Abstract
Oxidized low-density lipoprotein (ox-LDL)-induced endothelial-mesenchymal transition (EndMT), inflammation and apoptosis in endothelial cells play crucial roles in the progression of cardiovascular diseases including atherosclerosis. Vaccarin is a flavonoid glycoside from vaccariae semen associated with powerful cardiovascular protective effects. However, the effects of vaccarin on human umbilical vein endothelial cells (HUVEC) injury in response to ox-LDL remain unknown. Herein, we showed that treatment with vaccarin significantly suppressed ox-LDL-induced HUVEC inflammation, EndMT and apoptosis. Mechanistically, the HUVECs exposed to ox-LDL exhibited enlarged reactive oxygen species (ROS) production and p38 MAPK phosphorylation, which was counteracted by vaccarin. Importantly, ROS activator hydrogen peroxide (H2O2) and p38 MAPK activator anisomycin pretreatment prevent the protective effect of vaccarin on endothelial injury induced by ox-LDL. Our study suggested that vaccarin impeded ox-LDL-triggered HUVEC inflammation, EndMT and apoptosis via inhibition of ROS/p38 MAPK signaling pathway. Vaccarin may have a therapeutic effect on endothelial injury-related disorders.
Keywords: ox-LDL, HUVECs, vaccarin, inflammation, EndMT, apoptosis
Introduction
In normal blood vessels, the morphologic and functional integrality are the main features of vascular endothelial cells, which can swallow up the bacteria, necrotic and senescent tissue [1,2]. In various vascular pathologies, vascular endothelial cells may undergo phenotypic switch of endothelial-to-mesenchymal transition (EndMT) by downregulating endothelial markers including platelet endothelial cell adhesion molecule-1 (CD31), vascular epithelial calcitonin (VE-Cadherin) and upregulating mesenchymal markers, such as α-smooth muscle actin (α-SMA) and SM22α [3]. Accumulating evidence indicates that the appearance of EndMT is widely observed in atherosclerosis [4], myocardial infarction [5], vascular grift failure [6] and pulmonary hypertension [7]. Unraveling the potential mechanisms of EndMT may lead to new therapeutic target for interventions of cardiovascular diseases including atherosclerosis.
Endothelial cell inflammation and apoptosis play a pivotal role in the pathophysiology of cardiovascular diseases such as atherosclerosis [8], hypertension and diabetes [9]. The loss of the morphologic and functional integrality of vascular endothelial cells is closely related to its inflammation and apoptosis in atherosclerosis [10]. Therapeutic strategies against vascular endothelial cell EndMT, inflammation and apoptosis may lead to endothelial related pathological remission. Atherosclerosis proceeds through a multistep response that begins with endothelial cell injury induced by a plethora of injured stress signals, among which oxidized low-density lipoprotein (oxLDL) plays a critical role [11,12]. Ox-LDL binding to scavenger receptor can induce endothelial cell damages such as loss of endothelial integrity, inflammation and apoptosis, which is critically linked to pathophysiological events in the vascular endothelium [13].
Vaccarin, a naturally flavonoid glycoside, is isolated and purified from vaccariae semen [14], it stimulates endothelial cell proliferation, migration, vascularization via activation of AKT and ERK signaling pathways [15]. Vaccarin induces neovascularization via FGF2/FGFR-1 signaling activation in vitro and in vivo [16]. Vaccarin protects endothelial cell injury against hydrogen peroxide or high glucose through inhibition of Notch signaling [17,18]. Recently, we demonstrate that vaccarin protects human microvascular endothelial cells from apoptosis via suppressing reactive oxygen species accumulation and histone deacetylase1 (HDAC1) expression [19]. Bacterial cellulose-vaccarin membranes are expected to boost wound healing in rat skin models [20]. Vaccarin treatment ameliorates hypertension-related cardiovascular remodeling and nephropathy in renovascular hypertensive rats [21,22]. The existing evidence highlights the importance of vaccarin in cardiovascular functions. However, knowledge with regard to the effects of vaccarin on ox-LDL-induced endothelial injury is still unclear. Therefore, we aimed to explore the potential role and underlying molecular mechanisms of vaccarin in ox-LDL-induced EndMT, inflammation in human umbilical vein endothelial cells (HUVECs).
