenhagen sedentary (SED; n = 10) rats without FBS (-FBS) and with FBS (+FBS) on viable AT-1 cell number. There was a significant increase in cell number in SSM (+FBS) vs. SED SSM (-FBS) from Copenhagen SED rats at 48 and 96 h (P ≤ 0.05). Values are expressed as mean cell number (%) ± SEM. *P ≤ 0.05 vs. SSM (-FBS).
Viability of prostate cancer cells
AT-1 cells (1 × 104) were seeded in 96-well tissue culture plates with either SSM or PCM from SED and ET Copenhagen rats, while PC-3 cells (2 × 104) were seeded in 96-well tissue culture plates with either SSM or PCM from SED and ET Nude rats for 24, 48 and 96 h. AT-1 cell viability studies were performed in normoxic condition (via the mixture of room air with gaseous water and CO2 to form the incubator’s gas mixture of 70.2% N2, 18.6% O2, 5% CO2 and 6.2% H2O(gaseous water)) [29], while PC-3 cell viability studies were performed in normoxic and hypoxic (via the mixture of gaseous water and 90% N2, 5% O2 and 5% CO2 to form the hypoxia chamber’s gas mixture of 83.8% N2, 5% O2 and 5% CO2 and 6.2% H2O(gaseous water)) conditions [29]. Studies in hypoxia were performed in a customized airtight plexiglass chamber with a volume of 3.28 L. The hypoxia chamber was placed in the incubator for growth at 37°C. Viable cell number was quantified using the CellTiter 96 Non-Radioactive Cell Proliferation Assay (MTT) with a UV/Vis spectrophotometer (accuSkan GO) at 570 nm. Viable cell number was expressed as percentage of cell number in the SED group. Results presented are from two replicates (rat group x media type) of the experiments.
Cell exclusion zone assay
The migration rate was quantified using an assay by Platypus Technologies (Platypus Technologies Llc., Fitchburg, WI). Twenty thousand AT-1 cells in RPMI-1640 media with 10% FBS were seeded per well around the inserts to reach rapid confluence. After a 24 h incubation period, the inserts and media were removed, and SSM or PCM from SED and ET Copenhagen rats was added to each well of AT-1 cells and incubated for 24-96 h. Images of the center of the well were captured with a microscope (Micromaster; Fisher Scientific) at 40X magnification magnification for 24, 48 and 96 h incubation. Migration was determined with the ImageJ software (NIH), by quantifying cell number from the images. Results presented are from two replicates (rat group x media type) of the experiments.
Transwell migration assay
In the transwell cell migration assay, 24-well inserts from Corning (Corning Inc., Corning, NY) were used. AT-1 and PC-3 cells were re-suspended at a concentration of 5 × 105 cells/ml in the migration buffer consisting of RPMI-1640 media without FBS. SSM from Copenhagen SED and ET rats was used as the chemoattractant for AT-1 cells, while SSM or PCM from SED and ET Nude rats was used as the chemoattractant for PC-3 cells. Cells (2.5 × 105) suspended in 500 µl of migration buffer were placed upon the transwell membrane in the upper chamber and 750 µl of chemoattractant was added to the lower chamber. The cells were incubated for 24 h at 37°C with 5% CO2 and stained using the Diff Quik kit (Fisher Scientific). The stained cells were photographed with a microscope (Micromaster; Fisher Scientific) at 40X magnification magnification and subjected to cell counting using the ImageJ software. The migration was calculated as the mean ± SEM of cell number per field, and multiplied by 100.
Cell cycle analysis
AT-1 and PC-3 cells, in either SSM or PCM from SED and ET Copenhagen rats, or SED and ET Nude rats, respectively, were each seeded in 6-well tissue culture plates. AT-1 cells were plated at 2.5 × 105 cells/well for SSM or 7.5 × 105 cells/well for PCM. AT-1 cells had a relatively slow growth rate in PCM than SSM, therefore the cells were seeded at a higher density in PCM to obtain the threshold cell count required for quantification by the flow cytometer. PC-3 cells were plated at 2.5 × 105 cells/well for all conditioned media types. At 80% confluence, cells were isolated via trypsinization, washed in cold PBS, and fixed by dropwise addition of ice-cold 70% ethanol. Cells were again washed twice in PBS, re-suspended in a propidium iodide/RNase solution (Thermo Fisher Scientific) and subjected to fluorescence-activated cell sorting (FACS) analysis, using LSR Fortessa × 20 equipment (BD Biosciences, San Jose, CA), yielding the relative number of cells in G0/G1, S and G2/M cell cycle phase.
Study 2: Effects of aerobic exercise training on 5α-reductase 2 and caspase-3 expression in the prostate of healthy rats
Immunohistochemical analyses
5α-reductase 2
Prostate tissue sections (4 µm) from Copenhagen rats in Study 1 were treated for 20 min at 100°C in EDTA buffer (Epitope Retrieval 2 pH 9.0; Leica Biosystems, Buffalo Grove, IL) for antigen retrieval. Immunohistochemical staining was performed on the Leica Bond-Max autostainer with commercial 5α-reductase 2 (5αR2) antibody (rabbit polyclonal, DPBT-65712RR; Creative Diagnostics, Shirley, NY) diluted 1:1000 with Bond Primary Antibody Diluent (Leica Biosystems), with an immunoenzyme peroxidase polymer detection system (HRP α-Rabbit IgG from the Bond Polymer Refine Detection kit, Leica Biosystems) and diaminobenzidine (DAB) chromogen (Refine Polymer Detection kit, Leica Biosystems). Slides were counterstained with hematoxylin. Rabbit Flex Universal negative control (IS60061-2; Dako, Glostrup, Denmark) was used for negative control.
Caspase-3
For detection of caspase-3 positive cells in prostate tissue, sections (4 µm) were stained with rabbit-anti-cleaved caspase-3 by routine immunohistochemical methods. Paraffin sections were heated in a pressure cooker (10 min at 100°C) for antigen retrieval and incubated with anti-caspase-3 antibody (ab4051; diluted 1:50 dilution; ABCAM, Cambridge, MA) overnight at 4°C, followed by incubation for 20 min with biotinylated goat-anti-rabbitIgG secondary antibody (BA-1000; 1:200 dilution; Vector Laboratories, Inc., Burlingame, CA) and VECTASTAIN Elite ABC-HRP Kit (PK-6100; Vector Laboratories, Inc.). Bound antibodies were visualized by use of Nova Red (Vector Laboratories, Inc.) and counterstained with hematoxylin. Rabbit Flex Universal negative control (IS60061-2; Dako) was used for negative control.
Quantification of positive staining
A board-certified pathologist (CKG) identified areas of positive staining for 5αR2 and caspase-3 in prostate sections. These areas were scanned using a Pannoramic Midi scanner (3D Histech, Budapest, Hungary) with a 20 × objective, giving 2-megapixel resolution. Scanned 5αR2 images were then annotated using HALO software (Indica Labs, Coralles, NM) and quantified using the HALO CytoNuclear immunohistochemistry module (Indica Labs); which was set to identify nuclei based on hematoxylin, and 5αR2 positive cells based on the intensity of DAB staining in the cytoplasm. Six equal random fields from images were used for quantification. Thresholds for positivity were defined so that they matched what was seen visually. This was done by observing images across the range of intensities so that the defined thresholds best fit the entire group rather than shifting the threshold image by image. Caspase-3 positive cells based on the intensity of Nova Red staining in the cytoplasm were quantified manually under a Nikon Eclipse E600 microscope (Nikon Instruments, Inc., Melville, NY).
