Abstract
Biotechnological applications of protein complexes require detailed information about their structure and composition, which can be challenging to obtain for proteins from natural sources. Prominent examples are the ring‐shaped phycoerythrin (PE) and phycocyanin (PC) complexes isolated from the light‐harvesting antennae of red algae and cyanobacteria. Despite their widespread use as fluorescent probes in biotechnology and medicine, the structures and interactions of their noncrystallizable central subunits are largely unknown. Here, we employ ion mobility mass spectrometry to reveal varying stabilities of the PC and PE complexes and identify their closest architectural homologues among all protein assemblies in the Protein Data Bank (PDB). Our results suggest that the central subunits of PC and PE complexes, although absent from the crystal structures, may be crucial for their stability, and thus of unexpected importance for their biotechnological applications.
Keywords: structural mass spectrometry, protein interactions, collision cross sections, protein complex stability, ion mobility, red algae
Abbreviations
- APC
allophycocyanin
- IM‐MS
ion mobility mass spectrometry
- PC
phycocyanin
- PE
phycoerythrin
- CCS
collision cross section
Introduction
Phycobilisomes are large (up to 20 MDa) light‐harvesting complexes found mainly in red algae and cyanobacteria.1 Their main components are ring‐shaped heteromeric phycobiliprotein complexes composed of an equal number of α‐ and β‐subunits that each contain multiple tetrapyrrole chromophores.2 These complexes are grouped into the pink phycoerythrins (PE) and the blue phycocyanins (PC), as well as the blue allophycocyanin (APC). They are among the brightest fluorescent molecules known, and have therefore numerous applications as probes in biotechnology, food coloring additives, and even anticancer agents.3, 4 Although recombinant production has been explored, their purification from natural sources has remained the production standard for all applications.5, 6, 7 As a result, there is a significant effort to find new species of red algae for the extraction of phycobiliproteins with different properties, and methods that enable reliable characterization of their structure and integrity are in high demand.8, 9
In the native algal phycobilisome, PC and PE rings are assembled into rods and connected to a central APC core with the help of a heterogeneous family of linker polypeptides. PE and PC interact with a number of linker proteins, including one occupying the central cavity of the ring. PE commonly co‐purifies with its central linker, referred to as the γ‐subunit, as an α6β6γ complex (Fig. 1). This γ‐subunit is seen in the center of the α6β6 ring as a diffuse electron density in high‐resolution structures.10, 11 PC is known to associate with a central linker protein similar to the γ‐subunit in PE, but again, detailed crystallographic evidence for the interaction is lacking.12 The structure of the complex between PE and PC and their γ‐subunits remains unsolvable in crystallography because it lacks the symmetry of the α6β6 ring, which yields rotational averaging of its electron density.
Figure 1.
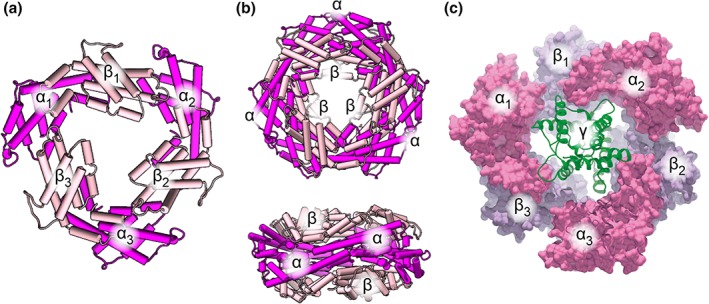
Architecture of the α6β6γ PE complex. (a) The basic unit of the PE complex is a ring‐shaped trimer composed of three αβ dimers. α‐ and β‐subunits are rendered in magenta and pink, respectively. (b) The α‐subunits of two α3β3 trimers stack together to form the α6β6 ring, shown as top and side view. (c) The ring cavity is occupied by the γ‐subunit (green), shown here based on an individual PE complex from the cryo‐EM structure of the entire phycobilisome (PDB ID 5Y6P) with the top α3β3 trimer removed.
