Abstract
Obsessive-compulsive disorder (OCD) causes severe distress and is therefore counted by the World Health Organisation (WHO) as one of the 10 most impairing illnesses. There is evidence for a strong genetic underpinning especially in early onset OCD (eoOCD). Though several genes involved in neurotransmission have been reported as candidates, there is still a need to identify new pathways. In this study, we focussed on genetic variants of the Neuropeptide Y (NPY) system. NPY is one of the most abundant neuropeptides in the human brain with emerging evidence of capacity to modulate stress response, which is of high relevance in OCD. We focussed on tag-SNPs of NPY and its receptor gene NPY1R in a family-based approach. The sample comprised 86 patients (children and adolescents) with eoOCD with both their biological parents. However, this first study on genetic variants of the NPY-system could not confirm the association between the investigated SNPs and eoOCD. Based on the small sample size results have to be interpreted as preliminary and should be replicated in larger samples. However, also in an additional GWAS analysis in a large sample, we could not observe an associations between NPY and OCD. Overall, these preliminary results point to a minor role of NPY on the stress response of OCD.
Keywords: NPY, obsessive-compulsive, children, anxiety, neuropeptide
Introduction
Obsessive-compulsive disorder (OCD) has a life-time prevalence of 2%–3.3%, both in adults and children and has its peaks of onset around 12 years (early onset OCD, eoOCD) and in early adulthood (late onset). The occurring obsessions/compulsions are interfering significantly with the patients’ everyday life and cause severe distress and anxiety. Additionally, 75% of the patients have at least one comorbidity. In eoOCD these are in particular attention deficit hyperactivity disorder, major depression and anxiety disorders (Fireman et al., 2001). OCD is the fourth most psychiatric disorder and due to its frequently severe impact on affected patients’ lives, the World Health Organisation (WHO) counts OCD to the 10 most impairing illnesses (Karno et al., 1988; Weissman et al., 1994; Lopez and Murray, 1998).
Evidence for a strong genetic component in the development of OCD derives from twin and family genetic studies as well as segregation analyses. Prevalence in first-degree relatives of OCD patients is about four times increased and even about eight times higher in eoOCD patients (Pauls et al., 1995; Alsobrook et al., 1999; Hanna et al., 2005). A higher familial load of eoOCD was observed in general, suggesting a greater importance of genetic factors (Pauls et al., 1995; Nestadt et al., 2000). In the search for genetic underpinnings in OCD, mainly genes of the serotonergic system were focussed, driven by the pharmacological effectivity of selective serotonin reuptake inhibitors (SSRIs). Several studies aimed at the gene encoding the serotonin-transporter (Bengel et al., 1999). Nonetheless, study results are heterogeneous and replications and a recent meta-analysis revealed a rather low effect size for the serotonin transporter (Walitza et al., 2014). Further, evidence emerged that the glutamate system is involved in OCD and might be a potential alternative pharmacological treatment target, especially since the association with the glutamate receptor gene SLC1A1 has been replicated in several studies (Wendland et al., 2009; Stewart et al., 2013; Grados et al., 2015). However, variance explained by the known candidates is still rather small and given the clinical complexity of OCD there is an understanding that many genes are involved in the disease’s pathogenesis.
In the search for further neuronal messengers involved, the Neuropeptide Y (NPY) is a highly interesting candidate (Tatemoto et al., 1982). It is one of the most abundant neuropeptides in the human brain and has multiple regulating effects in the nervous system. NPY, long known as a neuropeptide modulating feeding behavior and energy homeostasis (Morton and Schwartz, 2001), has been reported influencing neuronal processes relevant in psychiatric disorders. For instance, there is a rising evidence that the NPY-system including the NPY-receptors is involved in the development of alcohol and drug dependency (NPY1R), stress coping (NPY1R, NPY2R, NPY5R) and anxiolysis (NPY1R, NPY2R; Gerald et al., 1996; Movafagh et al., 2006; Hirsch and Zukowska, 2012; Pedragosa-Badia et al., 2013). Especially regulation of stress and anxiety levels are crucial elements in the development and maintenance of OCD. Patients affected by OCD suffer fear and simultaneously triggered stress when they experience the feeling “something is not in order.” NPY is released in many brain areas that participate in stress response. These are, for example, the adrenergic and noradrenergic (NA) neurons of the brainstem, the corticotropin-releasing factor (CRF)-neurons of the nucleus paraventricularis, the amygdala and the hypothalamus as well as an impact on the hypothalamic-pituitary-adrenal (HPA)-axis (Heilig, 2004; Alldredge, 2010). The NPY1R-receptor was shown to modulate anxious behavior and stress which can be reversed by NPY administration (Kormos and Gaszner, 2013). Interestingly, there is also an interaction of NPY and the serotonergic system (Diksic and Young, 2001; Pittenger and Bloch, 2014). SSRI administration leads to a higher NPY release in stressed depressive mice and a mediating role of NPY on SSRI effects is discussed (Caberlotto et al., 1998; Redrobe et al., 2005; Christiansen et al., 2011). This interplay of SSRI and NPY showed for depression might be of relevance in OCD as well, especially due to the high comorbidity rate of the two illnesses (Torres et al., 2016).