Study 3: Effects of aerobic exercise training on prostate cancer cell growth characteristics in serum or prostate-conditioned media from tumor-bearing rats
Orthotopic model of prostate cancer
The PC-3 cells were used in this study for orthotopic tumor implantation in tumor-bearing (TB) groups of Nude rats placed in SED (SED-TB; n = 4) or ET (ET-TB; n = 4) groups. Under anesthesia (isoflurane, 2%/O2 balance), cells were injected into the prostate of SED-TB and ET-TB rats. The bladder and prostate complex were exposed through a small incision (< 1 cm) lateral to the midline of the abdomen. Using sterile syringes (26 gauge), 0.1 ml of cell stock solution (1 × 106 cells) was injected into the ventral lobe of the prostate. Following the injection, closure of the abdominal wall (4-0, polyglycolic acid coated; DemeTECH, Miami Lakes, FL) incisions was performed, and the animal was allowed to recover. All procedures were performed under aseptic conditions. Post-operative monitoring of the animals was performed daily until animals recovered. One week after tumor cell injection, animals in the ET-TB group began exercise training as described in Study 1 (see “Exercise Training”).
Viability of prostate cancer cells, transwell migration assay, and cell cycle analysis
The same methods as described above in Study 1 were used for studies with PC-3 cells.
Citrate synthase activity
The red portion of the gastrocnemius, and soleus muscle, were used for determination of citrate synthase activity, a mitochondrial enzyme and marker of muscle oxidative potential, according to the method of Srere [30]. Briefly, 15 µl and 30 µl samples were diluted using 210 µl and 195 µl of tris buffer, respectively, and 15 µl of acetyl Co-A (Cayman Chemical) and 30 µl of DTNB (Thermo Fisher Scientific) were added to each sample. All samples were incubated for 5 min in a UV/Vis spectrophotometer (accuSkan GO; Fisher Scientific) at 30°C. Readings were taken with a UV/Vis spectrophotometer at 412 nm once per min for 5 min, after which, 30 µl of oxaloacetic acid (OAA; Sigma-Aldrich, St. Louis, MO) was added to all samples and immediately analyzed again. The absorbance values recorded pre-OAA addition represent citrate synthase activity before citrate formation, while the absorbance values recorded post-OAA addition represent citrate synthase activity following the reaction of acetyl-CoA and OAA to form citrate. Citrate synthase activity is reported as µmol/min/g wet weight.
Statistical analysis
Body weight, tissue weight, relative tissue weights, tumor weights, skeletal muscle citrate synthase activity, cell viability, cell migration, percentage of cells in cell cycle phase, 5αR2 and caspase-3 expression levels between rat groups were analyzed with unpaired Student’s t-test, utilizing GraphPad Prism 7.04 (GraphPad Software, Inc., La Jolla, CA). A two-way ANOVA using Holm-Sidak’s multiple comparison test was used to compare cell migration (cell exclusion zone assay) at the different time points within SED and ET groups. Data are presented as mean ± SEM, with P ≤ 0.05 considered statistically significant.
Results
Study 1
In Copenhagen rats, relative heart weight was significantly increased in ET compared to SED rats (Table 1). There were no significant changes in body weight or absolute and relative prostate weight between groups. There was a significant increase in citrate synthase activity in soleus and red portion of the gastrocnemius muscle for ET compared to SED rats, confirming the efficacy of the exercise training program. In Nude rats, there was no significant difference in body weight, relative heart weight, and absolute and relative prostate weight between SED and ET rat groups (Table 1). There was a significant increase in citrate synthase activity in the red portion of the gastrocnemius muscle for ET compared to SED rats, confirming the efficacy of the exercise training program (Table 1).
Table 1.
Body weight, tissue weight, relative tissue weights, and skeletal muscle citrate synthase activity of SED and ET Copenhagen rats, and SED, ET, SED-TB and ET-TB Nude rats
| Animal Characteristics | Copenhagen rats | Nude rats | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| SED (n = 10) | ET (n = 10) | p-value | SED (n = 4) | ET (n = 6) | p-value | SED-TB (n = 4) | ET-TB (n = 4) | p-value | |
| Body weight (BW) (g) | 340.7 ± 7.1 | 333.8 ± 10.4 | 0.59 | 403.8 ± 12.6 | 373.0 ± 13.9 | 0.16 | 403.3 ± 20.0 | 379.8 ± 19.5 | 0.43 |
| Prostate weight (PW) (g) | 0.56 ± 0.03 | 0.58 ± 0.04 | 0.70 | 0.69 ± 0.09 | 0.72 ± 0.05 | 0.79 | 0.41 ± 0.04 | 0.69 ± 0.13 | 0.09 |
| PW/BW (%) | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.51 | 0.17 ± 0.02 | 0.19 ± 0.02 | 0.44 | 0.10 ± 0.00 | 0.18 ± 0.04 | 0.07 |
| Heart weight/BW (%) | 0.24 ± 0.00 | 0.27 ± 0.01 | 0.0017* | 0.28 ± 0.00 | 0.29 ± 0.01 | 0.67 | 0.27 ± 0.01 | 0.30 ± 0.01 | 0.28 |
| Tumor weight (g) | - | - | - | - | - | - | 0.26 ± 0.12 | 0.26 ± 0.10 | 0.97 |
| Skeletal Muscle Citrate Synthase Activity (µmol/min/g) | |||||||||
| Soleus | 27.5 ± 0.7 | 38.6 ± 2.2 | < 0.0001** | 9.86 ± 0.55 | 13.91 ± 1.83 | 0.12 | 10.01 ± 0.20 | 14.54 ± 1.55 | 0.02* |
| Red gastrocnemius | 42.8 ± 2.2 | 50.9 ± 2.2 | 0.02* | 9.04 ± 0.30 | 10.93 ± 1.00 | 0.02* | 8.68 ± 0.83 | 11.07 ± 1.43 | 0.73 |
Values are expressed as mean ± SEM. n, number of rats; SED, sedentary (control); ET, exercise-trained; SED-TB, sedentary tumor-bearing; ET-TB, exercise-trained tumor-bearing.
P ≤ 0.05 vs. SED or SED-TB;
P ≤ 0.0001 vs. SED.
Effects of SSM and PCM on cell viability and migration
In SSM (Figure 2A) and PCM (Figure 2B) from ET rats, there was a significant reduction in AT-1 viable cell number at 24, 48 and 96 h incubation compared to that from SED rats. No significant differences were observed with training for the cell exclusion zone (migration) assay with SSM (Figure 3A) and PCM (Figure 3B) (24, 48 and 96 h incubation in each media type), or transwell migration assay with SSM (0.40 ± 0.02 vs. 0.43 ± 0.01 cell count/total area (pixels2); P > 0.05) in ET vs. SED rats, respectively, after 24 h incubation. Cell migration increased over time (i.e., at 24 vs. 48, 24 vs. 96, and 48 vs. 96 h incubation) in SSM of SED and ET rats (Figure 3A) and at 24 vs. 48, 24 vs. 96, and 48 vs. 96 incubation in PCM of SED rats, and at 24 vs. 96, and 48 vs. 96 h incubation in PCM of ET rats (Figure 3B). In PC-3 cells under normoxic condition, no significant differences were observed between SED and ET rat groups for cell viability with SSM (Figure 4A) and PCM (Figure 4B) (24, 48 and 96 h incubation in each media type). Under hypoxic condition, no significant differences were observed between SED and ET rat groups for PC-3 cell viability with SSM (Figure 5A) and PCM (Figure 5B) (24, 48 and 96 h incubation in each media type). There was no significant difference between SED and ET rat groups for transwell migration assay with SSM (0.75 ± 0.03 vs. 0.69 ± 0.05 cell count/total area (pixels2); P > 0.05) and PCM (0.62 ± 0.04 vs. 0.75 ± 0.06 cell count/total area (pixels2); P > 0.05), respectively, after 24 h incubation in each media type.