We therefore turned to mass spectrometry (MS) as an alternative strategy to assess the structural integrity of PE and PC complexes with their central linker subunits. Here, protein complexes can be gently transferred from near‐physiological solutions to the gas phase, often without significant distortions of their native structures or interactions. This enables us to determine the masses and abundances of intact complexes, or their individual components following dissociation inside the mass spectrometer.13, 14 In combination with ion mobility (IM) measurements, the approach can provide information about their overall structure and connectivity.15 Since MS has been used to monitor pH and concentration effects on the assembly light‐harvesting complexes,16, 17 we expect IM‐MS to be able to unravel the architectures of native phycobiliprotein assemblies.
Results and Discussion
As first step, we analyzed the stoichiometry of PE by MS. Using instrumental conditions optimized for the transfer of intact protein complexes to the gas phase,18 we obtained well‐resolved spectra of PE with no signs of complex dissociation [Fig. 2(a)]. The spectra show two overlapping series of peaks with narrow charge state distributions centered around the 35+ ions, indicating non‐denatured conformations.19 The masses of 262,279 ± 65 and 265,964 ± 108 Da are both in good agreement with α6β6γ complexes (Table S1). Leney et al. found that PE may adopt variable stoichiometries with 5 and 7 α‐ and β‐subunits.16 However, this does not explain the 3.6 kDa difference between the two populations observed here. Instead, SDS‐polyacrylamide gel electrophoresis (PAGE) analysis clearly shows the presence of two different γ‐subunits separated by less than 5 kDa (Fig. S1). Similar observations were previously interpreted as PE containing two γ‐subunits per complex.20 Our results on the other hand clearly show that purified PE exists as α6β6γ complexes with either a 28‐ (γ′) or a 32‐kDa subunit (γ″). The different γ‐subunits likely distinguish PE from the distal or middle regions of the light‐harvesting rod.21 In addition, a minor population of (α6β6γ)2 dimers with 55+ charges can be detected; however, their γ‐composition could not be resolved (Fig. S2a).
Figure 2.
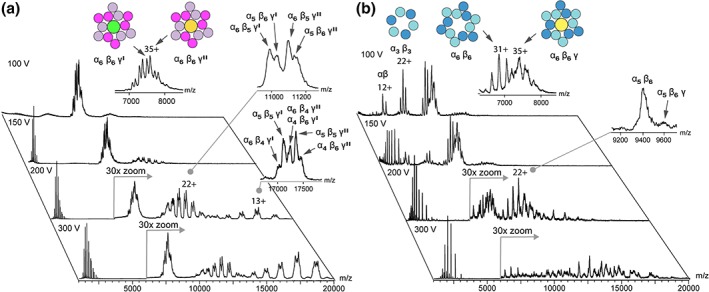
Mass spectrometric analysis of PE and PC. (a) Intact mass and gas‐phase dissociation of PE. At low cone voltages, PE exhibits a homogeneous α6β6γ stoichiometry containing either a 28 or 32 kDa γ‐subunits (γ′ or γ″). Increasing the cone voltage releases α6β5γ and subsequently α5β5γ sub‐complexes, but cannot dissociate all α6β6γ assemblies. (b) Intact mass and gas‐phase dissociation of PC. Under gentle ionization conditions, MS shows the presence of PC complexes with and without a γ‐subunit. Raising the cone voltage induces dissociation of both species, with the α6β6 and the α6β6γ complexes fully dissociated at 200 and 300 V, respectively.
Next, we subjected the intact PE complexes to gas‐phase dissociation, which provides information about subunit connectivity and stability. By raising the voltage of the sample cone at the entrance to the vacuum region of the mass spectrometer, the desolvated protein assemblies are accelerated and undergo dissociation via collisions with residual gas molecules prior to MS analysis. Upon increasing the cone voltage to 150 V, additional peaks corresponding to free β‐subunits and α6β5γ complexes were detected, indicating that the β‐subunit interactions are the most easily disrupted ones [Fig. 2(a), Figs. S2b, S3]. When the cone voltage is increased further, a second fragment series corresponding to PE complexes that have lost another subunit can be observed, although a significant amount of intact α6β6γ persists. Notably, PE with γ′‐ or γ″‐subunits exhibited the same fragment peak intensities, suggesting similar gas‐phase stabilities irrespective of which γ‐subunit is present (Fig. S1b). Taken together, the fragmentation pattern indicates that for the PE complex investigated here, β‐subunits dissociate first, followed by dissociation of an α‐ or a second β‐subunit from the core containing the γ‐subunit.