Material and methods
Reagents and chemicals
Vaccarin (Figure S1A) was purchased from Shanghai Shifeng technology Co., Ltd., China. Dulbeccoo’s modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were obtained from Gibco BRL (Carlsbad, CA, USA). Ox-LDL, N-acetyl-L-cysteine (NAC), 2’,7’-dichlorofluorescein diacetate (DCFH-DA) and dimethyl sulfoxide (DMSO) were purchased from Sigma Chemical Co. (St Louis, MO, USA). Anisomycin was purchased from Cell Signaling Technology (Danvers, MA, USA). DAPI (4’,6-diamidino-2-phenylindole) and Cell Counting Assay Kit-8 (CCK-8) were purchased from Beyotime Biotechnology Research Institute (Shanghai, China). Annexin V-FITC/PI Apoptosis Detection Kit was purchased from Shanghai Yisheng technology (Shanghai, China). Enzyme linked-immuno-sorbent assay (ELISA) kits for monocyte chemotactic protein 1 (MCP-1), interleukin-6 (IL-6), vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion molecule-1 (ICAM-1) were purchased from Boster biological technology company (Wuhan, China). Antibodies against total p38 MAPK, p65-NFκB, SM22α, β-actin and goat anti-rabbit IgGH&L (Alexa Fluor® 488) antibodies were acquired from Abcam (Cambridge, MA, USA). Rabbit monoclonal antibody against phosphorylated p38 MAPK was obtained from Cell Signaling Technology (Beverly, MA, USA). The specific primers were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China).
Cell culture and treatments
Human Umbilical Vein Endothelial Cells (HUVECs; FuDan IBS Cell Center, Shanghai, China) were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. The culture medium was replaced every 1-2 days, cells at 85-90% confluence were passaged at a ratio of 1:3 confluence. The cells were used between passages two and five in all experiments. To detect the possible protective effects of vaccarin in the context of ox-LDL, HUVECs were treated with 5 μM vaccarin for 12 h prior to ox-LDL (100 μg/mL) challenge for 24 h in the following experiments according to previous reports [18,19,23].
Cell viability assay
The cell viability of HUVECs was detected by CCK-8 kit according to previous reports [24]. Briefly, HUVECs were seeded on 96-well plates and treated with ox-LDL (0, 10, 20, 50, 100, 200 μg/mL) or vaccarin (0, 1, 2, 5, 10 μM) for 24 h. Then, 10 μL CCK-8 was added to each well for 2 h incubation at 37°C and absorbance was measured at 450 nm by Biotek microplate reader (Winooski, VT, USA). For per experiment, 5 duplicate wells were examined for each group.
Quantitative real time-PCR
Total RNA from HUVECs was extracted using Trizol reagent following the manufacturer’s instructions. Briefly, RNA purity was assessed by spectrophotometric analysis (CWBIO, Taizhou, China) wherein the A260/280 ratios were between 1.8 and 2.2. Equal RNA was used HiScriptQ RT SuperMix for qPCR (Vazyme, Nanjing, China) to generate cDNA. The real-time quantitative PCR used ChamQTM SYBR® qPCR Master Mix (Vazyme, Nanjing, China). For per experiment, 5 duplicate wells were examined for each group. The average cycle thresholds (Ct) were calculated by using 2-ΔΔCT method which was reported to calculate relative gene expression levels [25]. The primer sequences for IL-6: TTCTCCACAAGCGCCTTCGGTCCA (Forward), AGGGCTGAGATGCCGTCGAGGATGTA (Reverse); MCP-1: GCTCATAGCCACCTTCATTC (Forward), GGACACTTGCTGCTGGTGATTC (Reverse); VCAM-1: TTTGACAGGCTGGAGATAGACT (Forward), TCAATGTGTAATTTAGCTCGGCA (Reverse); ICAM-1: TTGGGCATAGAGACCCCGTT (Forward), GCACATTGCTCAGTTCATACACC (Reverse); β-actin: ATCATGTTTGAGACCTTCAACA (Forward), CATCTCTTGCTCGAAGTCCA (Reverse).
Enzyme linked-immunosorbent assay (ELISA)
The conditioned medium concentrations of MCP-1, IL-6, VCAM-1, ICAM-1 in HUVECs by ELISA assays according to the manufacturer’s protocols. The color absorbance was measured at 450 nm by Biotek microplate reader (Winooski, VT, USA).