Therefore, in this molecular genetic study, we aimed at the NPY-system as a messenger system with potential influence on the pathogenesis of eoOCD. Due to their previously reported functions in the regulation of anxiety and stress, we focussed on NPY and its receptor NPY1R in a family-based approach. Genetic variants of NPY and NPY1R were genotyped in a German family-based sample. The present study is the first investigating genes of the NPY-system in eoOCD.
Materials and Methods
Subjects
All patients were recruited at the Department of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, University Hospital Würzburg, Germany. Patients and parents were all of Caucasian descent and agreed to participate in the study. All participants and, in the case of minors, their parents, gave written informed consent. The study was approved by the Ethics Committees of the University of Würzburg.
Patients were included in the study after they had fulfilled the diagnostic criteria for eoOCD according to DSM-IV (American Psychiatric Association, 2000), which was valid at the time of recruitment, and ICD-10 (Dilling, 2015). Patients and parents were interviewed separately for childrens’ psychiatric disorders with the German semi-structured clinical “Diagnostic Interview for Psychiatric Disorders in Children and Adolescents” (DIPS; Schneider et al., 1995). Subsequently, the severity of symptoms was assessed by the Childrens’ Yale-Brown Obsessive Compulsive Scale (cY-BOCS; Goodman et al., 1989; Scahill et al., 1997). Subjects with comorbid disorders were only included when OCD was the main psychiatric diagnosis. Senior clinicians or psychologists performed all interviews and ratings.
Exclusion criteria were a lifetime history of psychotic disorders, Tourette’s syndrome, autism spectrum disorders, alcohol dependence or mental retardation (IQ ≤ 70).
The sample comprised 86 patients (children and adolescents) with eoOCD and their parents. Fifty-one children were female, 35 were male. The patients’ mean age was 10.7 years (SD = 2.8) at the onset of disease in a range from 3 to 15 years. In 32 patients onset was earlier than the age of 10 years. Eleven patients had tic-disorders as comorbidities. Further comorbidities as depression, ADHD or anorexia nervosa existed frequently in the patients’ medical histories but were not clinically relevant at the time of study inclusion. The sample was part of previous genetic analyses and described further in previous publications (e.g., Walitza et al., 2008).
Based on a power analysis with alpha 0.05 and beta 0.80 (according to Neumann et al., 2014) a sample size of at least N = 105 would have been required to unravel significant effects. Therefore, we additionally analyzed associations of NPY and OCD with the recent GWAS by Arnold et al. (2018).
To enlarge the sample size of OCD patients we used the data of the most recent GWAS meta-analysis on OCD including 2,688 individuals affected by OCD and 7,037 controls by the Psychiatric Genomics Consortium, and searched the results for each of the SNPs investigated in our study. The GWAS by the Psychiatric Genomics Consortium comprised 2,688 individuals affected by OCD and 7,037 controls. The meta-analysis comprised children as well as adults (Arnold et al., 2018).