Having established the oligomeric state and dissociation behavior of PE, we then employed the same MS approach to investigate PC, whose fold and assembly are near‐identical to that of PE. However, to our surprise, mass spectra of PC recorded using gentle ionization conditions did not show uniform oligomers like PE, but four series of peaks [Fig. 2(b)]. Based on their masses, we identified them as αβ‐heterodimers (37,526 ± 56 Da), α3β3‐heterohexamers (112,456 ± 48 Da), the complete α6β6‐assembly (225,280 ± 79 Da), and a complex corresponding to the α6β6‐ring with an additional 33 kDa component similar to the intact α6β6γ PE complex (259,135 ± 199 Da) (Table S1). We observe that the presence of the linker protein causes significant peak broadening, suggesting that the extra subunit may be more heterogeneous than the α‐ and β‐units. The main charge states are 12+ for αβ, 22+ for α3β3, and 31+ for α6β6. Since the charges of all dissociation products have to add up to the charge for the parent ion, the αβ and α3β3 populations cannot arise from gas‐phase dissociation of the intact α6β6 complex, but likely exist in solution. In addition, we detected minor populations of (α6β6)2 and (α6β6γ)2 dimers, which may arise from concentration‐dependent self‐association (Fig. S2a). Together, these findings provide clear evidence that PC, unlike PE, can exist as a α6β6 complex without retaining a linker protein as γ‐subunit. At the same time, our spectra show that a significant proportion of the PC complexes retain a phycobilisomal linker protein, which we here refer to as PC γ‐subunit in analogy to the linker in the intact PE complex.
When subjected to gas‐phase dissociation, the PC investigated here predominantly releases α‐, not β‐subunits, resulting in a mixture of low‐intensity populations including α5β6 and α5β6γ complexes [Fig. 2(b), Fig. S2c]. Notably, at 200 V, the α6β6 complexes had readily dissociated, as had the α6β6γ complex at 300 V. Together, the observations suggest that PC has a lower resilience to gas‐phase dissociation than PE, which is even further reduced in the absence of the γ‐subunit.
In the phycobilisome, the linker protein scaffold connects the PE and PC rods to central α3β3 rings of APC.1 We therefore extended our MS analysis to APC to investigate whether this complex, like PE and PC, can retain linker proteins. However, no additional subunits except for APC‐specific α and β were observed, and the α3β3 complex fully dissociated at 200 V (Fig. S4). We therefore conclude that retention of the linker subunits is not due to their overall abundance in the phycobilisome, but specific to PE and PC.
Since we found that PE and PC, despite their near‐identical ring structures, differ significantly in their γ‐subunit interactions, we asked how these interactions affect the overall architecture of the complexes. To answer this question, we employed IM‐MS, which informs about the shape of ions by measuring their drift time through an inert buffer gas under the influence of a weak electric field. Long and short drift times indicate loosely packed or compact shapes, respectively. The influence of an ion's structure is contained in its collision cross section (CCS), which can be inferred from the drift time, the ion's net charge, and the instrument parameters. The CCS is a measure to how much of the protein is exposed for collisions with the buffer gas, and like the drift time, it decreases with the ion's compactness.22