Intracellular reactive oxygen species (ROS) measurement
Intracellular ROS generation was detected by a fluorescence probe DCFH-DA as previously described [26]. In short, HUVECs were washed 3 times with PBS and then incubated with 10 μM DCFH-DA for 30 min in dark at 37°C. After washing the cells with PBS, the photographs were captured on a fluorescence microscope (80i, Nikon, Japan). The mean fluorescence intensity was analyzed and averaged using the IMAGE-PRO PLUS 6.0 (Version 6.0, Media Cybernetics, Bethesda, Maryland, USA) by using the same parameters.
Apoptosis analysis
The cell apoptosis was detected by a flow cytometry (BD Accuri C6, USA), HUVECs in 6-well plates stain with PI and Annexin V-fluorescein isothiocyanate (FITC) according to manufacturer’s protocols [19]. The apoptotic HUVECs were analyzed with FlowJo_V10 software.
Immunofluorescence
The stimulated HUVECs were fixed with 4% paraformaldehyde for 30 min, and then were permeabilized with 0.1% Triton X-100 in PBS for 15 min at room temperature. Blocking of aspecific antibody, the cells was used 5% BSA for 1 h at room temperature, and then incubated with primary antibody at 4°C overnight. After washing with PBS three times, the cells were incubated with goat anti-rabbit IgG H&L Alexa Fluor® 488. Finally, the cells washed with PBS three times and then stained with DAPI for 10 min at room temperature. The fluorescent images were photographed by a fluorescence microscope (80i, Nikon, Tokyo, Japan). The data were analyzed by the IMAGE-PRO PLUS 6.0 (Version 6.0, Media Cybernetics, Bethesda, Maryland, USA).
Western blot analysis
Total protein was isolated using RIPA lysis buffer (CWBIO, Taizhou, China) as our previously described [19]. The nuclear protein and cytoplasmic protein of HUVECs were extracted with nuclear and cytoplasmic extraction kit (CWBIO, Taizhou, China) following the manufacturer’s instructions. The protein concentrations were quantified through bicinchoninic acid (BCA) protein assay kit (Beyotime, Nanjing, China). The expression of proteins blots was detected as our previous [21,22]. The resulting bands were semi-quantified with Image J software (National Institutes of Health, Bethesda, MD, USA) and Image Lab software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All results are expressed as mean ± S.D. Comparisons within two groups were performed using Student’s t-test. Comparisons within multiple groups were determined using ANOVA followed by Dunnet t-test. Differences with P value < 0.05 were regarded as significant. All the experiments were repeated three times at least.
Results
Vaccarin retarded ox-LDL-induced cell viability decline and EndMT in HUVECs
To mimic lipid accumulation-disrupted normal functionality of the endothelium endothelial in atherosclerosis, we treated HUVECs with different doses of ox-LDL (10, 20, 50, 100, 200 μg/mL) for 24 h. Cell viability progressively reduced along with the increase of ox-LDL concentration, and cell viability decreased to 51.59% ± 6.23% after treated with 100 μg/mL ox-LDL (Figure S1B). Therefore, the ox-LDL concentration of 100 μg/mL was used for further experiments. To evaluate the actions of vaccarin on ox-LDL-induced HUVECs injury, the cells were incubated with vaccarin (0, 1, 2, 5, 10 μM) in the presence of 100 μg/mL ox-LDL for 24 h. In ox-LDL-treated cells, the cell viability was partially restored from 52.43% ± 7.41% to 81.93% ± 8.01% with 5 μM vaccarin (Figure S1C). Therefore, the vaccarin concentration for 5 μM was used for the following experiments. Immunofluorescence staining showed that incubation of HUVECs with ox-LDL decreased expression of the endothelial marker CD31 (Figure 1A and 1C) and increased expression of the mesenchymal marker SM22α (Figure 1B and 1D), which were mitigated by vaccarin. Consistently, western blot showed that vaccarin attenuated the upregulated SM22α protein level in HUVECs induced by ox-LDL (Figure 1E and 1F).
Figure 1.
