Abstract
Rubus alceaefolius Poir. has been used for the treatment of nasopharyngeal carcinoma (NPC) in China for many years. Euscaphic acid is an active component of Rubus alceaefolius Poir. However, the mechanism of action of euscaphic acid in NPC remains unclear. In this study, Euscaphic acid inhibited the proliferation of NPC cells, induced apoptosis, and led to cell cycle arrest in the G1/S phase. In addition, euscaphic acid inhibited the expression of phosphatidylinositide 3-kinases (PI3K), phosphorylated protein kinase B (p-AKT), and phosphorylated mammalian target of rapamycin (p-mTOR) p-mTOR in NPC cells. The activation of the PI3K/AKT/mTOR signaling pathway by IGF-1 promoted cell proliferation, inhibited apoptosis, and cell cycle arrest in NPC cells. In conclusion, we demonstrated that euscaphic acid reduced cell proliferation and induced apoptosis and cell cycle arrest in NPC cells by suppressing the PI3K/AKT/mTOR signaling pathway.
Keywords: Euscaphic acid, nasopharyngeal carcinoma, PI3K, AKT, mTOR
Introduction
Nasopharyngeal carcinoma (NPC) is an uncommon disease in most countries, with and age-adjusted incidence for both sexes of less than one person per population of 100000 [1]. However, the disease much more frequently in southern China, northern Africa, and Alaska. The people living in the province of Guangdong are especially prone to the disease [2]. Chemotherapy and radiotherapy are still the most common therapies for NPC: radiotherapy is the main treatment, but the 5-year survival rate is only approximately 50% [3]; and chemotherapy still has an important role in treatment, especially in advanced cases. However, these therapies may lead to side-effects [4,5]. Therefore, it is necessary to look for more effective and less toxic drugs for NPC.
Traditional Chinese medicines (TCMs) have a long history of use for cancer treatment in China. In recent years, their use has become more accepted worldwide and the body of evidence supporting the anticancer effect of TCMs increased [6-9]. Previous studies have reported demonstrating the anticancer effects of celastrol, oridonin, and cinnamic acid through the promotion of apoptosis and the inhibition of proliferation of NPC cells [10-12]. Rubus alceaefolius Poir. is a traditional Chinese medicine compound found in Jiangsu, Fujian, Taiwan, Guangdong, and the Guangxi Province. It has wide range of pharmacological activities, including antibiotic [13,14], anti-inflammatory [15], antitumor [16,17], hepatoprotective [18,19] effects. Traditionally, it is used in the treatment of NPC in southern China [20]. Euscaphic acid is a triterpene from the root of the R. alceaefolius Poir. It has been shown that euscaphic acid has DNA polymerase inhibitory activity and inhibits cell growth [21]. However, the role and mechanism of euscaphic acid against NPC remain unclear.
The PI3K/AKT/mTOR pathway is an important intracellular signal transduction pathway. It has already been reported to be related to cell activities such as proliferation, migration, and invasion [22-24]. Signaling through the PI3K/AKT/mTOR pathway can be initiated by several mechanisms, all of which increase the activation of the pathway in cancer cells. We aimed to identify the relationship between euscaphic acid and the PI3K/AKT/mTOR signaling pathway.
In this study, we investigated the effects of euscaphic acid on the proliferation, cell cycle, and apoptosis of NPC cells. Subsequently, we analyzed the potential regulatory mechanism of the PI3K/AKT/mTOR signaling pathway in NPC cells.
Materials and methods
Cell cultures and drugs treatment
NP69 (non-transformed nasopharyngeal epithelial cells derived from the human nasopharynx), C666-1, and CNE-1 human NPC cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The three cell lines were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin and 100 μg/ml streptomycin (GIBCO BRL, NewYork, USA). All NPC cells were cultured at 37°C in a humidified incubator with at atmosphere containing 5% CO2. Euscaphic acid was purchased from PUSH BIO-TECHNOLOGY (http://www.push-herbchem.com/, C30H48O5, Chengdu, Sichuan, China; Chemical structural formula was showed in Figure 1B) and was dissolved in absolute ethanol. A euscaphic acid stock solution (1 mg/mL) was prepared with a final ethanol concentration of ≤2% and sterilized by passing through a membrane filter with a pore size of 0.22 µm.