We determined the CCS values (Table S2) of PE to be 11,320 Å2 for α6β6γ′ and 11,450 Å2 for α6β6γ″, with a standard deviation of less than 50 Å2 between all charge states [Fig. 3(a)]. PC gave a CCS of 11,360 ± 42 Å2 for the α6β6γ complex and 10,380 ± 242 Å2 for the α6β6 ring, with 10,047 Å2 for the lowest charge being more than 10% below the values for the complexes containing γ‐subunits [Fig. 3(b)]. Since no crystal structure of an intact α6β6γ complex of PE or PC has been reported, we calculated the theoretical CCS for the crystal structures without γ‐subunits as 11,369 Å2 for algal PE (PDB ID 1LIA) and 11,286 Å2 for PC (PDB ID 1GHO). These values are in good agreement with the CCSs determined for the α6β6γ complexes, but significantly higher than that measured for α6β6 PC. However, in the absence of a matching reference structure, it was not possible to determine whether the presence of the γ‐subunit increases the CCS by protruding from the complex or stabilizing its overall architecture against compaction in the gas phase. We therefore reasoned that the combination of molecular weight and experimental CCS might constitute a signature for complexes with certain architectures. Because the theoretical CCSs have already been computed for all biological assemblies in the PDB,23 we could use a protein's experimental mass and CCS to identify the nearest neighbors within the known structural proteome [Fig. 3(c)]. To test this approach, we first used the mass of PE dodecamer together with the calculated CCS and noted that the majority of neighbors were ring‐shaped (Fig. S5). Another few were otherwise hollow, and yet another few had other architectures. Having demonstrated the feasibility of the search strategy, we used the values for α6β6γ′ and α6β6γ″ PE, which strikingly returned mostly ring‐ or disc‐shaped complexes among the structural homologues, with a large overlap between the two sets. A selection made using the CCS and molecular weight of α6β6γ PC also contained rings and more extended architectures, while α6β6 PC, on the other hand, identified exclusively globular protein complexes [Fig. 3(d)]. These observations suggest that PE is able to retain its solution structure in the gas phase, while PC with its γ‐subunit may undergo partial collapse, and without γ‐subunit total collapse. PC therefore exhibits similar behavior as other labile ring‐shaped complexes, which easily rearrange into more compact shapes when subjected to desolvation and collisional activation.24, 25
Figure 3.
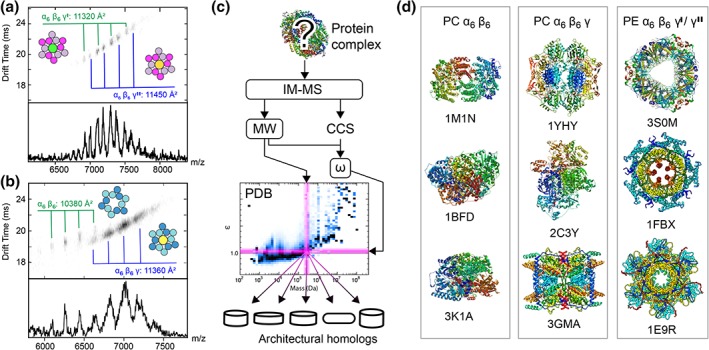
Using IM‐MS data to mine the known structural proteome distinguishes ring‐like architectures and structural collapse in the absence of specific reference structures. (a, b) Mobiligrams of the intact PE and PC complexes show narrow arrival time distributions. The average CCS values for each ion series are indicated. (c) Illustration of the IM‐MS‐based PDB search strategy. (d) The structures of the top three architectural homologues identified based on the molecular weights and experimental CCSs of PC and PE complexes reveal that α6β6γ′and α6β6γ″ PE retain their ring architectures, while α6β6γ PC undergoes partial collapse and α6β6 PC complete structural collapse.
Our observations from IM‐MS are in striking agreement with the relative gas‐phase stabilities of both complexes (Fig. 2), indicating that the central linker protein in PC provides overall less structural support of the ring than that in PE. Furthermore, we find that using IM‐MS data to mine the PDB makes it possible to identify likely complex architectures without a need for theoretical CCS calculations of generated candidate structure models. Hence, it enables us to directly assess their structural integrity for, for example, biotechnological applications.
Conclusions
Numerous studies have demonstrated functional roles for the linker proteins that form the γ‐subunits in PE‐ and PC, most notably as regulators of different conformational states and fluorescence properties.12, 26 Therefore, preserving γ‐subunit interactions in phycobiliprotein complexes, particularly their less stable association with PC, is likely crucial for maintaining their desired spectroscopic properties. The possibilities offered by native MS to assess integrity and composition of phycobiliproteins as demonstrated here are evident, considering the widespread applications of phycobiliproteins as fluorescent probes in biotechnology, and particularly the continued efforts to find new natural sources.