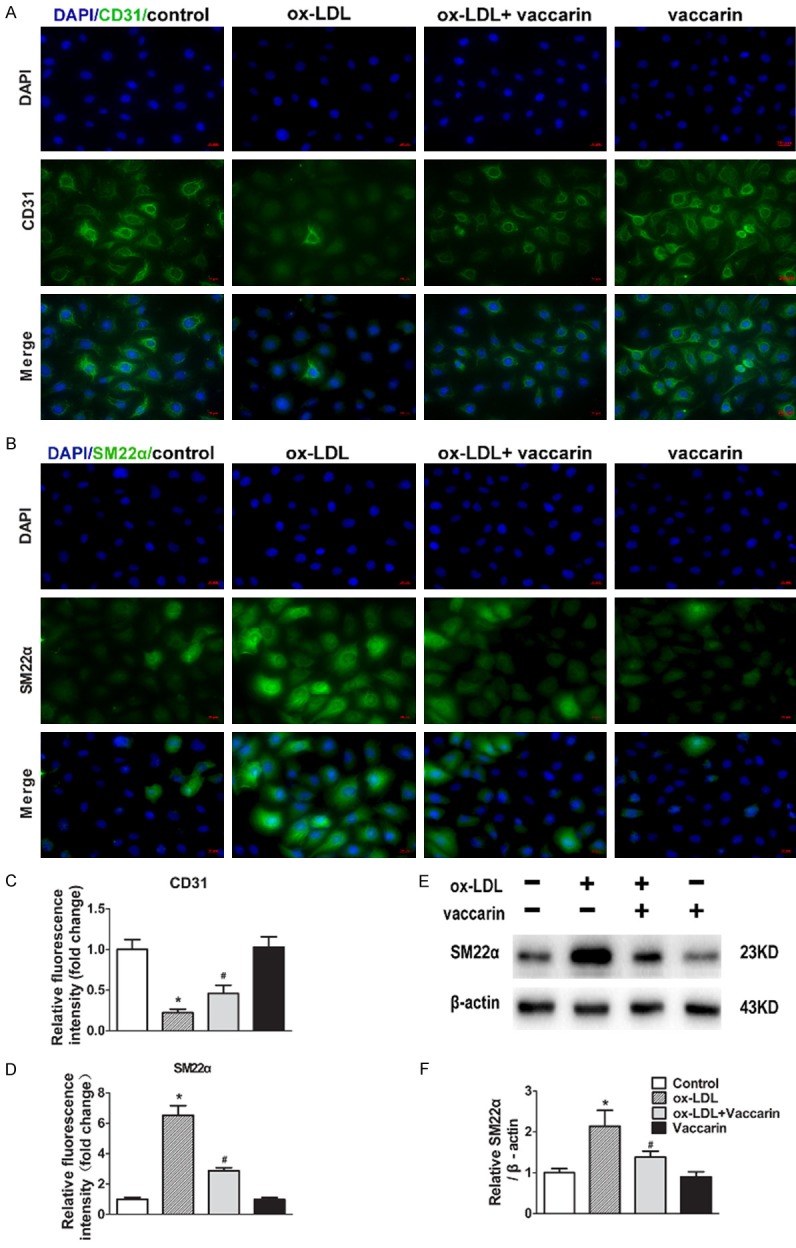
Vaccarin retarded ox-LDL induced HUVEC EndMT. HUVECs treated with ox-LDL (100 μg/ml) with or without vaccarin (5 μM) for 24 h. A. Immunofluorescence analysis of the protein expression of CD31 (×400). B. Fluorescent images showing the protein expression of SM22α (×400). C. Quantification of immunofluorescence analysis of CD31. D. Quantification of immunofluorescence analysis of SM22α. E. Representative immunoblots showing intracellular the protein expression of SM22α. F. Bar graph showing quantification of SM22α. Values are mean ± S.D. *P < 0.05 vs control, #P < 0.05 vs ox-LDL, n = 3/group.
Vaccarin abrogated ox-LDL-induced inflammation in HUVECs
In the presence of ox-LDL, the mRNA levels of IL-6, MCP-1, VCAM-1, ICAM-1 were obviously upregulated in HUVECs, while vaccarin treatment reversed these aberrant changes (Figure 2A). Likewise, the similar results were further confirmed by ELISA (Figure 2B). It has been reported that ox-LDL induces the production of proinflammatory cytokines by the transcription factor NFκB p65 activation [27]. Translocation of p65 of nuclear factor kappa beta (NFκB) from cytoplasm to nucleus is recognized as a prerequisite for transcription [28]. As expected, vaccarin treatment significantly suppressed the ox-LDL-evoked NFκB p65 nuclear translocation, as manifested by decreased NFκB p65 in the nucleus (Figure 2C and 2D) and increased NFκB p65 in the cytoplasm (Figure 2C and 2D).
Figure 2.