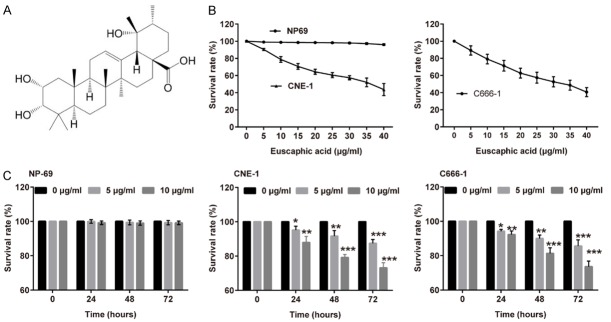
Figure 1.
Euscaphic acid inhibited the proliferation of CNE-1 and C666-1 cells. A. The chemical structural formula of Euscaphic acid. B. CNE-1, C666-1, and NP69 cells were analyzed in the CCK8 assay after exposure to different concentrations of euscaphic acid. C. NP69 cells, CNE-1 cells, and C666-1 cells were analyzed in the CCK8 assay after treatment with euscaphic acid for 24, 48, and 72 hours. *P<0.05, **P<0.01, and ***P<0.001 vs 0 μg/ml group.
Proliferation assays
Cell Counting Kit-8 (CCK-8; Beyotime, Shanghai, China) was used to evaluate the growth of CNE-1, C666-1, and NP69 cells were incubated with different concentrations of euscaphic acid (0, 5, 10, 15, 20, 25, 30, 35, and 40 μg/mL) in accordance with the manufacturer’s instructions. CNE-1, C666-1, and NP69 cells (4×103 cells/well) were seeded into 96-well plates in a final volume of 100 µL medium and cultured for 24, 48, or 72 h. At the end of the incubation period, CCK-8 solution (10 µL) was added to each well, mixed gently, and incubated for 4 h at 37°C. Subsequently, the optical density at 450 nm was measured by using a microplate reader. The survival rate of the non-treatment cells (0 μg/ml Euscaphic acid) was defined as 100%, and the survival rate of cells from all other concentrations of Euscaphic acid was calculated separately from that of the control group. All assays were performed in triplicate.
Cell cycle analysis
The cell cycle was detected by using flow cytometry. Briefly, the cells were treated with euscaphic acid at various concentrations (0, 5, and 10 μg/mL) for 48 h, and then harvested, washed twice with 1× PBS, and re-suspended in 200 µL 1× PBS. The cells were fixed in 4 mL ice-cold 75% ethanol at 4°C overnight and stained with 200 µL propidium iodide (50 µg/mL, Sigma-Aldrich), 20 µL RNase (1 mg/mL, Sigma-Aldrich) was added, and the cells were incubated in a 37°C water bath for 15-20 min to remove RNA. The cells were then analyzed by using a flow cytometer (Cytomics FC 500 MPL, Beckman Coulter). All assays were performed in triplicate.
Apoptosis assays
The cells were incubated with euscaphic acid at different concentrations (0, 5, and 10 μg/mL) for 48 h and assayed to determine the degree of induction of apoptosis. After incubation, the cells were harvested and washed twice with cold 1× PBS, after which 1×105 cells/mL were re-suspended in 200 μL binding buffer, stained with 5 μL Annexin-V and PI (BD Biosciences) for 15 min in the dark at 25°C, and analyzed by using a flow cytometer (Cytomics FC 500 MPL, Beckman Coulter).
Western blotting
Total cellular protein was extracted by using pre-chilled RIPA buffer (Beyotime) containing protease inhibitors. Equal amounts of protein samples were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes. Non-specific binding to the membrane was blocked by incubation in PBS with 0.1% Tween-20 (PBST) containing 5% non-fat milk at 25°C for 2 h. For the protein analysis, the membrane was incubated with the corresponding primary antibody overnight at 4°C, washed with PBST, and then incubated with the secondary the horseradish peroxidase (HRP)-conjugated antibody (1:10,000) at 25°C for 2 h. The membranes were rinsed and the protein bands were visualized by the application of enhanced chemiluminescence reagent (Thermo Scientific). GAPDH was used as the loading control. The sections were incubated with the primary antibodies (anti-human phosphorylated PI3K (p-PI3K) polyclonal antibody (1:10,000; Santa Cruz), anti-human total PI3K (t-PI3K) polyclonal antibody (1:4,000; Santa Cruz), anti-human phosphorylated AKT (p-AKT) polyclonal antibody (1:10,000; Santa Cruz), anti-human total AKT (t-AKT) polyclonal antibody (1:4,000; Santa Cruz), anti-human mTOR polyclonal antibody (1:5,000; Santa Cruz), and anti-human GAPDH polyclonal antibody (1:1,0000; Abcam), respectively.