Figure 2.
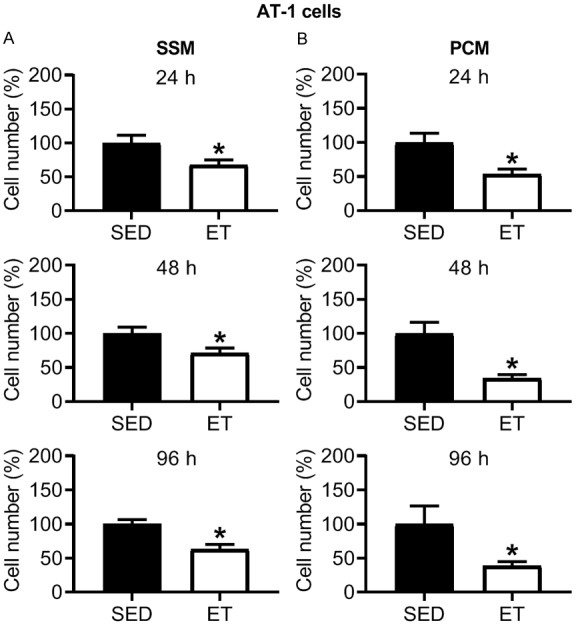
Effect of 24, 48 and 96 h incubation with serum-supplemented media (SSM; A) and prostate-conditioned media (PCM; B) from sedentary (SED; n = 6-10) and exercise-trained (ET; n = 9-10) Copenhagen rats on viable AT-1 cell number. There was a significant decrease in cell number in ET vs. SED for SSM and PCM at 24-96 h (P ≤ 0.05). Values are expressed as mean cell number (%) ± SEM. *P ≤ 0.05 vs. SED, within SSM or PCM treatment group.
Figure 3.
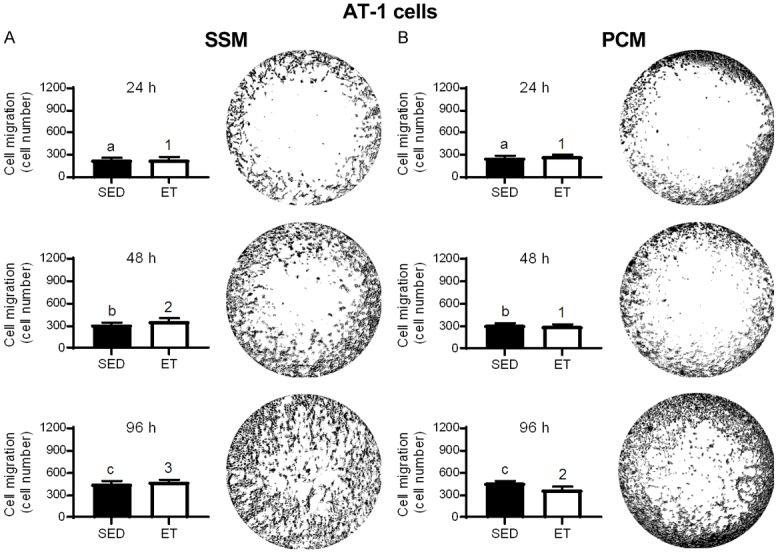
Effect of 24, 48 and 96 h incubation with serum-supplemented media (SSM; A) and prostate-conditioned media (PCM; B) from sedentary (SED; n = 6-7) and exercise-trained (ET; n = 8-9) Copenhagen rats on migration of AT-1 cells. A representative image of cell migration utilizing a cell exclusion zone technique in a 96-well tissue culture plate is illustrated to the right of each bar graph. No significant difference in cell migration between SED and ET groups was observed in SSM or PCM from 24-96 h (P > 0.05). Bars with different letters are statistically different from one another within SED group over time. Bars with different numbers are statistically different from one another within ET group over time. Values are expressed as mean cell number ± SEM. P ≤ 0.05 for 24 vs. 48, 24 vs. 96, and 48 vs. 96 h within SED or ET group.
Figure 4.
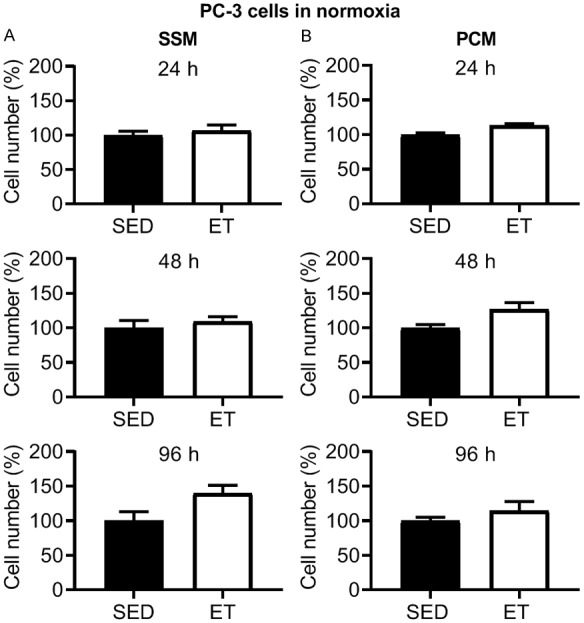
Effect of 24, 48 and 96 h incubation with serum-supplemented media (SSM; A) and prostate-conditioned media (PCM; B) from sedentary (SED; n = 4) and exercise-trained (ET; n = 4-6) Nude rats on viable PC-3 cell number in normoxia. There was no significant difference in cell number in ET vs. SED group for SSM or PCM at 24-96 h (P > 0.05). Values are expressed as mean cell number (%) ± SEM.
Figure 5.
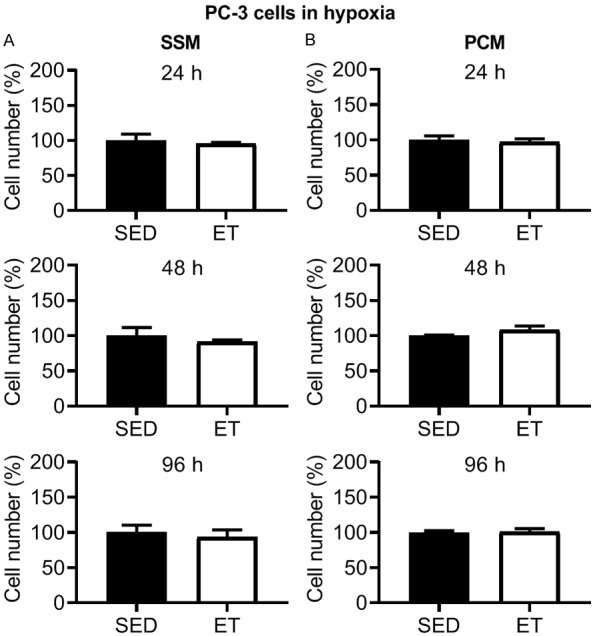
Effect of 24, 48 and 96 h incubation with serum-supplemented media (SSM; A) and prostate-conditioned media (PCM; B) from sedentary (SED; n = 3-4) and exercise-trained (ET; n = 4-6) Nude rats on viable PC-3 cell number in hypoxia. There was no significant difference in cell number in ET vs. SED group for SSM or PCM at 24-96 h (P > 0.05). Values are expressed as mean cell number (%) ± SEM.
Cell cycle analysis
In AT-1 cells, there was no significant change in percentage of cells in G0/G1, S, and G2/M cell cycle phase after treatment with SSM (Figure 6A) or PCM (Figure 6B) in ET compared to SED rats. Similarly, in PC-3 cells under normoxic condition, no significant differences in percentage of cells in G0/G1, S, and G2/M cell cycle phase were observed between SED and ET rat groups after treatment with SSM (Figure 6C) or PCM (Figure 6D).