Gene Loci
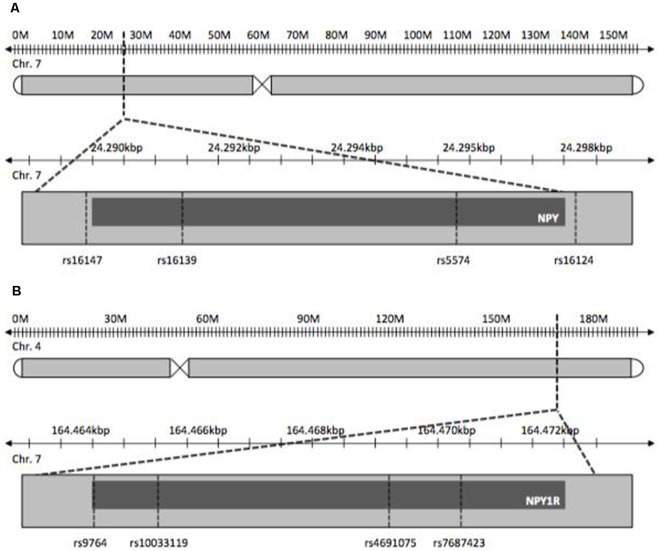
NPY and NPY1R SNPs chosen for this study were tag-SNPs and previously published SNPs with reported influence on psychiatric disorders. The latter were studied with regard to stress-related diseases, as OCD is postulated to be, like depression, ADHD and obesity (Peterson et al., 2001; Tiwari et al., 2013). Tag-SNPs were determined using HaploView® to cover both genes completely on the basis of SNP data provided by the International HapMap-Project (International HapMap Consortium, 2003; Barrett et al., 2005; Figure 1). Tagger settings included a minor allele frequency >0.1 and r2 = 0.9. Additionally, SNPs known from previously published studies pertaining to other disorders were included in the tagging process. For NPY, SNPs rs5574, rs16124, rs16139, rs16147 were determined, and SNPs rs9764, rs4691075, rs7687423 and rs10033119 were found for NPY1R.
Figure 1.
Gene loci of Neuropetide Y (NPY, A) and NPY-receptor (NPY1R, B).
Genotyping
Genomic DNA was extracted from whole blood following standard protocols. DNA was amplified by standard PCR using specific reverse- and forward-primers for each of the eight SNPs. After amplification genotypes were determined by enzymatic digestion and gel-electrophoresis. Further detailed information on primers and procedures is available upon request.
Statistics
Association between the included markers of NPY and NPY1R and eoOCD was tested by the Transmission Disequilibrium Test (TDT; Spielman et al., 1993). TDT was performed for all eight SNPs using the software FamHAP® (Herold and Becker, 2008). A p-value < 0.05 was defined as the significance level. Each of the trios was tested for the hypothesis that their tested gene variants of either: (1) NPY or (2) NPY1R are associated with eoOCD. The examination pattern used for this analysis was a genotype-wise model considering every heterozygous parental genotype separately and corresponds to a test of the global null-hypothesis of transmission equilibrium of both alleles in every parental genotype. All eight SNPs were checked for Mendelian Errors which were exclusion criteria. Moreover, all parental genotype distributions were tested for Hardy-Weinberg equilibrium (HWI).
Results
In one of the NPY1R-SNPs, rs4691074, occurred a deviation from the HWI (p = 0.04). The remaining seven SNPs were unremarkable (p > 0.05). No transmission disequilibrium was observed for the NPY-SNPs. The p-values exceeded the defined significance level α (Table 1). For rs5574, 34 heterogeneous parental couples could be examined ( = 1.058; p = 0.303), for rs16124, 85 ( = 0.576; p = 0.448), for rs16139, 12 ( = 0.333; p = 0.564) and for rs16147, 76 ( = 0.842; p = 0.359). Moreover, no transmission disequilibrium could be assessed for the NPY1R-SNPs. For rs9764, 72 heterogeneous parental couples could be analyzed ( = 0.000; p = 1.000), for rs4691075, 33 ( = 1.485; p = 0.223), for rs7687423, 89 ( = 0.101; p = 0.75) and for rs10033119, 14 ( = 0.000; p = 1.000).
Table 1.
Transmission Disequilibrium Test (TDT) results.
| SNP | Gene | Allele | T | NT | pTDT | |
|---|---|---|---|---|---|---|
| rs5574 | NPY | 2 | 20 | 14 | 1.059 | 0.303 |
| 4 | 14 | 20 | ||||
| rs16124 | NPY | 3 | 39 | 46 | 0.576 | 0.448 |
| 4 | 46 | 39 | ||||
| rs16139 | NPY | 2 | 7 | 5 | 0.333 | 0.564 |
| 4 | 5 | 7 | ||||
| rs16147 | NPY | 2 | 34 | 42 | 0.842 | 0.359 |
| 4 | 42 | 34 | ||||
| rs9764 | NPY1R | 1 | 36 | 36 | 0.000 | 1.000 |
| 3 | 36 | 36 | ||||
| rs4691075 | NPY1R | 1 | 20 | 13 | 1.485 | 0.223 |
| 3 | 13 | 20 | ||||
| rs7687423 | NPY1R | 2 | 46 | 43 | 0.101 | 0.750 |
| 4 | 43 | 46 | ||||
| rs10033119 | NPY1R | 2 | 7 | 7 | 0.000 | 1.000 |
| 4 | 7 | 7 |
Note: Column 1 lists the SNP names, column 2 the correspondent gene, column 3 shows the present allele: 1 equal to adenine, 2 equal to cytosine, 3 equal to guanine, 4 equal to thymine. Column 4 shows the number of transmissions (T) for each gene, column 5 shows the number of non-transmitted (NT) alleles. Column 6 represents the chi square value, column 7 the p-value.