Statistical analysis
The SPSS 19.0 software package (IBM, Chicago, IL, USA) was used to calculate statistical analyses. All data were presented as the mean ± SD, and Student’s t-test or one-way analysis of variance (ANOVA) was used to compare the difference between two or more groups. P values of <0.05 were considered statistically significant. Graphs were created by using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). IC50 was calculated using GraphPad Prism 7.
Results
Euscaphic acid inhibited the proliferation of CNE-1 and C666-1 cells
CNE-1, C666-1, and NP69 cells were treated with euscaphic acid (0, 5, 10, 15, 20, 25, 30, 35, 40 μg/mL) for 48 h, and cell viability was determined using the CCK8 assay (Figure 1A). The CCK8 assay showed that proliferation was significantly suppressed by an increase in the concentration of euscaphic acid in both CNE-1 and C666-1 cells compared to that in NP69 cells. Additionally, result showed that for C666-1 and CNE-1 cells, the Euscaphic acid concentration to reduce cell viability to 50% was about 36.86 μg/ml (IC50=36.86) and 33.39 μg/ml (IC50=33.39), respectively. We chose 0, 5, and 10 μg/mL euscaphic acid for the subsequent study as these concentrations exerted only low cytotoxicity against the cells, thereby excluding the anti-proliferation effect of high-dose euscaphic acid in cancer cells. To investigate whether euscaphic acid inhibited the proliferation of CNE-1, C666-1, and NP69 cells, the CCK8 assay was performed significantly indicated that proliferation was inhibited in CNE-1 and C666-1cells arrest in a dose- and time-dependent manner, but not in NP69 cells (Figure 1B-D).
Euscaphic acid induces apoptosis and cell cycle arrest in CNE-1 and C666-1 cells
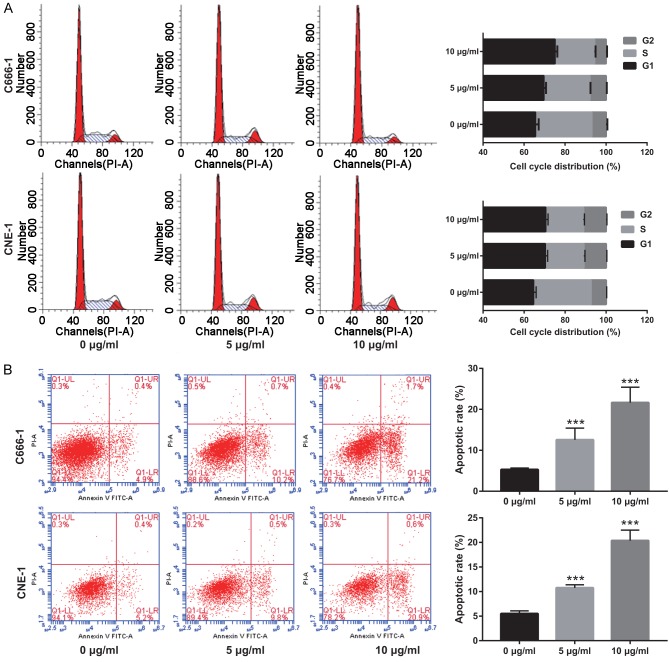
To investigate the effect of euscaphic acid on the induction of apoptosis and cell cycle arrest in CNE-1 and C666-1 cells, flow cytometric analysis was used after treatment with different concentrations of euscaphic acid (0, 5, and 10 μg/mL). At 0 µg/mL, no effects on the cell cycle of NPC cells were observed. At 5 or 10 µg/mL, the proportion of cell cycle arrest in the G1/S phase was altered (Figure 2A). In addition, the results indicated that an increase in euscaphic acid promoted the induction of apoptosis in CNE-1 cells (Figure 2B). The proportion of apoptotic cells increased with an increase in euscaphic acid concentration. Similar results were found in the C666-1 cells. The results confirmed that euscaphic acid significantly promoted apoptosis and inhibited the progression of the cell cycle in CNE-1 and C666-1 cells in a dose-dependent manner. Apoptotic and cell cycle-associated proteins were measured by western blotting. The results clearly illustrated that Bax, caspase-3, p21, and p27 were increased, whereas Bcl-2 and cyclin D1 were decreased after euscaphic acid treatment (Figure 3).
Figure 2.
Euscaphic acid (0, 5, and 10 μg/mL) induces apoptosis and cell cycle arrest in CNE-1 and C666-1 cells. A. The effect of euscaphic acid on the cell cycle of CNE-1 and C666-1 cells was detected by flow cytometry. B. The effect of euscaphic acid on apoptosis in CNE-1 and C666-1 cells was detected by flow cytometry. ***P<0.001.