Figure 6.
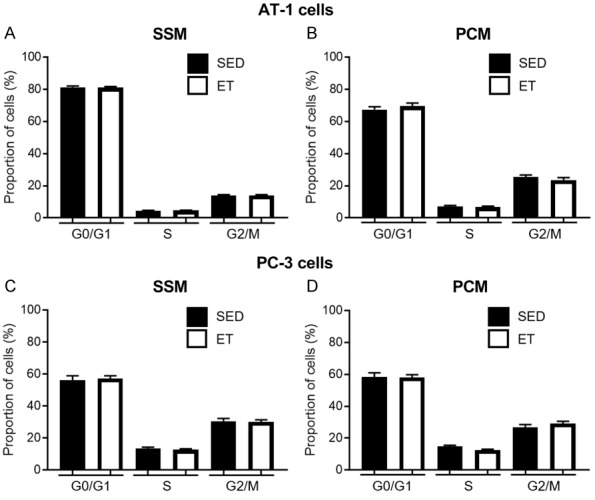
Bar graph representing the percentage of cells in G0/G1, S, and G2/M cell cycle phase after treatment of AT-1 cells with serum-supplemented media (SSM; A) and prostate-conditioned media (PCM; B) from sedentary (SED; n = 5-10) and exercise-trained (ET; n = 9-10) Copenhagen rats, or PC-3 cells (in normoxia) with SSM (C) and PCM (D) from SED (n = 4) and ET (n = 6) Nude rats. There was no significant effect of media type on AT-1 or PC-3 cell population in different cell cycle phase, in SED vs. ET rats (P > 0.05). Values are expressed as mean proportion of cells (%) ± SEM.
Study 2
5α-reductase 2 and caspase-3
Representative images of immunohistochemical sections stained for 5αR2 (A and B) and caspase-3 (C and D) in the prostate of SED and ET rats, respectively, are shown in Figure 7. Prostate 5αR2 expression showed no significant difference in staining intensity between the groups (Figure 7E). However, caspase-3 expression was significantly increased (Figure 7F) with exercise training compared to SED group.
Figure 7.
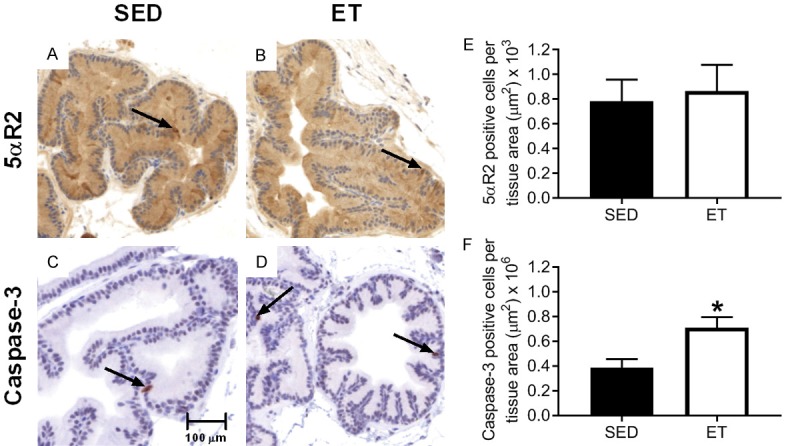
Representative images of immunohistochemical sections stained for 5αR2 (A and B) and caspase-3 (C and D) in the prostate of SED and ET Copenhagen rats, respectively. The arrows within the tissues are representative stained sections showing 5αR2 and caspase-3 staining. Images were captured at 200X magnification. Bar graph representing prostate 5αR2 (E) and caspase-3 (F) expression between sedentary (SED; n = 10) and exercise-trained (ET; n = 10) Copenhagen rats. No significant difference in 5αR2 between SED and ET groups was observed (P > 0.05). Caspase-3 was significantly increased in ET vs. SED group (*P ≤ 0.05 vs. SED). Values are expressed as mean positive cells per tissue area ± SEM.
Study 3
Training efficacy and tissue weights
In Nude tumor-bearing rats, there was no significant difference in body weight, relative heart weight, absolute and relative prostate weight, and tumor weights between SED-TB and ET-TB rat groups (Table 1). There was a significant increase in citrate synthase activity in soleus for ET-TB compared to SED-TB rats confirming the efficacy of the exercise training program (Table 1).
Effects of SSM-TB and PCM-TB on cell viability and migration
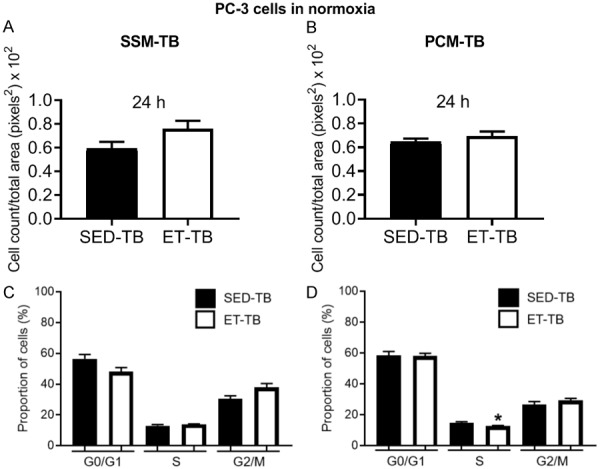
There was no significant difference in viable PC-3 cell number in ET-TB vs. SED-TB rats at 24, 48 and 96 h incubation with SSM-TB in normoxia (Figure 8A). However, there was a significant decrease in viable cell number in ET-TB vs. SED-TB rats at 48 and 96 h incubation with PCM-TB (Figure 8B). Under hypoxic condition, no significant differences were observed between SED-TB and ET-TB rat groups for PC-3 cell viability with SSM-TB (Figure 9A) and PCM-TB (Figure 9B) (24, 48 and 96 h incubation in each media type). There was no significant difference between SED-TB and ET-TB rat groups for transwell migration assay with SSM-TB (24 h incubation) (Figure 10A) and PCM-TB (24 h incubation) (Figure 10B).
Figure 8.
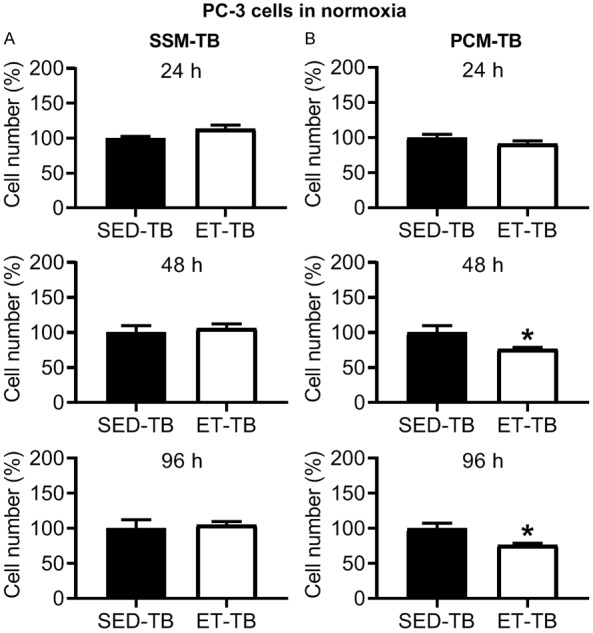
Effect of 24, 48 and 96 h incubation with serum-supplemented media from tumor-bearing rats (SSM-TB; A) and prostate-conditioned media from tumor-bearing rats (PCM-TB; B) from sedentary tumor-bearing (SED-TB; n = 4) and exercise-trained tumor-bearing (ET-TB; n = 4) Nude rats on viable PC-3 cell number in normoxia. There was no significant difference in cell number in ET-TB vs. SED-TB group for SSM-TB at 24-96 h (P > 0.05). However, a significant decrease in cell number in ET-TB vs. SED-TB group was observed for PCM-TB at 48 and 96 h (P ≤ 0.05). Values are expressed as mean cell number (%) ± SEM. *P ≤ 0.05 vs. SED-TB, within PCM-TB.