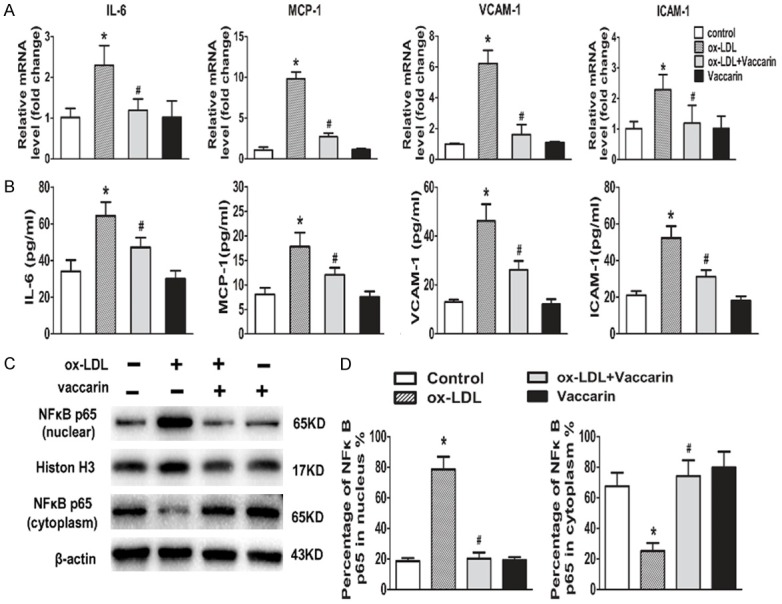
Vaccarin abrogated ox-LDL-induced inflammation in HUVECs. HUVECs treated with ox-LDL (100 μg/ml) with or without vaccarin (5 μM) for 24 h. A. RT-PCR showing the intracellular mRNA levels of MCP-1, IL-6, VCAM-1 and ICAM-1. B. The concentrations of MCP-1, IL-6, VCAM-1 and ICAM-1 in HUVECs cultured supernatants determined by ELISA. C. Represented immunoblots showing the protein expression of NFκB p65 in nuclear and in cytoplasm. D. Bar graph showing percentage of NFκB p65 in nucleus and in cytoplasm. Values are mean ± S.D. *P < 0.05 vs control, #P < 0.05 vs ox-LDL, n = 3/group.
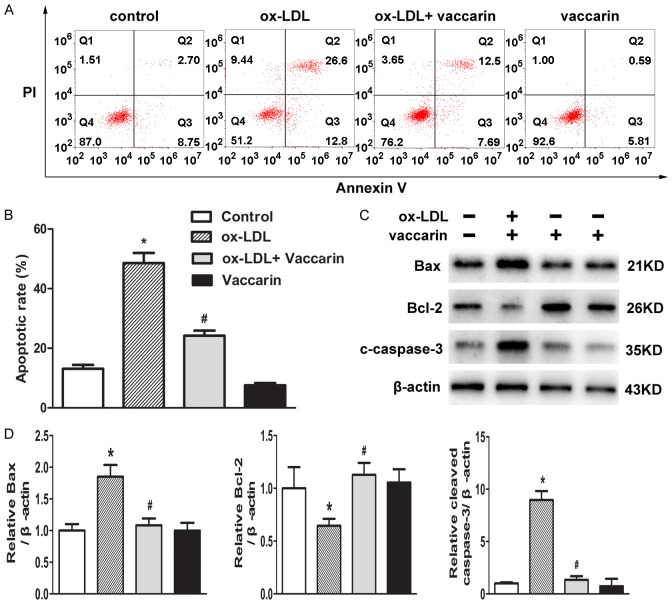
Vaccarin abrogated ox-LDL-induced apoptosis in HUVECs
The increased apoptosis of endothelial cells in response to ox-LDL are intimately linked to atherosclerosis [29]. To investigate the effect of vaccarin on HUVEC apoptosis, HUVECs were co-cultured with vaccarin in ox-LDL-containing medium. Flow cytometry results showed that ox-LDL-induced HUVECs apoptosis were markedly rescued by vaccarin (Figure 3A and 3B). Similarly, treatment with vaccarin counteracted the upregulated Bax and cleaved-caspase-3 protein levels, and downregulated Bcl-2 protein level in ox-LDL-induced HUVECs (Figure 3C and 3D). These above findings indicated that vaccarin abrogated ox-LDL-induced EndMT, inflammation and apoptosis in HUVECs.
Figure 3.