Figure 3.
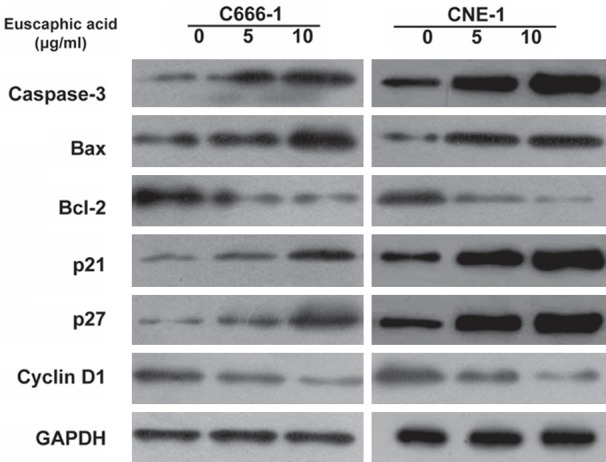
Western blotting of apoptosis-associated and cell cycle-associated proteins after euscaphic acid treatment.
Euscaphic acid inhibited the PI3K/AKT/mTOR signaling pathway
The PI3K/AKT/mTOR pathway is an important signaling pathway for the regulation of cell proliferation and the induction of apoptosis in cancer cells. To determine the effects of euscaphic acid, we detected the proteins in this pathway after treatment of cells with different concentrations of euscaphic acid (0, 5, and 10 μg/mL) by western blotting. As shown in Figure 4, euscaphic acid clearly suppressed the protein expression of PI3K, p-AKT/AKT, and p-mTOR/mTOR in a dose-dependent manner in CNE-1 and C666-1 cells. Euscaphic acid inhibited the NPC through the PI3K/AKT/mTOR signaling pathways. The expression of the components of the PI3K/AKT/mTOR pathways in both CNE-1 and C666-1 cell lines was detected by western blotting after treatment with different concentrations of euscaphic acid (0, 5, and 10 μg/mL).
Figure 4.
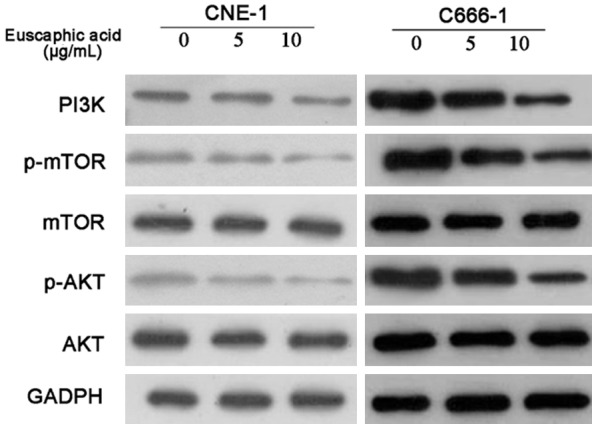
Western blotting of PI3K, p-AKT, AKT, p-mTOR, and mTOR after euscaphic acid treatment.