The GWAS meta-analysis also showed no association of any NPY- nor NPY1R-SNPs with regard to the development of OCD (Table 2).
Table 2.
Meta-analysis results.
| Chr. | SNP | bp | A1 | A2 | Info | OR | SE | p |
|---|---|---|---|---|---|---|---|---|
| 7 | rs5574 | 24329133 | T | C | 0.9750 | 0.967152 | 0.0949 | 0.7248 |
| 7 | rs16124 | 24331799 | T | G | 0.9735 | 1.00944 | 0.0335 | 0.7802 |
| 7 | rs16139 | 24324879 | T | C | 0.9737 | 0.983144 | 0.0335 | 0.6112 |
| 7 | rs16147 | 24323410 | T | C | 0.9720 | 1.01572 | 0.0335 | 0.6421 |
| 4 | rs9764 | 164245405 | T | C | 1.0030 | 1.03345 | 0.0382 | 0.3881 |
| 4 | rs4691075 | 164249485 | T | C | 0.9948 | 0.937255 | 0.0504 | 0.1991 |
| 4 | rs7687423 | 164250797 | A | G | 0.9800 | 1.001 | 0.0347 | 0.9766 |
| 4 | rs10033119 | 164245854 | A | G | 0.8709 | 0.962809 | 0.0851 | 0.6562 |
Note: Column 1 lists the respective chromosomes (hg 19), column 2 the correspondent marker name, column 3 shows the base pair location (hg 19), column 4 shows the reference allele for OR (may or may not be minor allele), column 5 the alternative allele. Column 6 represents the imputation information score, column 7 the Odds ratio for the effect of the A1 allele, column 8 the standard error of the log(OR) and column 9 the p-value for the association test in the meta-analysis.
Discussion
This is the first molecular genetic study on potential functional variants of the candidate gene NPY and its receptor NPY1R in eoOCD. In this study, association was not detected. Thus, we could not confirm a major role of the NPY system in OCD with childhood onset.
As our number of trios was smaller than postulated by the power-analysis, the negative outcome might foremost be due to the sample size. That is why we used the data of the most recent and aforementioned GWAS meta-analysis on OCD by the Psychiatric Genomics Consortium, and searched the results for the investigated SNPs. Though our sample was part of this meta-analysis, a potential effect over a large sample including different ages of onset might have been observable. However, also this meta-analysis showed no association of any NPY- nor NPY1R-SNPs (Arnold et al., 2018). Our study had been designed as a family-based study to avoid stratification effects, nonetheless, a case-control study, which had not been performed so far with regard to the studied gene loci, would be highly interesting.
However, the NPY system, which is involved in stress response, might still be of relevance in OCD subgroups, although undetected due to the study design. The selected SNPs were either already known from earlier studies to other stress-related psychiatric disorders or tag-SNPs that were selected out of the HapMap data with the help of the programme Haploview® (Barrett et al., 2005) in order to cover NPY and NPY1R. Nonetheless, the selected SNPs are infrequent in the population, thus statistically evaluable transmissions in the sample were rather small.
Reflecting the NPY-system, other effectors like the Y2-receptor and the Y5-receptor might also be valuable targets in OCD. The Y1-receptor was the first choice for this study due to its various anxiolytic and stress-reducing effects and its wide spread dissemination in stress regulating brain regions. Anxious behavior and stress can be reversed with NPY administration (Kormos and Gaszner, 2013). Nevertheless, the influence of the remaining receptors should not be excluded a priori. Especially the Y5-receptor shares a similar effect spectrum with the tested Y1 (Kormos and Gaszner, 2013). Even though its role is not fully understood yet, an effect on the fear and stress system seems to be obvious and further research worthwhile. The Y2-receptor, however, could indirectly influence the delicate equilibrium of neurotransmission with its impact on the release of other neurotransmitters (Upadhya et al., 2009; Kormos and Gaszner, 2013). A reduced inhibition of glutamate release, for example, could be a correlate for the increased thalamic and striatal glutamate activity in untreated OCD-children and could explain the upregulation of Y2-receptors in a state of anxiety in the mouse model (Leckman et al., 1997; Upadhya et al., 2009).