Figure 9.
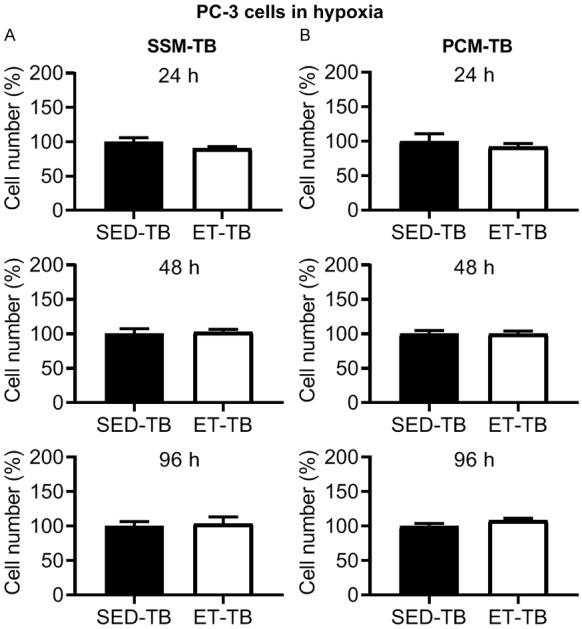
Effect of 24, 48 and 96 h incubation with serum-supplemented media from tumor-bearing rats (SSM-TB; A) and prostate-conditioned media from tumor-bearing rats (PCM-TB; B) from sedentary tumor-bearing (SED-TB; n = 4) and exercise-trained tumor-bearing (ET-TB; n = 4) Nude rats on viable PC-3 cell number in hypoxia. There was no significant difference in cell number in ET-TB vs. SED-TB group for SSM-TB or PCM-TB at 24-96 h (P > 0.05). Values are expressed as mean cell number (%) ± SEM.
Figure 10.
Effect of 24 h incubation with serum-supplemented media from tumor-bearing rats (SSM-TB; A) and prostate-conditioned media from tumor-bearing rats (PCM-TB; B) from sedentary tumor-bearing (SED-TB; n = 3-4) and exercise-trained tumor-bearing (ET-TB; n = 4) Nude rats on migration (via Transwell migration assay) of PC-3 cells in normoxia. No significant difference in cell migration between groups was observed (P > 0.05). Values are expressed as mean cell count/total area (pixels2) ± SEM. Bar graph representing the percentage of PC-3 cells in G0/G1, S, and G2/M cell cycle phase after treatment of PC-3 cells with serum-supplemented media from tumor-bearing rats (SSM-TB; C) and prostate-conditioned media from tumor-bearing rats (PCM-TB; D) from sedentary tumor-bearing (SED-TB; n = 4) and exercise-trained tumor-bearing (ET-TB; n = 4) Nude rats in normoxia. There was no significant effect of SSM-TB on cell population in different cell cycle phase in SED-TB vs. ET-TB rats (P > 0.05). PCM-TB however caused a significant decrease of cells in S phase in ET-TB vs. SED-TB (P ≤ 0.05). Values are expressed as mean proportion of cells (%) ± SEM. *P ≤ 0.05 vs. SED-TB, within PCM-TB.
Cell cycle analysis
No significant differences in percentage of cells in G0/G1, S, and G2/M cell cycle phase were observed between SED-TB and ET-TB groups after treatment with SSM-TB (Figure 10C). However, there was a significant decrease of PC-3 cells in S phase in ET-TB vs. SED-TB rats after treatment with PCM-TB (P ≤ 0.05) (Figure 10D).
Discussion
There are several novel findings from this series of investigations. Specifically, exercise training in healthy Copenhagen rats reduced prostate cancer (AT-1 cells) cell viability in conditioned media from the prostate and serum versus that from sedentary counterparts. Further, there was an upregulation of caspase-3 expression within the prostate of exercise-trained versus sedentary groups. This suggests a potential preventative effect of exercise on the establishment and viability of cancerous cells in the prostate and/or circulation. However, in conditioned media from healthy Nude rats, exercise training did not alter prostate cancer cell (PC-3 cells) growth characteristics. This suggests potential cell and/or immune-status variability. For example, exercise training may not reduce prostate cancer cell viability to the same degree in subjects with a chronically suppressed immune system as it does in immune-competent subjects. Importantly and clinically relevant to the prostate cancer patient, once the tumor was established, we demonstrated that exercise training can reduce the viability of tumor cell (PC-3 cells) treated with conditioned media from the prostate surrounding the tumor. Further, cell cycle analysis from the PCM of the exercise-trained tumor bearing animals demonstrated a lower portion of cells in the active S phase. Collectively, these studies suggest that exercise training may cause systemic changes in serum or modify the growth-supporting environment of the prostate to reduce prostate cancer cell viability in healthy individuals, and that aerobic exercise may alter the local environment of the prostate surrounding a tumor to reduce cell viability and replication, thus slowing the progression of the disease. The latter provides one potential mechanism for the increased survivorship of patients with prostate cancer that engage in exercise training [7].
Exercise training effects on the anti-cancer potential of serum
Various training intensities and/or durations (chronic or acute exercise) can elicit differential responses in the levels of insulin-like growth factor-1 (IGF-1) and insulin-like growth factor-binding protein-1 (IGFBP-1), two growth factors that play significant roles in tumor cell growth [31]. Although we did not quantify these growth factors, previous studies using serum from long-term endurance trained humans identified reduced levels of IGF-1 and increased levels of IGFBP-1 as factors responsible for the inhibitory effect of exercise-conditioned serum on LNCaP cell growth [11,32,33]. Previous human studies evaluated the effect of acute exercise training on serum [12], whereas, we utilized chronic exercise training to mimic the long-term effects of exercise on serum and the local prostate environment. Therefore, results in this study represent how moderate exercise training may impact prostate cancer cell viability and thereby suppress tumor progression over the disease’s long latency period [34]. Further, we chose androgen-insensitive prostate cancer cell lines [35,36] to avoid potential effects of altered testosterone concentrations that occur with long-term exercise training. Specifically, acute exercise usually increases serum testosterone [37]; whereas, long-term aerobic exercise decreases serum testosterone levels [38]. Thus, the inhibitory effect of serum from exercise-trained rats on prostate cancer (AT-1 cells) cell viability found herein would not result from altered serum androgen concentrations. In general, circulating tumor cells do not survive well in the systemic circulation due, in part, to the chaotic nature of cardiovascular physical forces (e.g., significant hemodynamic shear stress) [39]. However, those that do survive may ultimately result in metastatic disease. The current results suggest that exercise training may inhibit the viability of some cancer cell types when exposed to components of the blood (i.e., serum), possibly diminishing the metastatic potential of such cells once they have migrated into the systemic circulation.