Vaccarin prevented ox-LDL-induced HUVECs apoptosis. HUVECs treated with ox-LDL (100 μg/ml) with or without vaccarin (5 μM) for 24 h. A. The cell apoptosis was analyzed by flow cytometry. B. Bar graph showing the apoptosis rates by the ratio of Annexin-V-positive and Annexin-V/PI-double positive to the total cells. C. The protein expressions of Bax, Bcl-2, and cleaved-caspase-3 detected by western blot. D. Quantification of the related bands of Bax, Bcl-2 and cleaved-caspase-3. Values are mean ± S.D. *P < 0.05 vs control, #P < 0.05 vs ox-LDL, n = 3/group.
Vaccarin prevented ox-LDL-induced ROS/p38 MAPK signaling
Activation of p38 MAPK signaling participates in ox-LDL-induced cell apoptosis, and ROS generation in HUVECs [30]. Inhibition of ROS/p38 MAPK signaling effectively prevents the progression of EndMT in human renal proximal tubular epithelial cells exposed to ox-LDL [31]. ROS scavenger is sufficient to inhibit tumor necrosis-α (TNF-α)-induced phosphorylation of p38 MAPK and its downstream proinflammatory response in human aortic endothelial cells [32]. Thus, we hypothesized that inactivation of ROS/p38 MAPK signaling was involved in the protective effects of vaccarin against endothelial cell injury induced by ox-LDL. As observed, vaccarin had no significant effect on basal ROS production and phosphorylated p38 MAPK protein level, but markedly diminished ROS accumulation (Figure 4A and 4B) and p38 MAPK phosphorylation (Figure 4C and 4D) in HUVECs response to ox-LDL.
Figure 4.
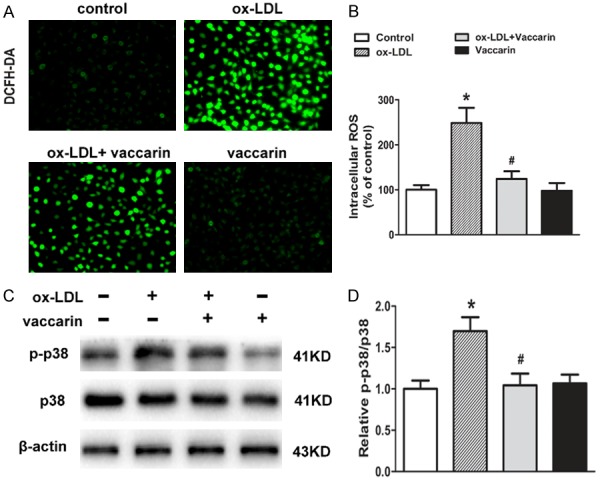
Vaccarin inhibited ox-LDL-induced ROS production and p38 MAPK signaling activation in HUVECs. HUVECs treated with ox-LDL (100 μg/ml) with or without vaccarin (5 μM) for 24 h. A. Intracellular levels of ROS were detected by DCFH-DA fluorescence (×200). B. Intracellular ROS fluorescence values. C. The phosphorylated and total p38 protein levels. D. Quantification of the related bands. Values are mean ± S.D. *P < 0.05 vs control, #P < 0.05 vs ox-LDL, n = 3/group.
Vaccarin prevented ox-LDL-induced EndMT via inactivation of ROS/p38 MAPK signaling
Importantly, pretreatment with p38 MAPK activator anisomycin suppressed the upregulated endothelial marker CD31 protein expression and the down-regulated mesenchymal marker SM22α protein expression in vaccarin-treated HUVECs, as evidenced by immunofluorescence staining (Figure 5A and 5C). Similar to the results from anisomycin, H2O2, an activator of ROS, abolished the inhibitory effect of vaccarin on EndMT induced by ox-LDL (Figure 5D and 5F). As observed above, vaccarin suppressed the phosphorylated p38 MAPK protein level in ox-LDL-incubated HUVECs, but these effects were blocked by both anisomycin (Figure 5B) and H2O2 (Figure 5E). These results hinted that vaccarin may suppress ROS/p38 MAPK signaling pathway to ameliorate ox-LDL-induced HUVEC EndMT.
Figure 5.