Our sample consists of children and adolescents only, resembling an OCD subgroup with early onset and a significant number of children with an onset before the age of 10 years. A stronger impact of genetic factors is reported for eoOCD and our sample might differ from adult samples regarding genetic underpinnings and impact of life-events (Nestadt et al., 2000; Walitza et al., 2008). The responsivity of the NPY system after stress exposure during the development has been shown in animal models and could be of higher relevance in failed coping and development of OCD in adults (Serova et al., 2017; Yam et al., 2017).
Moreover, comorbidities as e.g., ADHD or depression were accepted in this study. Nonetheless, comorbidities in OCD might indicate distinct neurobiological OCD subgroups with divergent etiologies (Taurines et al., 2010). An analysis reflecting the comorbidities was not applicable due to the sample size. Especially an analysis regarding comorbidity with depression would be of interest (Caberlotto et al., 1998; Redrobe et al., 2005; Christiansen et al., 2011).
Due to the sample size, we were not able to examine an influence of gender, which was reported for other candidate genes in previous studies. In the meta-analyses of the glutamate transporter gene SLC1A1, the SNP rs12682807 was found associated only in male probands (Thiele et al., 1998; Arnold et al., 2006; Wendland et al., 2009). Other genetic publications also stated gender differences (Thiele et al., 1998; Dickel et al., 2006; Canals et al., 2012). Gender differences are also found in the clinical perspective with the prevalence of subclinical OCD-symptoms twice as high in boys than in girls (Canals et al., 2012). Furthermore, the two sexes differ in the clinical manifestation which comprises an earlier age of onset as well as a higher prevalence of symptoms belonging to the entity of symmetry and ordering among males and increased symptoms of cleanliness and washing among females (Bogetto et al., 1999; Stewart et al., 2007).
Though the sample size was rather small for a genetic study, regarding eoOCD it has a considerable size. Since the sample was collected in one facility, it provides a clear stringency regarding a precisely defined phenotype and restrictive exclusion of severe comorbid disorders to assure the predominance of definite OCD in contrast to obsessive-compulsive symptoms in other psychiatric disorders.
In conclusion, our family-based study on genetic variants of NPY and NPY1R could not confirm association with OCD with childhood onset correlating with the outcomes for adult NPY and OCD by Altemus et al. (1999). Unfortunately, there is overall a paucity of studies on NPY and OCD and we can only add another hint for non-existing significant effects. We only know so far that NPY is involved in stress and other anxiety disorders. Therefore, we hope that we could deliver with our study a contribution to the question about an association of NPY with OCD. The major impact of our study is that we could show in our sample—and comprehending the forementioned GWAS-data—that NPY effects on OCD should be very small or not present. The very impact of our study is a sample consisting of early onset patients and therefore of fundamental impact on the database in this specific subgroup. Moreover, the outcome has a meaning for the understanding of the stress response in OCD in general which might be different from other anxiety disorders with an association to NPY. Indeed, in DSM V OCD is no longer listed under anxiety disorders. If our null finding is replicated in larger studies, we might conclude that NPY has no effect on the stress response in OCD and probably might also be a less valuable target for pharmacological research in OCD.
Author Contributions
All authors certify that they have participated sufficiently in the work to take public responsibility for the content. TR was responsible for conception and design of the study. TR and MF carried out data acquisition and analysis. TR, MF and AC were mainly involved in drafting the article. TR, MF, AC, EG, AMW, SW, CW, MR, AW and HS contributed to data interpretation and manuscript draft and revision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alldredge B. (2010). Pathogenic involvement of neuropeptides in anxiety and depression. Neuropeptides 44, 215–224. 10.1016/j.npep.2009.12.014 [DOI] [PubMed] [Google Scholar]