Effects of exercise training on the local environment within the prostate of non-tumor bearing animals
During moderate-intensity exercise (< 70% VO2max), blood flow to the prostate does not change considerably in rats [40], demonstrating the lack of change in the organ’s metabolic activity during exercise of this intensity. However, given that the beneficial effects of exercise training extend beyond those of the actively-contracting muscle [41], we had hypothesized that exercise training will induce both a systemic effect on serum as well as a local effect on the prostate. Consistent with our hypothesis, PCM from exercise-trained healthy rats (i.e., immune-competent) caused an inhibitory effect on prostate cancer (AT-1 cells) cell viability, which is consistent with other studies demonstrating an altered tumor growth-supporting environment of some tissues after exercise training. For example, Theriau et al [14] demonstrated that in rats fed a high-fat diet, voluntary physical activity mitigated several MCF7 cell growth characteristics in conditioned media from visceral (epidydimal) adipose tissue, including decreasing the percentage of cells in S phase. There are several potential differences between studies, including the comparison of the tissues for the conditioned media, exercise training, and cancer type. Specifically, we investigated the effect of moderate-intensity exercise training on the host-tissue (prostate) of prostate cancer; whereas, Theriau et al [14] investigated the effect of voluntary physical activity on visceral (epidydimal) adipose tissue which, although not the tissue of origin for breast cancer cells, may act in a paracrine fashion to support breast cancer growth [42]. Whether exercise training alters the mammary duct(s) (predominant site of breast cancer neoplasms) is unknown. The current results suggest that exercise training changes some facets of the growth-supporting environment of the prostate on prostate cancer cell growth; however, in the PCM and SSM from the Nude (immune-deficient) non-tumor bearing rats, exercise training did not alter PC-3 cell growth characteristics and the mechanisms that contribute to the differences between immune-deficient and immune-competent rats are unknown. It is possible that the depressed immune function of the Nude rats countered any potential local or systemic changes in the prostate or serum, respectively that could impact tumor cell viability. Further, the exercise capacity of the Nude rats was considerably lower, as was the oxidative capacity of locomotory skeletal muscle (Table 1), mandating a lower absolute intensity of exercise that may have also influenced those results.
Exercise training and 5αR2
To our knowledge, this is the first study to evaluate the effect of exercise training on prostate 5αR2 expression, an essential component of androgen metabolism in prostate cancer [16]. Given that an increased 5αR2 may convert testosterone to DHT to potentially stimulate prostate tumor development and progression [16], we had hypothesized that exercise training will decrease the enzyme’s expression in the prostate. Contrary to our hypothesis, 5αR2 in the prostate was not significantly changed with exercise training, suggesting that in the prostate, exercise training may not modulate local bioactive androgen metabolism. Serum testosterone levels are known to decrease with endurance aerobic exercise training [38]; however, this may not affect local concentrations in the prostate [43]. In fact, the prostate can maintain a functional level of androgen regardless of serum androgen levels [43]. The level of 5αR2 in the prostate of ET rats was probably sufficient to convert any prostate testosterone levels to DHT such that 5αR2 expression in the prostate remained unaltered. Some studies report an increase in 5α-reductase 1 (5αR1) in skeletal muscles following acute exercise [44] or an increase in 5α-reductase activity in blood following high-intensity interval exercise [45] in response to an elevated uptake and utilization of testosterone [46]. There are several potential differences between studies, including the comparison of the isoenzymes, exercise training, and tissue types. Our study measured 5αR2 in the prostate of moderate-intensity ET rats; whereas, Aizawa et al [44] measured 5αR1 in skeletal muscles of a single period of acute exercise-trained rats. We would not expect an increased uptake and/or utilization of testosterone in the prostate during exercise training.
Exercise training and caspase-3
Consistent with our hypothesis, there was an increase in caspase-3 in the prostate with exercise training. This is similar to findings in a study demonstrating increased apoptosis in the rat ventral prostate after exercise [47], thus potentially eliminating cells with increased potential for malignancy (e.g., cells with damaged DNA or aberrant cell cycling) [48,49]. A reduction in circulating levels of testosterone, as occurs with aerobic exercise training [38], was found to increase caspase-3 in human prostate cancer xenografts [50]. Previous studies also demonstrating an inhibition of MCF7 cell growth in exercise serum-conditioned media suggest this was associated with an induction of apoptosis [51]. Such increase in apoptosis has been associated with a reduction in circulating IGF-1 and an increase in IGFBP-1 [11]. IGF-1 is known to promote cell proliferation and inhibit apoptosis [52]. Although we did not determine levels of these growth factors, these may be underlying mechanisms for the upregulation of caspase-3 in the prostate. The induction of apoptosis has been associated with tumor regression [53]. Although exercise training does not consistently decrease tumor growth versus sedentary counterparts [54], exercise training may lead to a less aggressive phenotype once the tumor is established. PCM’s inhibitory effect on cell viability suggests caspase-3 activity in the prostate may be required to induce significant changes in the tumor growth-supporting environment of the prostate.
Exercise of exercise training on the prostate from tumor-bearing animals
One of the most intriguing findings from the current study was the influence of exercise training on reducing tumor cell (human PC-3 cells) viability in prostate-conditioned media from the prostate surrounding the orthotopic tumor. The inhibition of PC-3 cells in the PCM of the exercise-trained tumor-bearing animals may, in part, result from S phase deceleration, as observed in this study, and evidenced by others [14,55] as a possible mechanism for the anti-proliferative effects of exercise training. It is also noteworthy that this effect was not observed in the PCM from the non-tumor bearing nude rats. This may be due to a higher relative intensity of exercise between the two groups. Recently we have demonstrated that prostate cancer, independent of treatment, lowers endurance capacity [56] leading to premature fatigue, which may be associated with cardiac dysfunction with the disease [57]. Therefore, given the lower exercise capacity of the tumor-bearing versus non-tumor-bearing animal, for a given speed and incline on a treadmill, the tumor-bearing rats would be at a higher relative intensity (i.e., higher % of aerobic capacity). These findings in the exercise-trained immune-deficient animals are clinically relevant given the depressed immune function that occurs in many human patients treated with traditional anti-cancer therapies. Indeed, data suggest that in men diagnosed with prostate cancer, exercise training can reduce the severity of the disease [7], although the precise mechanisms remain unknown.
The decrease in tumor cell viability induced by PCM from exercise-trained tumor bearing animals was abolished when the PC-3 cells were incubated in a hypoxic environment. This highlights the importance of hypoxia on tumor cell characteristics and the aggressiveness of the tumor microenvironment [40]. Importantly, we have demonstrated a reduction in tumor hypoxia after training [54], suggesting the results from cells incubated in normoxia may better recapitulate the local environment in the prostate after training. In breast cancer, Betof and collegaues have also demonstrated a more homogenous basal tumor perfusion after training [58]; collectively suggesting the beneficial effects of exercise may be conserved across multiple cancers.
Taken together, our results suggest that exercise could provide therapeutic benefits for prostate cancer patients. Our results show that exercise training was beneficial in modifying the prostate environment to diminish prostate cancer cell viability in vitro. Further, exercise training may impact the prostate tumor microenvironment by mitigating tumor hypoxia [3], a well-known driver of tumor aggressiveness and therapeutic resistance.
Limitations
Exercise training can affect circulating stress hormone (cortisol) levels in rats, with the latter affecting immune responses [59]. However, it should be noted that in humans, exercise intensities above 40% of VO2max significantly increase circulating cortisol levels [60], similar to what is observed in animals that exercise [59]. Generally, the lack of differences in cell cycle phases in conditioned media types between rat groups may have resulted from cells not attaining the threshold size where cells irreversibly commit to at least one round of division, thereby not achieving adequate size required for DNA synthesis and mitosis [61]. The use of genetically matched cell line (AT-1 cells) specific to the immunocompetent Copenhagen rat may have avoided any potential effects of immune responses on cell growth, hence the differences in cell viability with SSM and PCM between rat groups [62]. On the other hand, PC-3 cells are not a genetically matched cell line specific to Nude rats, and this could have affected immune responses on cell growth and resulted in the lack of differences we observed in cell viability with SSM, PCM and SSM-TB in normoxia, or with all conditioned media types in hypoxia. Due to limited amount of SSM and/or PCM from Copenhagen rats, we were unable to perform transwell migration assay and hypoxia studies with AT-1 cells.