Perhaps most importantly, we show that IM‐MS data can be used to directly determine the effects of the γ‐subunits on the overall architecture of the different phycobiliprotein complexes without a need for a specific reference structure. Although the present approach would be less well‐suited for complexes with more globular structures, which may give only subtle differences in their CCS‐MW relation, or complexes with unique architectures without close structural homologues in the PDB, it has considerable potential for the analysis of protein complexes without prior structural information. More generally, the use of IM‐MS data to find related structures in the PDB can provide classification of unknown protein complexes beyond biotechnological applications, adding another dimension to MS‐driven protein structure analyses.
Materials and Methods
Protein preparation
Phycocyanin (Cat. number 52468), R‐phycoerythrin (Cat. number 52412), and APC (Cat. number A7472) were purchased from Sigma. Proteins were dissolved in PBS to a final concentration of approximately 0.2 mg/mL, corresponding to about 2 μM. For MS analysis, proteins were buffer‐exchanged into 1 M ammonium acetate, pH 7.5, using Biospin 6 microcentrifuge columns (BioRad, CA).
Mass spectrometry
Mass spectra were acquired on a Micromass LCT ToF modified for analysis of intact protein complexes (MS Vision, The Netherlands) equipped with an offline nanospray source. Samples were introduced via coated borosilicate capillaries (Thermo Scientific, Germany). The capillary voltage was 1.5 kV. Collisional activation was performed by ramping the cone voltage between 100 and 300 V. The radio frequency (RF) lens voltage was 1.5 kV. The source pressure was maintained at 9.0 mbar to prevent in‐source dissociation. The mass scale was calibrated using cesium iodide.
Ion mobility MS spectra were acquired on a Synapt G2S travelling wave ion mobility mass spectrometry (TWIMS) MS equipped with an offline nanospray source. The capillary voltage was 1.5 kV and the source temperature was maintained at 120°C and the sample cone at 80 V. The pressures were as follows: backing 3.29 mbar, source 5.8 × 10−3 mbar, trap 4.41 × 10−2 mbar, and IMS 4.16 mbar. The ion mobility spectrometry (IMS) settings were as follows: wave height 20 V and wave velocity 550 m/s. The drift gas was nitrogen with a flow rate of 50 mL/min and the collision voltages in the ion trap and transfer were 5 and 2 V, respectively. The trap DC bias was 45 V and the IMS DC bias was 3 V. TWIMS calibration was performed using the 33+ to 37+ charge states of pyruvate kinase as sole calibrant, since it very closely matches the molecular weight and average charge as intact PE and PC.27 Data were analyzed using the MassLynx 4.1 and PULSAR (pulsar.chem.ox.ac.uk 28) software packages. Average masses and standard deviations between charge states were determined using a Microsoft Excel file kindly provided by the Benesch Laboratory, University of Oxford.
Structure analysis
For theoretical CCS calculations, crystal structures of α6β6 PE (PDB ID 1LIA) and PC (PDB ID 1GHO) were used. Theoretical trajectory‐method‐corrected CCS values for the α6β6 complexes of PE and PC were determined by averaging 10 separate calculations using IMPACT (impact.chem.ox.ac.uk).23 Protein structures were visualized and edited using UCSF Chimera.29
CCS‐mass‐based modeling using IMPACT
Masses and CCSs were used as input to the python script find_omega_neighbours.py, which is distributed alongside IMPACT23, with default parameters. In the script, the CCSs are converted to reduced cross‐sections (ω), where a ω above (or below) 1.0 signifies a higher (or lower) CCS than expected for a protein of the given mass (see Reference 23). The script returns the 10 protein complexes that best match the target mass and ω, and consequently, the CCS. The script was run using (i) the CCS inferred from the trimmed structure model of the PE α6β6 together with the expected mass calculated from the masses of its monomers, (ii) the experimental data for the PE α6β6γ′ and α6β6γ″ complexes, (iii) the experimental data for the PC α6β6γ complex, and (iv) the experimental data for the PC α6β6 complex.