Activation of the PI3K/AKT/mTOR pathway to reverse the effect of euscaphic acid
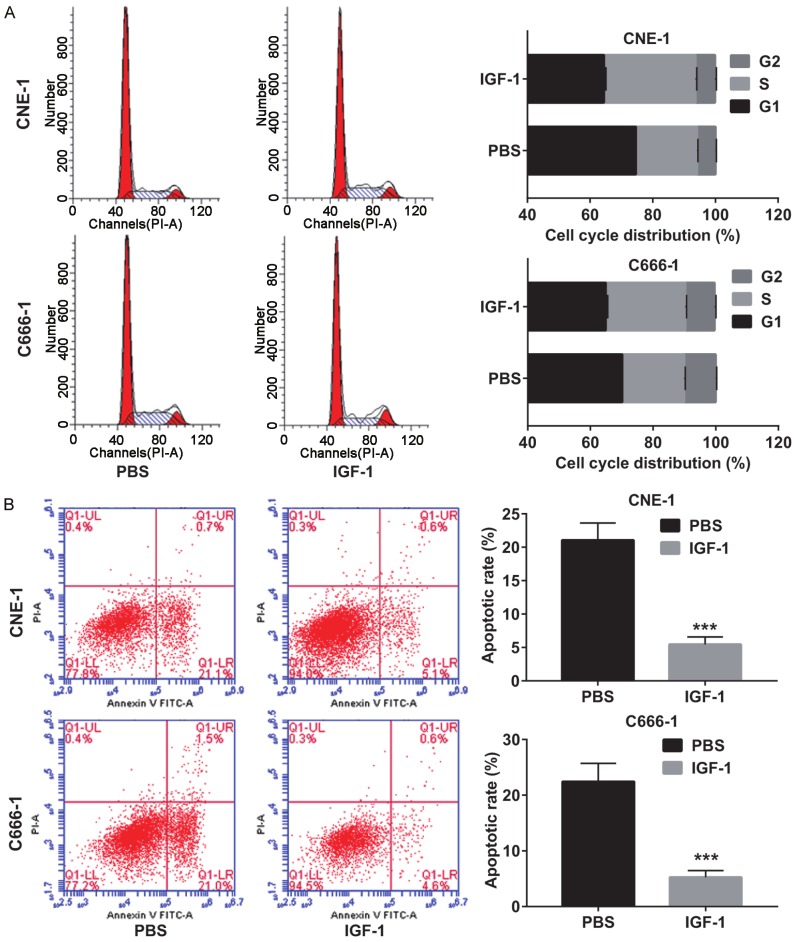
To confirm if euscaphic acid induced apoptosis and cell cycle arrest in CNE-1 and C666-1 cells through the suppression of the PI3K/AKT/mTOR signaling pathway, we further assessed whether the activation of the PI3K/AKT/mTOR signaling pathway by insulin-like growth factors-1 (IGF-1), a PI3K/AKT/mTOR signaling pathway activating agent, could reverse the effects of euscaphic acid in CNE-1 and C666-1 cells. First, we measured the expression of PI3K, p-AKT/AKT, and p-mTOR/mTOR in CNE-1 and C666-1 cells after treatment with euscaphic acid (10 µg/mL) and when supplemented with IGF-1 or PBS. The results clearly showed that supplementation with IGF-1 increased the expression of PI3K, p-AKT/AKT, and p-mTOR/mTOR in CNE-1 and C666-1 cells compared to that when supplemented with PBS (Figure 5). The CCK8 assay suggested that supplementation with IGF-1 significantly promoted cell proliferation in both CNE-1 and C666-1 cells compared to that when supplemented with PBS (Figure 6). The flow cytometry assay indicated that supplementation with IGF-1 significantly promoted cell cycle progression and inhibited cell apoptosis both in CNE-1 and C666-1 cells (Figure 7). The results of the western blot analysis clearly showed that the expression of Bcl-2 and Cyclin D1 in the cells supplemented with IGF-1 was significantly increased, whereas the expression of Bax, caspase-3, p21, and p27 was decreased, compared to that in cells supplemented with PBS (Figure 8).
Figure 5.
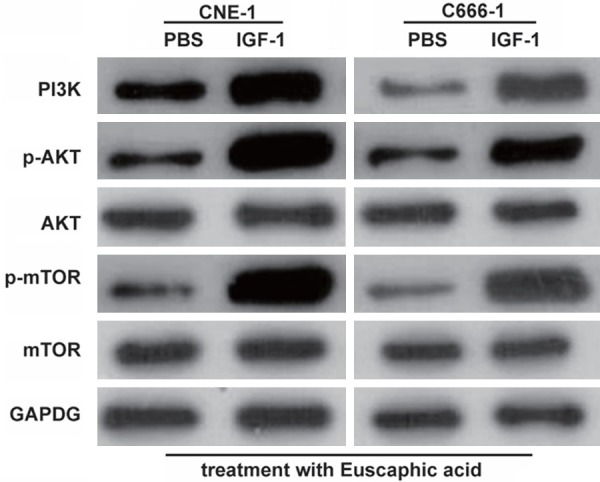
Activation of the PI3K/AKT/mTOR pathway by IGF-1. The effect of euscaphic acid treatment supplemented with IGF-1 on the expression of PI3K, p-AKT/AKT, and p-mTOR/mTOR in CNE-1 and C666-1 cells as measured by western blotting.
Figure 6.
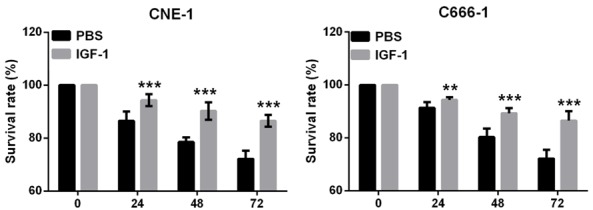
Activation of the PI3K/AKT/mTOR pathway reversed the effect of euscaphic acid on cell proliferation. The effect of euscaphic acid supplemented with IGF-1 on the ability of cell proliferation in CNE-1 and C666-1 cells that were detected by CCK8 analysis. **P<0.01, ***P<0.001.