Conclusions
Overall, our findings suggest that moderate-intensity exercise training significantly reduced prostate cancer cell viability in the serum and prostate of trained rats but did not modify several other key prostate tumor cell characteristics (e.g., migration, cell cycle except in S phase of PC-3 cells in PCM-TB). Importantly, once the tumor was established, exercise training reduced tumor cell viability in the surrounding prostate, which may help explain the reduced severity of the disease in patients that exercise.
Acknowledgements
We thank Dr. Timothy Musch and Dr. David Poole of the Departments of Kinesiology, and Anatomy and Physiology, Kansas State University for allowing us to use their treadmill to train the rats, and for providing technical assistance with the treadmill. We gratefully acknowledge Savannah Ardery, Joe Pyle, Jen Thompson, Charles Schneider, Eric Heffern, Cameron Jackson, Taylor Rand, Olivia Schmidtberger and Vanessa-Rose Turpin at Kansas State University for their support during rat training and cell migration studies. We also thank Jennifer Phinney, of the Kansas State University Histology and Immunohistochemistry laboratory, for the support during the immunohistochemistry experiments, and Kaori Knights, of the College of Veterinary Medicine, Kansas State University for assisting with flow cytometry. American Cancer Society Grant RSG-14-150-01-CCE (BJB).
Disclosure of conflict of interest
None.
References
- 1.American Cancer Society. Cancer Facts and Figures. 2019 [Google Scholar]
- 2.Ferrara F, Staquicini DI, Driessen WHP, D’Angelo S, Dobroff AS, Barry M, Lomo LC, Staquicini FI, Cardó-Vila M, Soghomonyan S, Alauddin MM, Flores LG, Arap MA, Lauer RC, Mathew P, Efstathiou E, Aparicio AM, Troncoso P, Navone NM, Logothetis CJ, Marchiò S, Gelovani JG, Sidman RL, Pasqualini R, Arap W. Targeted molecular-genetic imaging and ligand-directed therapy in aggressive variant prostate cancer. PNAS. 2016;113:12786–12791. doi: 10.1073/pnas.1615400113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiggins JM, Opoku-Acheampong AB, Baumfalk DR, Siemann DW, Behnke BJ. Exercise and the tumor microenvironment: potential therapeutic implications. Exerc Sport Sci Rev. 2018;46:56–64. doi: 10.1249/JES.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 4.Littman AJ, Kristal AR, White E. Recreational physical activity and prostate cancer risk (United States) Cancer Causes Control. 2006;17:831–841. doi: 10.1007/s10552-006-0024-8. [DOI] [PubMed] [Google Scholar]
- 5.Wiklund F, Lageros YT, Chang E, Bälter K, Johansson J, Adami H, Grönberg H. Lifetime total physical activity and prostate cancer risk: a population-based case-control study in Sweden. Eur J Epidemiol. 2008;23:739–746. doi: 10.1007/s10654-008-9294-7. [DOI] [PubMed] [Google Scholar]
- 6.Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889–3895. doi: 10.1158/0008-5472.CAN-10-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh AA, Jones LW, Antonelli JA, Gerber L, Calloway EE, Shuler KH, Freedland SJ, Grant DJ, Hoyo C, Bañez LL. Association between exercise and primary incidence of prostate cancer: does race matter? Cancer. 2013;119:1338–1343. doi: 10.1002/cncr.27791. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Jacobs EJ, Gapstur SM, Maliniak ML, Gansler T, McCullough ML, Stevens VL, Patel AV. Recreational physical activity in relation to prostate cancer-specific mortality among men with nonmetastatic prostate cancer. Eur Urol. 2017;72:931–939. doi: 10.1016/j.eururo.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States) Cancer Causes Control. 2002;13:929–935. doi: 10.1023/a:1021911517010. [DOI] [PubMed] [Google Scholar]
- 12.Rundqvist H, Augsten M, Strömberg A, Rullman E, Mijwel S, Kharaziha P, Panaretakis T, Gustafsson T, Östman A. Effect of acute exercise on prostate cancer cell growth. PLoS One. 2013;8:e67579. doi: 10.1371/journal.pone.0067579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dethlefsen C, Hansen LS, Lillelund C, Andersen C, Gehl J, Christensen JF, Pedersen BK, Hojman P. Exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 2017;77:4894–4904. doi: 10.1158/0008-5472.CAN-16-3125. [DOI] [PubMed] [Google Scholar]
- 14.Theriau CF, Shpilberg Y, Riddell MC, Connor MK. Voluntary physical activity abolishes the proliferative tumor growth microenvironment created by adipose tissue in animals fed a high fat diet. J Appl Physiol. 2016;121:139–153. doi: 10.1152/japplphysiol.00862.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenzo PI, Arnoldussen YJ, Saatcioglu F. Molecular mechanisms of apoptosis in prostate cancer. Crit Rev Oncog. 2007;13:1–38. doi: 10.1615/critrevoncog.v13.i1.10. [DOI] [PubMed] [Google Scholar]
- 16.Imamoto T, Suzuki H, Yano M, Kawamura K, Kamiya N, Araki K, Komiya A, Nihei N, Naya Y, Ichikawa T. The role of testosterone in the pathogenesis of prostate cancer. Int J Urol. 2008;15:472–480. doi: 10.1111/j.1442-2042.2008.02074.x. [DOI] [PubMed] [Google Scholar]
- 17.Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 18.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–6. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 19.O’Neill AJ, Boran SA, O’Keane C, Coffey RN, Hegarty NJ, Hegarty P, Gaffney EF, Fitzpatrick JM, Watson RW. Caspase 3 expression in benign prostatic hyperplasia and prostate carcinoma. Prostate. 2001;47:183–188. doi: 10.1002/pros.1061. [DOI] [PubMed] [Google Scholar]
- 20.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Cancer invasion and metastasis: molecular and cellular perspective. Landes Bioscience; 2013. [Google Scholar]
- 21.Fadaka A, Ajiboye B, Ojo O, Adewale O, Olayide I, Emuowhochere R. Biology of glucose metabolism in cancer cells. J Oncol Sci. 2017:45–51. [Google Scholar]
- 22.Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Pinover WH, Greenberg RE, Stobbe C, Hanks GE. Hypoxia in human prostate carcinoma: an Eppendorf PO2 study. Am J Clin Oncol. 2001;24:458–461. doi: 10.1097/00000421-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Parker C, Milosevic M, Toi A, Sweet J, Panzarella T, Bristow R, Catton C, Catton P, Crook J, Gospodarowicz M, McLean M, Warde P, Hill RP. Polarographic electrode study of tumor oxygenation in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:750–757. doi: 10.1016/S0360-3016(03)01621-3. [DOI] [PubMed] [Google Scholar]
- 24.Movsas B, Chapman JD, Horwitz EM, Pinover WH, Greenberg RE, Hanlon AL, Iyer R, Hanks GE. Hypoxic regions exist in human prostate carcinoma. Urology. 1999;53:11–18. doi: 10.1016/s0090-4295(98)00500-7. [DOI] [PubMed] [Google Scholar]