Fluorescence measurements
Fluorescence spectra of 2 μM PE or PC in PBS with 0–5% formic acid were recorded on a Tecan Spark 20 M multimode reader (Tecan Instruments, Switzerland) using Corning COSTAR black flat bottom 96‐well plates (Sigma‐Aldrich, Germany). For PE, excitation wavelength was 530 nm; emission was recorded between 550 and 750 nm in 1 nm steps with 5 nm bandwidth and an integration time of 40 μs. For PC, excitation wavelength was 595 nm; emission was recorded between 630 and 750 nm in 1 nm steps with 5 nm bandwidth and an integration time of 40 μs. Data were visualized using the Magellan software package (Tecan Instruments, Switzerland).
Supporting information
Figure S1. (a) SDS‐PAGE analysis of PE shows the presence of two linker proteins (γ’ and γ”) which differ by less than 5 kDa. The indicated masses were calculated from the intact PE complexes (Table S1). (b) Comparison of the ratio of intact α6β6γ and α6β6γ” PE for each charge state at each cone voltage shows similar gas‐phase stabilities for both complexes.
Figure S2. PE and PC complexes form dimers and release different subunits. (a) Top: The enlarged high m/z region of the spectrum in Figure 1(a) shows peaks indicating complexes of approximately 530 kDa in size (blue dots). Bottom: Zoom in the high m/z region of the spectrum in Figure 1(b) reveals the presence of two distinct dimeric PC complexes corresponding in mass to two α6β6 (red dots) or two α6β6γ complexes (yellow dots). (b) Zoom of the low m/z region of the spectra in Figure 1(a) indicates sequential release of β‐ and then α‐subunits (bright and dark pink, respectively). (c) Zoom of the low m/z region of the spectra in Figure 1(b) shows predominant release of α‐subunits over β‐subunits (bright and dark blue, respectively) in response to collisional dissociation.
Figure S3. (a) Fluorescence spectroscopy was used to monitor acid‐induced dissociation of PE and PC. (b) MS spectra of the completely dissociated complexes show that α‐ and β‐subunits of PE and PC give similar MS responses.
Figure S4. Intact mass analysis and gas‐phase dissociation of APC. Using gentle ionization conditions, a homogeneous population of intact APC hetero‐hexamers with no additional components can be detected. Raising the cone voltage to induce gas‐phase dissociation releases individual α‐ and β‐subunits (insert).
Figure S5. Top ten structural neighbors of the different PE and PC complexes identified by the PDB search. First column, matches for the theoretical CCS and MW of α6β6γ PE. Second column, matches for the experimental CCS and MW of α6β6γ PC. Third column, matches for α6β6 PC. Fourth and fifth column, matches for α6β6γ’ and α6β6γ” PE.
Table S1. Masses for PE and PC complexes and dissociation products. Standard deviations indicate the mass error between charge states. Predicted masses are based on the experimentally determined masses for α‐ and β‐subunits.
Table S2. CCS values for PE and PC complexes
Acknowledgments
The authors would like to extend special thanks to Nicklas Österlund and Prof. Leopold Ilag, Stockholm University, for access to the Synapt G2S IM‐MS instrument, and to Prof. Dame Carol Robinson and Prof. Justin Benesch, University of Oxford, for encouragement and helpful discussions. M.L. gratefully acknowledges technical support from MS Vision, NL. M.L. is supported by an Ingvar Carlsson Award from the Swedish Foundation for Strategic Research, a KI faculty‐funded Career Position, and a KI‐StratNeuro starting grant. E.G.M. holds a Marie Skłodowska Curie International Career Grant from the European Commission and the Swedish Research Council (2015‐00559). Special thanks to Prof. Sir David P. Lane, Karolinska Institutet, for support through Swedish Research Council Grant 2013_08807.
Contributor Information
Erik G. Marklund, Email: erik.marklund@kemi.uu.se.
Michael Landreh, Email: michael.landreh@ki.se.