Figure 7.
Activation of the PI3K/AKT/mTOR pathway reversed the effect of euscaphic acid on cell cycle and apoptosis. A. The effect of euscaphic acid supplemented with IGF-1 on the cell cycle arrest of CNE-1 and C666-1 cells as detected by flow cytometry. B. The effect of euscaphic acid supplemented with IGF-1 on apoptosis in CNE-1 and C666-1 cells was detected by flow cytometry. ***P<0.001.
Figure 8.
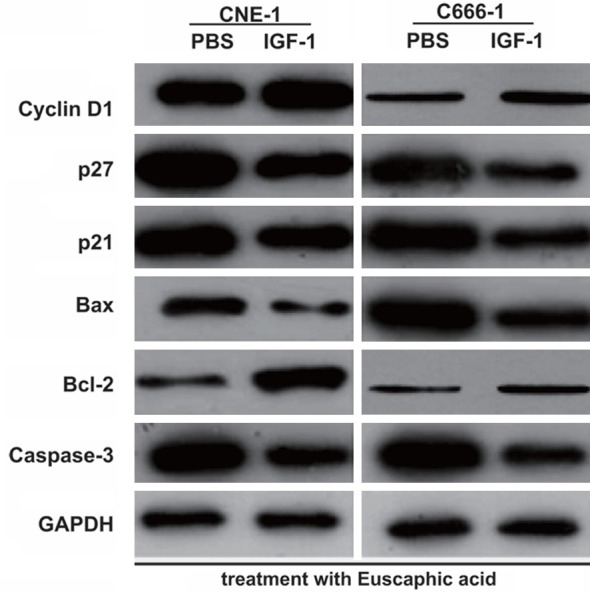
Effect of euscaphic acid supplemented with IGF-1 on the expression of apoptosis-associated and cell cycle regulatory proteins in CNE-1 and C666-1 cells as measured by western blotting after activation of the PI3K/AKT/mTOR pathway.
Discussion
The antitumor activity of euscaphic acid has been previously reported. Banno et al. [25] confirmed the inhibitory effect of euscaphic acid on in vivo two-stage mouse skin carcinogenesis. Kim et al. [26] reported that tumor cells treated with different concentrations of euscaphic acid exhibited a significant decrease in proliferation in a dose-dependent manner. Rocha et al. [27] confirmed the antitumor activity of euscaphic acid in different types of cancer and showed that it induced apoptosis in a caspase-dependent manner. Nonetheless, there has been little research on the effects of euscaphic acid on NPC. In this study, we found that euscaphic acid inhibited the proliferation of CNE-1 and C666-1 cells, and induced apoptosis and cell cycle arrest, unlike in NP69 cells, in a dose- and time-dependent manner. We also found that euscaphic acid treatment clearly increased the expression of Bax, caspase-3, p21, and p27, whereas the expression of Bcl-2 and cyclin D1 was reduced. The results suggested that euscaphic acid exerts anticancer effects through the inhibition of proliferation and the induction of apoptosis in NPC.
Subsequently, we analyzed the antitumor effects of euscaphic acid in the NPC cells through the changes in the expression of PI3K, p-AKT, and p-mTOR to evaluate changes in the PI3K/AKT/mTOR signaling pathway. The PI3K/AKT/mTOR signaling pathway has an important role in NPC, promoting cell proliferation and inhibiting apoptosis [28]. In recent studies, many mechanisms of silencing the PI3K/AKT/mTOR signaling pathway by traditional Chinese medicine have been reported to be related to anticancer effects [29,30]. We found that the activation of the PI3K/AKT/mTOR signaling pathway by IGF-1 reversed the effect of euscaphic acid on proliferation, the cell cycle, and apoptosis. These results showed that euscaphic acid regulated the PI3K/AKT/mTOR signaling pathway to exert anticancer effects in NPC cells.
In summary, we demonstrated the euscaphic acid inhibits the proliferation and promotes apoptosis in NPC cells through the silencing of the PI3K/AKT/mTOR signaling pathway. We believed that euscaphic acid has the potential for development as an anticancer drug for NPC. However, before euscaphic acid can be developed as an anticancer agent, its antitumor activity should be investigated in vivo and in clinical settings.