- Alsobrook J. P., II., Leckman J. F., Goodman W. K., Rasmussen S. A., Pauls D. L. (1999). Segregation analysis of obsessive-compulsive disorder using symptom-based factor scores. Am. J. Med. Genet. 88, 669–675. [DOI] [PubMed] [Google Scholar]
- Altemus M., Jacobson K. R., Debellis M., Kling M., Pigott T., Murphy D. L., et al. (1999). Normal CSF oxytocin and NPY levels in OCD. Biol. Psychiatry 45, 931–933. 10.1016/s0006-3223(98)00263-7 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders. 4th Edn. Washington, DC: American Psychiatric Association Publishing. [Google Scholar]
- Arnold P. D., Askland K. D., Barlassina C., Bellodi L., Bienvenu O. J., Black D., et al. (2018). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry 23, 1181–1188. 10.1038/mp.2017.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold P. D., Sicard T., Burroughs E., Richter M. A., Kennedy J. L. (2006). Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch. Gen. Psychiatry 63, 769–776. 10.1001/archpsyc.63.7.769 [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Fry B., Maller J., Daly M. J. (2005). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265. 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- Bengel D., Greenberg B. D., Corá-Locatelli G., Altemus M., Heils A., Li Q., et al. (1999). Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Mol. Psychiatry 4, 463–466. 10.1038/sj.mp.4000550 [DOI] [PubMed] [Google Scholar]
- Bogetto F., Venturello S., Albert U., Maina G., Ravizza L. (1999). Gender-related clinical differences in obsessive-compulsive disorder. Eur. Psychiatry 14, 434–441. 10.1016/S0924-9338(99)00224-2 [DOI] [PubMed] [Google Scholar]
- Caberlotto L., Fuxe K., Overstreet D. H., Gerrard P., Hurd Y. L. (1998). Alterations in neuropeptide Y and Y1 receptor mRNA expression in brains from an animal model of depression: region specific adaptation after fluoxetine treatment. Mol. Brain Res. 59, 58–65. 10.1016/s0169-328x(98)00137-5 [DOI] [PubMed] [Google Scholar]
- Canals J., Hernández-Martínez C., Cosi S., Voltas N. (2012). The epidemiology of obsessive—compulsive disorder in Spanish school children. J. Anxiety Disord. 26, 746–752. 10.1016/j.janxdis.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Christiansen S. H., Olesen M. V., Wörtwein G., Woldbye D. P. D. (2011). Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav. Brain Res. 216, 585–591. 10.1016/j.bbr.2010.08.044 [DOI] [PubMed] [Google Scholar]
- Dickel D. E., Veenstra-VanderWeele J., Cox N. J., Wu X., Fischer D. J., Van Etten-Lee M., et al. (2006). Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch. Gen. Psychiatry 63, 778–785. 10.1001/archpsyc.63.7.778 [DOI] [PubMed] [Google Scholar]
- Diksic M., Young S. N. (2001). Study of the brain serotonergic system with labeled α-methyl-L-tryptophan. J. Neurochem. 78, 1185–1200. 10.1046/j.1471-4159.2001.00536.x [DOI] [PubMed] [Google Scholar]
- Dilling H. (2015). Internationale Klassifikation Psychischer Störungen. Göttingen: Hogrefe AG. [Google Scholar]
- Fireman B., Koran L. M., Leventhal J. L., Jacobson A. (2001). The prevalence of clinically recognized obsessive-compulsive disorder in a large health maintenance organization. Am. J. Psychiatry 158, 1904–1910. 10.1176/appi.ajp.158.11.1904 [DOI] [PubMed] [Google Scholar]
- Gerald C., Walker M. W., Criscione L., Gustafson E. L., Batzl-Hartmann C., Smith K. E., et al. (1996). A receptor subtype involved in neuropeptide-Y-induced food intake. Nature 382, 168–171. 10.1210/en.137.8.3177 [DOI] [PubMed] [Google Scholar]
- Goodman W. K., Price L. H., Rasmussen S. A., Mazure C., Fleischmann R. L., Hill C. L., et al. (1989). The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch. Gen. Psychiatry 46, 1006–1011. 10.1001/archpsyc.1989.01810110048007 [DOI] [PubMed] [Google Scholar]
- Grados M., Atkins E., Kovacikova G. I., McVicar E. (2015). A selective review of glutamate pharmacological therapy in obsessive-compulsive and related disorders. Psychol. Res. Behav. Manag. 8, 115–131. 10.2147/prbm.s58601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna G. L., Himle J. A., Curtis G. C., Gillespie B. W. (2005). A family study of obsessive-compulsive disorder with pediatric probands. Am. J. Med. Genet. B Neuropsychiatr. Genet. 134B, 13–19. 10.1002/ajmg.b.30138 [DOI] [PubMed] [Google Scholar]