References
- 1. Watanabe M, Ikeuchi M (2013) Phycobilisome: architecture of a light‐harvesting supercomplex. Photosynth Res 116:265–276. [DOI] [PubMed] [Google Scholar]
- 2. Adir N (2005) Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth Res 85:15–32. [DOI] [PubMed] [Google Scholar]
- 3. Wan DH, Zheng BY, Ke MR, Duan JY, Zheng YQ, Yeh CK, Huang JD (2017) C‐Phycocyanin as a tumour‐associated macrophage‐targeted photosensitiser and a vehicle of phthalocyanine for enhanced photodynamic therapy. Chem Commun 53:4112–4115. [DOI] [PubMed] [Google Scholar]
- 4. Klein B, Buchholz R. Microalgae as sources of food ingredients and nutraceuticals, (2013) Microbial production of food ingredients, enzymes and nutraceuticals, Amsterdam, NL: Elsevier, pp 559–570. [Google Scholar]
- 5. Eriksen NT (2008) Production of phycocyanin ‐ a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14. [DOI] [PubMed] [Google Scholar]
- 6. Guan X, Qin S, Su Z, Zhao F, Ge B, Li F, Tang X (2007) Combinational biosynthesis of a fluorescent cyanobacterial holo‐α‐phycocyanin in Escherichia coli by using one expression vector. Appl Biochem Biotechnol 142:52–59. [DOI] [PubMed] [Google Scholar]
- 7. Cuellar‐Bermudez SP, Aguilar‐Hernandez I, Cardenas‐Chavez DL, Ornelas‐Soto N, Romero‐Ogawa MA, Parra‐Saldivar R (2015) Extraction and purification of high‐value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. J Microbial Biotechnol 8:190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonani RR (2016) Recent advances in production, purification and applications of phycobiliproteins. World J Biol Chem 7:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumay J, Morançais M, Munier M, Le Guillard C, Fleurence J (2014) Phycoerythrins: Valuable proteinic pigments in red seaweeds. Adv Bot Res 71:321–343. [Google Scholar]
- 10. Chang WR, Jiang T, Wan ZL, Zhang JP, Yang ZX, Liang DC (1996) Crystal structure of R‐phycoerythrin from Polysiphonia urceolata at 2.8 Å resolution. J Mol Biol 262:721–731. [DOI] [PubMed] [Google Scholar]
- 11. Ritter S, Hiller RG, Wrench PM, Welte W, Diederichs K (1999) Crystal structure of a phycourobilin‐containing phycoerythrin at 1.90‐Å resolution. J Struct Biol 126:86–97. [DOI] [PubMed] [Google Scholar]
- 12. David L, Marx A, Adir N (2011) High‐resolution crystal structures of trimeric and rod phycocyanin. J Mol Biol 405:201–213. [DOI] [PubMed] [Google Scholar]
- 13. Benesch JL, Robinson CV (2006) Mass spectrometry of macromolecular assemblies: preservation and dissociation. Curr Opin Struct Biol 16:245–251. [DOI] [PubMed] [Google Scholar]
- 14. Lössl P, van de Waterbeemd M, Heck AJ (2016) The diverse and expanding role of mass spectrometry in structural and molecular biology. EMBO J 35:2634–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ben‐Nissan G, Sharon M (2018) The application of ion‐mobility mass spectrometry for structure/function investigation of protein complexes. Curr Opin Chem Biol 42:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leney AC, Tschanz A, Heck AJR (2018) Connecting color with assembly in the fluorescent B‐phycoerythrin protein complex. FEBS J 285:178–187. [DOI] [PubMed] [Google Scholar]
- 17. Eisenberg I, Harris D, Levi‐Kalisman Y, Yochelis S, Shemesh A, Ben‐Nissan G, Sharon M, Raviv U, Adir N, Keren N, Paltiel Y (2017) Concentration‐based self‐assembly of phycocyanin. Photosynth Res 134:39–49. [DOI] [PubMed] [Google Scholar]
- 18. Sobott F, Hernández H, McCammon MG, Tito MA, Robinson CV (2002) A tandem mass spectrometer for improved transmission and analysis of large macromolecular assemblies. Anal Chem 74:1402–1407. [DOI] [PubMed] [Google Scholar]
- 19. Hall Z, Robinson CV (2012) Do charge state signatures guarantee protein conformations? J Am Soc Mass Spectrom 23:1161–1168. [DOI] [PubMed] [Google Scholar]