Acknowledgements
The study was granted by research funding project of Guangdong Provincial Administration of traditional Chinese Medicine (NO. 20110178).
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Muir CS. Cancer incidence in five continents. Comparability and quality of data. IARC Sci Publ. 1992:45–173. [PubMed] [Google Scholar]
- 2.Nielsen NH, Mikkelsen F, Hansen JP. Nasopharyngeal cancer in Greenland. The incidence in an Arctic Eskimo population. Acta Pathol Microbiol Scand A. 1977;85:850–858. [PubMed] [Google Scholar]
- 3.Teo P, Yu P, Lee WY, Leung SF, Kwan WH, Yu KH, Choi P, Johnson PJ. Significant prognosticators after primary radiotherapy in 903 nondisseminated nasopharyngeal carcinoma evaluated by computer tomography. Int J Radiat Oncol Biol Phys. 1996;36:291–304. doi: 10.1016/s0360-3016(96)00323-9. [DOI] [PubMed] [Google Scholar]
- 4.Chambers MS, Garden AS, Kies MS, Martin JW. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck. 2004;26:796–807. doi: 10.1002/hed.20045. [DOI] [PubMed] [Google Scholar]
- 5.McMillan AS, Pow EH, Leung WK, Wong MC, Kwong DL. Oral health-related quality of life in southern Chinese following radiotherapy for nasopharyngeal carcinoma. J Oral Rehabil. 2004;31:600–608. doi: 10.1111/j.1365-2842.2004.01383.x. [DOI] [PubMed] [Google Scholar]
- 6.Devi PU. Withania somnifera Dunal (Ashwagandha): potential plant source of a promising drug for cancer chemotherapy and radiosensitization. Indian J Exp Biol. 1996;34:927–932. [PubMed] [Google Scholar]
- 7.Han MQ, Liu JX, Gao H. Effects of 24 Chinese medicinal herbs on nucleic acid, protein and cell cycle of human lung adenocarcinoma cell. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15:147–149. [PubMed] [Google Scholar]
- 8.Lee KH. Novel antitumor agents from higher plants. Med Res Rev. 1999;19:569–596. doi: 10.1002/(sici)1098-1128(199911)19:6<569::aid-med7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Spaulding-Albright N. A review of some herbal and related products commonly used in cancer patients. J Am Diet Assoc. 1997;97:S208–215. doi: 10.1016/s0002-8223(97)00767-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P, Zhao SR, Liu F, Sun XJ, Liu H. Oridonin induces apoptosis in human nasopharyngeal carcinoma cells involving ROS generation. Folia Biol (Praha) 2017;63:155–163. doi: 10.14712/fb2017063040155. [DOI] [PubMed] [Google Scholar]
- 11.Lin HF, Hsieh MJ, Hsi YT, Lo YS, Chuang YC, Chen MK, Chien SY. Celastrol-induced apoptosis in human nasopharyngeal carcinoma is associated with the activation of the death receptor and the mitochondrial pathway. Oncol Lett. 2017;14:1683–1690. doi: 10.3892/ol.2017.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi G, Chen J, Shi C, Wang Y, Mi S, Shao W, Yu X, Ma Y, Ling J, Huang J. Cinnamic Acid (CINN) induces apoptosis and proliferation in human nasopharyngeal carcinoma cells. Cell Physiol Biochem. 2016;40:589–596. doi: 10.1159/000452572. [DOI] [PubMed] [Google Scholar]
- 13.Martini S, D’Addario C, Colacevich A, Focardi S, Borghini F, Santucci A, Figura N, Rossi C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int J Antimicrob Agents. 2009;34:50–59. doi: 10.1016/j.ijantimicag.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Thiem B, Goslinska O. Antimicrobial activity of Rubus chamaemorus leaves. Fitoterapia. 2004;75:93–95. doi: 10.1016/j.fitote.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Lee KT, Ha J, Yun SY, Ko CD, Jung HJ, Park HJ. Antinociceptive and antiinflammatory effects of Niga-ichigoside F1 and 23-hydroxytormentic acid obtained from Rubus coreanus. Biol Pharm Bull. 2003;26:1436–1441. doi: 10.1248/bpb.26.1436. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Ham YA, Choi SH, Im EO, Jung JH, Im KS, Kim DK, Xu Y, Wang MW, Kim ND. Activity of crude extract of Rubus crataegifolius roots as a potent apoptosis inducer and DNA topoisomerase I inhibitor. Arch Pharm Res. 2000;23:338–343. doi: 10.1007/BF02975444. [DOI] [PubMed] [Google Scholar]