- Heilig M. (2004). The NPY system in stress, anxiety and depression. Neuropeptides 38, 213–224. 10.1016/j.npep.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Herold C., Becker T. (2008). Genetic association analysis with FAMHAP: a major program update. Bioinformatics 25, 134–136. 10.1093/bioinformatics/btn581 [DOI] [PubMed] [Google Scholar]
- Hirsch D., Zukowska Z. (2012). NPY and stress 30 years later: the peripheral view. Cell. Mol. Neurobiol. 32, 645–659. 10.1007/s10571-011-9793-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap Consortium . (2003). The International Hapmap project. Nature 426, 789–796. 10.1038/nature02168 [DOI] [PubMed] [Google Scholar]
- Karno M., Golding J. M., Sorenson S. B., Burnam M. A. (1988). The epidemiology of obsessive-compulsive disorder in five US communities. Arch. Gen. Psychiatry 45, 1094–1099. 10.1001/archpsyc.1988.01800360042006 [DOI] [PubMed] [Google Scholar]
- Kormos V., Gaszner B. (2013). Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 47, 401–419. 10.1016/j.npep.2013.10.014 [DOI] [PubMed] [Google Scholar]
- Leckman J. F., Grice D. E., Boardman J., Zhang H., Vitale A., Bondi C., et al. (1997). Symptoms of obsessive-compulsive disorder. Am. J. Psychiatry 154, 911–917. 10.1176/ajp.154.7.911 [DOI] [PubMed] [Google Scholar]
- Lopez A. D., Murray C. C. (1998). The global burden of disease, 1990–2020. Nat. Med. 4, 1241–1243. 10.1038/3218 [DOI] [PubMed] [Google Scholar]
- Morton G. J., Schwartz M. W. (2001). The NPY/AgRP neuron and energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 25, S56–S62. 10.1038/sj.ijo.0801915 [DOI] [PubMed] [Google Scholar]
- Movafagh S., Hobson J. P., Spiegel S., Kleinman H. K., Zukowska Z. (2006). Neuropeptide Y induces migration, proliferation and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. FASEB J. 20, 1924–1926. 10.1096/fj.05-4770fje [DOI] [PubMed] [Google Scholar]
- Nestadt G., Samuels J., Riddle M., Bienvenu O. J., III., Liang K. Y., LaBuda M., et al. (2000). A family study of obsessive-compulsive disorder. Arch. Gen. Psychiatry 57, 358–363. 10.1001/archpsyc.57.4.358 [DOI] [PubMed] [Google Scholar]
- Neumann C., Taub M. A., Younkin S. G., Beaty T. H., Ruczinski I., Schwender H. (2014). Analytic power and sample size calculation for the genotypic transmission/disequilibrium test in case-parent trio studies. Biom. J. 56, 1076–1092. 10.1002/bimj.201300148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls D. L., Alsobrook J. P., II., Goodman W., Rasmussen S., Leckman J. F. (1995). A family study of obsessive-compulsive disorder. Am. J. Psychiatry 152, 76–84. 10.1176/ajp.152.1.76 [DOI] [PubMed] [Google Scholar]
- Pedragosa-Badia X., Stichel J., Beck-Sickinger A. G. (2013). Neuropeptide Y receptors: how to get subtype selectivity. Front. Endocrinol. 4:5. 10.3389/fendo.2013.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B. S., Pine D. S., Cohen P., Brook J. S. (2001). Prospective, longitudinal study of tic, obsessive-compulsive and attention-deficit/hyperactivity disorders in an epidemiological sample. J. Am. Acad. Child Adolesc. Psychiatry 40, 685–695. 10.1097/00004583-200106000-00014 [DOI] [PubMed] [Google Scholar]
- Pittenger C., Bloch M. H. (2014). Pharmacological treatment of obsessive-compulsive disorder. Psychiatr. Clin. North Am. 37, 375–391. 10.1016/j.psc.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe J. P., Dumont Y., Fournier A., Baker G. B., Quirion R. (2005). Role of serotonin (5-HT) in the antidepressant-like properties of neuropeptide Y (NPY) in the mouse forced swim test. Peptides 26, 1394–1400. 10.1016/j.peptides.2005.03.029 [DOI] [PubMed] [Google Scholar]
- Scahill L., Riddle M. A., McSwiggin-Hardin M., Ort S. I., King R. A., Goodman W. K., et al. (1997). Children’s yale-brown obsessive compulsive scale: reliability and validity. J. Am. Acad. Child Adolesc. Psychiatry 36, 844–852. 10.1097/00004583-199706000-00023 [DOI] [PubMed] [Google Scholar]
- Schneider S., Unnewehr S., Margraf J. (1995). Kinder-DIPS: Diagnostisches Interview Bei Psychischen Störungen Im Kindes- Und Jugendalter. Berlin: Springer. [DOI] [PubMed] [Google Scholar]