- 20. Wang L, Wang S, Fu X, Sun L (2015) Characteristics of an R‐phycoerythrin with two γ subunits prepared from red macroalga Polysiphonia urceolata . PLoS One 10:e0120333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang J, Ma J, Liu D, Qin S, Sun S, Zhao J, Sui SF (2017) Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 551:57–63. [DOI] [PubMed] [Google Scholar]
- 22. Marklund EG (2015) Molecular self‐occlusion as a means for accelerating collision cross‐section calculations. Int J Mass Spectrom 386:54–55. [Google Scholar]
- 23. Marklund EG, Degiacomi MT, Robinson CV, Baldwin AJ, Benesch JLP (2015) Collision cross sections for structural proteomics. Structure 23:791–799. [DOI] [PubMed] [Google Scholar]
- 24. Ruotolo BT, Giles K, Campuzano I, Sandercock AM, Bateman RH, Robinson CV (2005) Biochemistry: evidence for macromolecular protein rings in the absence of bulk water. Science 310:1658–1661. [DOI] [PubMed] [Google Scholar]
- 25. Hall Z, Politis A, Bush MF, Smith LJ, Robinson CV (2012) Charge‐state dependent compaction and dissociation of protein complexes: insights from ion mobility and molecular dynamics. J Am Chem Soc 134:3429–3438. [DOI] [PubMed] [Google Scholar]
- 26. Gwizdala M, Krüger TPJ, Wahadoszamen M, Gruber JM, Van Grondelle R (2018) Phycocyanin: one complex, two states, two functions. J Phys Chem Lett 9:1365–1371. [DOI] [PubMed] [Google Scholar]
- 27. Bush MF, Hall Z, Giles K, Hoyes J, Robinson CV, Ruotolo BT (2010) Collision cross sections of proteins and their complexes: a calibration framework and database for gas‐phase structural biology. Anal Chem 82:9557–9565. [DOI] [PubMed] [Google Scholar]
- 28. Allison TM, Reading E, Liko I, Baldwin AJ, Laganowsky A, Robinson CV (2015) Quantifying the stabilizing effects of protein–ligand interactions in the gas phase. Nat Commun 6:8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a) SDS‐PAGE analysis of PE shows the presence of two linker proteins (γ’ and γ”) which differ by less than 5 kDa. The indicated masses were calculated from the intact PE complexes (Table S1). (b) Comparison of the ratio of intact α6β6γ and α6β6γ” PE for each charge state at each cone voltage shows similar gas‐phase stabilities for both complexes.
Figure S2. PE and PC complexes form dimers and release different subunits. (a) Top: The enlarged high m/z region of the spectrum in Figure 1(a) shows peaks indicating complexes of approximately 530 kDa in size (blue dots). Bottom: Zoom in the high m/z region of the spectrum in Figure 1(b) reveals the presence of two distinct dimeric PC complexes corresponding in mass to two α6β6 (red dots) or two α6β6γ complexes (yellow dots). (b) Zoom of the low m/z region of the spectra in Figure 1(a) indicates sequential release of β‐ and then α‐subunits (bright and dark pink, respectively). (c) Zoom of the low m/z region of the spectra in Figure 1(b) shows predominant release of α‐subunits over β‐subunits (bright and dark blue, respectively) in response to collisional dissociation.
Figure S3. (a) Fluorescence spectroscopy was used to monitor acid‐induced dissociation of PE and PC. (b) MS spectra of the completely dissociated complexes show that α‐ and β‐subunits of PE and PC give similar MS responses.
Figure S4. Intact mass analysis and gas‐phase dissociation of APC. Using gentle ionization conditions, a homogeneous population of intact APC hetero‐hexamers with no additional components can be detected. Raising the cone voltage to induce gas‐phase dissociation releases individual α‐ and β‐subunits (insert).
Figure S5. Top ten structural neighbors of the different PE and PC complexes identified by the PDB search. First column, matches for the theoretical CCS and MW of α6β6γ PE. Second column, matches for the experimental CCS and MW of α6β6γ PC. Third column, matches for α6β6 PC. Fourth and fifth column, matches for α6β6γ’ and α6β6γ” PE.
Table S1. Masses for PE and PC complexes and dissociation products. Standard deviations indicate the mass error between charge states. Predicted masses are based on the experimentally determined masses for α‐ and β‐subunits.
Table S2. CCS values for PE and PC complexes