- 17.Zheng ZX, Zhang LJ, Huang CX, Huang QL, Wei XD, Wu XY, Zhou WM. Antitumour effect of total saponins of Rubus parvifolius on malignant melanoma. Zhongguo Zhong Yao Za Zhi. 2007;32:2055–2058. [PubMed] [Google Scholar]
- 18.Badr AM, El-Demerdash E, Khalifa AE, Ghoneim AI, Ayoub NA, Abdel-Naim AB. Rubus sanctus protects against carbon tetrachloride-induced toxicity in rat isolated hepatocytes: isolation and characterization of its galloylated flavonoids. J Pharm Pharmacol. 2009;61:1511–1520. doi: 10.1211/jpp/61.11.0011. [DOI] [PubMed] [Google Scholar]
- 19.Hong ZF, Li TJ, Zhao JY. Effect of total alkaloids of Rubus alceaefolius poiron on gene expressions of CYP2E1 and CYP3A1 in rats with acute liver injury. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29:711–715. [PubMed] [Google Scholar]
- 20.Mei Q, Sun X, Gao Y, Zhou X. Research progress on prevention and treatment of nasopharyngeal carcinoma with traditional Chinese medicine. China Pharmaceuticals. 2007:28–29. [Google Scholar]
- 21.Murakami C, Ishijima K, Hirota M, Sakaguchi K, Yoshida H, Mizushina Y. Novel anti-inflammatory compounds from Rubus sieboldii, triterpenoids, are inhibitors of mammalian DNA polymerases. Biochim Biophys Acta. 2002;1596:193–200. doi: 10.1016/s0167-4838(02)00227-3. [DOI] [PubMed] [Google Scholar]
- 22.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 23.Yang N, Qu YJ, Cheng Y, Liang T, Zhang MN, Zhang D, Dong LN, Wang XW, Zhang GM. Endoplasmic reticulum stress regulates proliferation, migration and invasion of human ovarian cancer SKOV3 cells through PI3K/AKT/mTOR signaling pathway. Cancer Biomark. 2017;19:263–269. doi: 10.3233/CBM-160424. [DOI] [PubMed] [Google Scholar]
- 24.Zhong JT, Yu J, Wang HJ, Shi Y, Zhao TS, He BX, Qiao B, Feng ZW. Effects of endoplasmic reticulum stress on the autophagy, apoptosis, and chemotherapy resistance of human breast cancer cells by regulating the PI3K/AKT/mTOR signaling pathway. Tumour Biol. 2017;39:1010428317697562. doi: 10.1177/1010428317697562. [DOI] [PubMed] [Google Scholar]
- 25.Banno N, Akihisa T, Tokuda H, Yasukawa K, Taguchi Y, Akazawa H, Ukiya M, Kimura Y, Suzuki T, Nishino H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol Pharm Bull. 2005;28:1995–1999. doi: 10.1248/bpb.28.1995. [DOI] [PubMed] [Google Scholar]
- 26.Kim YK, Yoon SK, Ryu SY. Cytotoxic triterpenes from stem bark of Physocarpus intermedius. Planta Med. 2000;66:485–486. doi: 10.1055/s-2000-8585. [DOI] [PubMed] [Google Scholar]
- 27.Rocha Gda G, Simoes M, Lucio KA, Oliveira RR, Coelho Kaplan MA, Gattass CR. Natural triterpenoids from Cecropia lyratiloba are cytotoxic to both sensitive and multidrug resistant leukemia cell lines. Bioorg Med Chem. 2007;15:7355–7360. doi: 10.1016/j.bmc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Lin YT, Wang HC, Chuang HC, Hsu YC, Yang MY, Chien CY. Pre-treatment with angiotensin-(1-7) inhibits tumor growth via autophagy by downregulating PI3K/Akt/mTOR signaling in human nasopharyngeal carcinoma xenografts. J Mol Med (Berl) 2018;96:1407–1418. doi: 10.1007/s00109-018-1704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanwei L, Yinli Y, Pan Z. Traditional herbal formula NPC01 exerts antiangiogenic effects through inhibiting the PI3K/Akt/mTOR signaling pathway in nasopharyngeal carcinoma cells. Evid Based Complement Alternat Med. 2018;2018:5291517. doi: 10.1155/2018/5291517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin YT, Wang HC, Hsu YC, Cho CL, Yang MY, Chien CY. Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071343. [DOI] [PMC free article] [PubMed] [Google Scholar]