- Serova L., Mulhall H., Sabban E. (2017). NPY1 receptor agonist modulates development of depressive-like behavior and gene expression in hypothalamus in SPS rodent PTSD model. Front. Neurosci. 11:203. 10.3389/fnins.2017.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman R. S., McGinnis R. E., Ewens W. J. (1993). Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am. J. Hum. Genet. 52, 506–516. [PMC free article] [PubMed] [Google Scholar]
- Stewart S. E., Fagerness J. A., Platko J., Smoller J. W., Scharf J. M., Illmann C., et al. (2007). Association of theSLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 1027–1033. 10.1002/ajmg.b.30533 [DOI] [PubMed] [Google Scholar]
- Stewart S. E., Mayerfeld C., Arnold P. D., Crane J. R., O’Dushlaine C., Fagerness J. A., et al. (2013). Meta-analysis of association between obsessive-compulsive disorder and the 3′ region of neuronal glutamate transporter gene SLC1A1. Am. J. Med. Genet. B Neuropsychiatr. Genet. 162B, 367–379. 10.1002/ajmg.b.32137 [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Carlquist M., Mutt V. (1982). Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 296, 659–660. 10.1038/296659a0 [DOI] [PubMed] [Google Scholar]
- Taurines R., Schmitt J., Renner T., Conner A. C., Warnke A., Romanos M. (2010). Developmental comorbidity in attention-deficit/hyperactivity disorder. Atten. Def. Hyperact. Disord. 2, 267–289. 10.1007/s12402-010-0040-0 [DOI] [PubMed] [Google Scholar]
- Thiele T. E., Marsh D. J., Ste Marie L., Bernstein I. L., Palmiter R. D. (1998). Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature 396, 366–369. 10.1038/24614 [DOI] [PubMed] [Google Scholar]
- Tiwari A. K., Brandl E. J., Weber C., Likhodi O., Zai C. C., Hahn M. K., et al. (2013). Association of a functional polymorphism in neuropeptide Y with antipsychotic-induced weight gain in schizophrenia patients. J. Clin. Psychopharmacol. 33, 11–17. 10.1097/jcp.0b013e31827d145a [DOI] [PubMed] [Google Scholar]
- Torres A. R., Fontenelle L. F., Shavitt R. G., Ferrão Y. A., do Rosário M. C., Storch E. A., et al. (2016). Comorbidity variation in patients with obsessive-compulsive disorder according to symptom dimensions: results from a large multicentre clinical sample. J. Affect. Disord. 190, 508–516. 10.1016/j.jad.2015.10.051 [DOI] [PubMed] [Google Scholar]
- Upadhya M. A., Dandekar M. P., Kokare D. M., Singru P. S., Subhedar N. K. (2009). Involvement of neuropeptide Y in the acute, chronic and withdrawal responses of morphine in nociception in neuropathic rats: behavioral and neuroanatomical correlates. Neuropeptides 43, 303–314. 10.1016/j.npep.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Walitza S., Marinova Z., Grünblatt E., Lazic S. E., Remschmidt H., Vloet T. D., et al. (2014). Trio study and meta-analysis support the association of genetic variation at the serotonin transporter with early-onset obsessive-compulsive disorder. Neurosci. Lett. 580, 100–103. 10.1016/j.neulet.2014.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitza S., Scherag A., Renner T. J., Hinney A., Remschmidt H., Herpertz-Dahlmann B., et al. (2008). Transmission disequilibrium studies in early onset of obsessive-compulsive disorder for polymorphisms in genes of the dopaminergic system. J. Neural Transm. 115, 1071–1078. 10.1007/s00702-008-0051-6 [DOI] [PubMed] [Google Scholar]
- Weissman M. M., Bland R. C., Canino G. J., Greenwald S., Hwu H. G., Lee C. K., et al. (1994). The cross national epidemiology of obsessive compulsive disorder. The cross national collaborative group. J. Clin. Psychiatry 55, 5–10. [PubMed] [Google Scholar]
- Wendland J. R., Moya P. R., Timpano K. R., Anavitarte A. P., Kruse M. R., Wheaton M. G., et al. (2009). A haplotype containing quantitative trait loci for SLC1A1 gene expression and its association with obsessive-compulsive disorder. Arch. Gen. Psychiatry 66, 408–416. 10.1001/archgenpsychiatry.2009.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam K. Y., Ruigrok S. R., Ziko I., De Luca S. N., Lucassen P. J., Spencer S. J., et al. (2017). Ghrelin and hypothalamic NPY/AgRP expression in mice are affected by chronic early-life stress exposure in a sex-specific manner. Psychoneuroendocrinology 86, 73–77. 10.1016/j.psyneuen.2017.09.006 [DOI] [PubMed] [Google Scholar]