- 25.Avila JJ, Kim SK, Massett MP. Differences in exercise capacity and responses to training in 24 inbred mouse strains. Front Physiol. 2017;8:974. doi: 10.3389/fphys.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spier SA, Laughlin MH, Delp MD. Effects of acute and chronic exercise on vasoconstrictor responsiveness of rat abdominal aorta. J Appl Physiol. 1999;87:1752–1757. doi: 10.1152/jappl.1999.87.5.1752. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka A, Hasegawa T, Chen Z, Okita Y, Okada K. A novel rat model of abdominal aortic aneurysm using a combination of intraluminal elastase infusion and extraluminal calcium chloride exposure. J Vasc Surg. 2009;50:1423–1432. doi: 10.1016/j.jvs.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc. 2013;8:2471–2482. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenger RH, Kurtcuoglu V, Scholz CC, Marti HH, Hoogewijs D. Frequently asked questions in hypoxia research. Hypoxia (Auckl) 2015;3:35–43. doi: 10.2147/HP.S92198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srere P. Methods in enzymology. 1969. Citrate synthase; pp. 1–11. [Google Scholar]
- 31.Nindl BC, Pierce JR. Insulin-like growth factor I as a biomarker of health, fitness, and training status. Med Sci Sports Exerc. 2010;42:39–49. doi: 10.1249/MSS.0b013e3181b07c4d. [DOI] [PubMed] [Google Scholar]
- 32.Barnard RJ, Ngo TH, Leung P, Aronson WJ, Golding LA. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate. 2003;56:201–206. doi: 10.1002/pros.10251. [DOI] [PubMed] [Google Scholar]
- 33.Ngo TH, Barnard RJ, Leung P, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144:2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 34.Arcangeli S, Pinzi V, Arcangeli G. Epidemiology of prostate cancer and treatment remarks. World J Radiol. 2012;4:241–246. doi: 10.4329/wjr.v4.i6.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaacs JT, Isaacs WB, Feitz WF, Scheres J. Establishment and characterization of seven dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate. 1986;9:261–281. doi: 10.1002/pros.2990090306. [DOI] [PubMed] [Google Scholar]
- 36.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 37.Cumming DC, Brunsting LA, Strich G, Ries AL, Rebar RW. Reproductive hormone increases in response to acute exercise in men. Med Sci Sports Exerc. 1986;18:369–373. [PubMed] [Google Scholar]
- 38.Hackney AC. Endurance exercise training and reproductive endocrine dysfunction in men: alterations in the hypothalamic-pituitary-testicular axis. Curr Pharm Des. 2001;7:261–273. doi: 10.2174/1381612013398103. [DOI] [PubMed] [Google Scholar]
- 39.Regmi S, Fu A, Luo KQ. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Scientific Reports. 2017;7:39975. doi: 10.1038/srep39975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullough DJ, Stabley JN, Siemann DW, Behnke BJ. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst. 2014;106:dju036. doi: 10.1093/jnci/dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCullough DJ, Davis RT, Dominguez JM, Stabley JN, Bruells CS, Behnke BJ. Effects of aging and exercise training on spinotrapezius muscle microvascular PO2 dynamics and vasomotor control. J Appl Physiol. 2011;110:695–704. doi: 10.1152/japplphysiol.01084.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patsialou A, Wyckoff J, Wang Y, Goswami S, Stanley ER, Condeelis JS. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009;69:9498–9506. doi: 10.1158/0008-5472.CAN-09-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Otto-Duessel M, He M, Markel S, Synold T, Jones JO. Low systemic testosterone levels induce androgen maintenance in benign rat prostate tissue. J Mol Endocrinol. 2013;51:143–153. doi: 10.1530/JME-13-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aizawa K, Iemitsu M, Maeda S, Otsuki T, Sato K, Ushida T, Mesaki N, Akimoto T. Acute exercise activates local bioactive androgen metabolism in skeletal muscle. Steroids. 2010;75:219–223. doi: 10.1016/j.steroids.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Hackney AC, Hosick KP, Myer A, Rubin DA, Battaglini CL. Testosterone responses to intensive interval versus steady-state endurance exercise. J Endocrinol Invest. 2012;35:947–950. doi: 10.1007/BF03346740. [DOI] [PubMed] [Google Scholar]
- 46.Cumming DC. The male reproductive system, exercise, and training. In: Warren MP, Constantini NW, editors. Contemporary Endocrinology. Sports Endocrinology. 2000. pp. 119–131. [Google Scholar]
- 47.Teixeira GR, Fávaro WJ, Pinheiro PF, Chuffa LG, Amorim JP, Mendes LO, Fioruci BA, Oba E, Martins OA, Martinez M, Martinez FE. Physical exercise on the rat ventral prostate: steroid hormone receptors, apoptosis and cell proliferation. Scand J Med Sci Sports. 2012;22:e86–92. doi: 10.1111/j.1600-0838.2012.01501.x. [DOI] [PubMed] [Google Scholar]
- 48.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 49.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 50.Smitherman AB, Gregory CW, Mohler JL. Apoptosis levels increase after castration in the CWR22 human prostate cancer xenograft. Prostate. 2003;57:24–31. doi: 10.1002/pros.10271. [DOI] [PubMed] [Google Scholar]
- 51.Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab. 2011;301:504–510. doi: 10.1152/ajpendo.00520.2010. [DOI] [PubMed] [Google Scholar]
- 52.Dupont J, Le Roith D. Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells: new insights into their synergistic effects. Mol Pathol. 2001;54:149–154. doi: 10.1136/mp.54.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staack A, Kassis AP, Olshen A, Wang Y, Wu D, Carroll PR, Grossfeld GD, Cunha GR, Hayward SW. Quantitation of apoptotic activity following castration in human prostatic tissue in vivo. Prostate. 2003;54:212–219. doi: 10.1002/pros.10179. [DOI] [PubMed] [Google Scholar]
- 54.McCullough DJ, Nguyen LM, Siemann DW, Behnke BJ. Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. J Appl Physiol. 2013;115:1846–1854. doi: 10.1152/japplphysiol.00949.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farioli-Vecchioli S, Mattera A, Micheli L, Ceccarelli M, Leonardi L, Saraulli D, Costanzi M, Cestari V, Rouault J, Tirone F. Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cells. Stem Cells. 2014;32:1968–1982. doi: 10.1002/stem.1679. [DOI] [PubMed] [Google Scholar]
- 56.Esau PJ, Gittemeier EM, Opoku-Acheampong AB, Rollins KS, Baumfalk DR, Poole DC, Musch TI, Behnke BJ, Copp SW. Prostate cancer reduces endurance exercise capacity in association with reductions in cardiac and skeletal muscle mass in the rat. Am J Cancer Res. 2017;7:2566–2576. [PMC free article] [PubMed] [Google Scholar]
- 57.Baumfalk DR, Opoku-Acheampong AB, Caldwell JT, Ade CJ, Copp SW, Musch TI, Behnke BJ. Effects of prostate cancer and exercise training on left ventricular function and cardiac and skeletal muscle mass. J Appl Physiol (1985) 2019;126:668–680. doi: 10.1152/japplphysiol.00829.2018. [DOI] [PubMed] [Google Scholar]
- 58.Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, Palmer G, Jones LW, Dewhirst MW. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jameel MK, Joshi AR, Dawane J, Padwal M, Joshi A, Pandit VA, Melinkeri R. Effect of various physical stress models on serum cortisol level in wistar rats. J Clin Diagn Res. 2014;8:181–183. doi: 10.7860/JCDR/2014/7210.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31:587–591. doi: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- 61.Guertin DA, Sabatini DM. Chapter 12 - Cell Growth. In: Mendelsohn J, Howley PM, Israel MA, Gray JW, Thompson CB, editors. The molecular basis of cancer. 3rd edition. Philadelphia, W.B.: Saunders; 2008. pp. 169–175. [Google Scholar]
- 62.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]