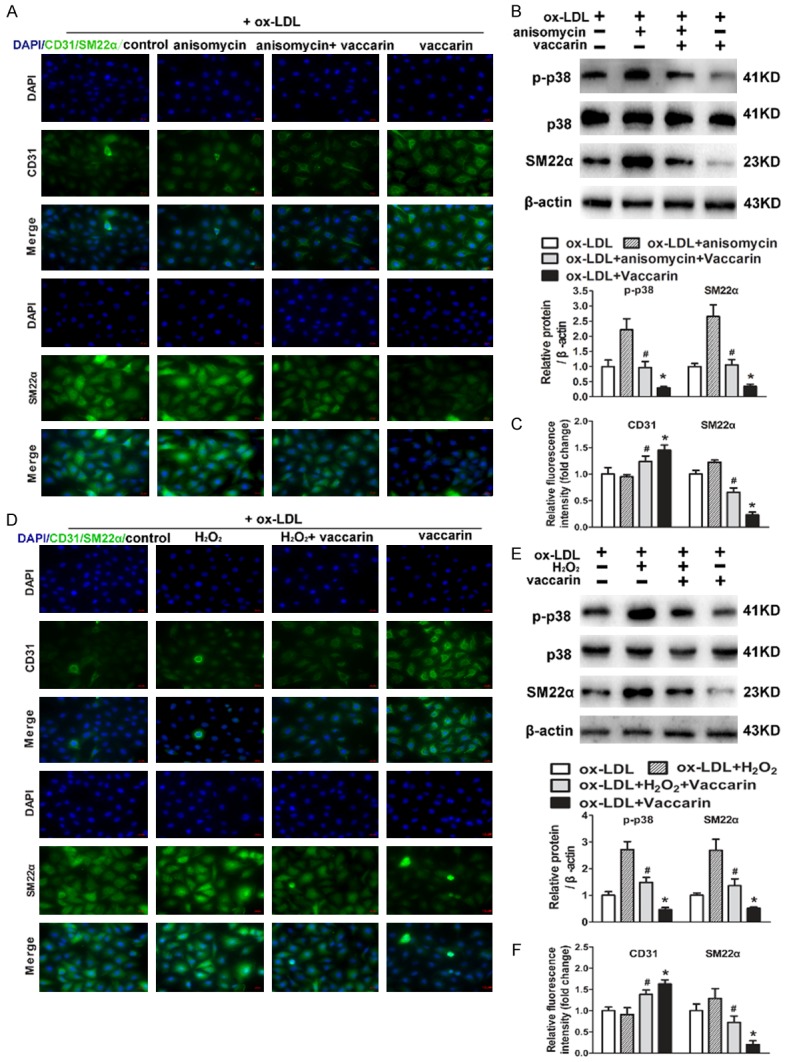
Anisomycin or H2O2 inhibited ox-LDL and vaccarin treated HUVEC EndMT via suppressing ROS/p38 MAPK signaling pathway. HUVECs were pretreated with anisomycin (25 μg/mL) for 30 min prior to ox-LDL (100 μg/mL) and vaccarin (5 μM) treatment for 24 h. A. Immunofluorescence analysis of the protein expression of CD31 and SM22α (×400). B. The expression of total and phosphorylated p38 MAPK and SM22α were detected by immunoblot assays. C. Quantification of Immunofluorescence analysis of CD31 and SM22α. HUVECs were treated with H2O2 (100 μM/mL) for 30 min before ox-LDL (100 μg/mL) and vaccarin (5 μM) for 24 h. D. Fluorescent images showing the protein expression of CD31 and SM22α (×400). E. Represented immunoblots showing the total and phosphorylated p38 MAPK and SM22α. F. Bar graph showing Quantification of CD31 and SM22α. Values are mean ± S.D. *P < 0.05 vs ox-LDL, #P < 0.05 vs ox-LDL+ vaccarin, n = 3/group.
Vaccarin prevented ox-LDL-induced inflammation via inactivation of ROS/p38 MAPK signaling
Activation of ROS/p38 MAPK signaling plays a central role in inflammation and apoptosis in endothelial cells [33]. Anisomycin pretreatment retarded that the suppressive effect of vaccarin on ox-LDL-induced the production of proinflammatory cytokines, as evidenced by RT-PCR and ELISA assays (Figure 6A and 6B). Moreover, vaccarin remarkably antagonized the translocation of NFκB p65 from cytoplasm to nucleus, which was impeded by anisomycin (Figure 6C). In similarity, H2O2 treatment also prohibited the antagonistic action of vaccarin on the expressions of proinflammatory cytokines (Figure 6D and 6E) and NFκB p65 nuclear translocation (Figure 6F) in ox-LDL-incubated HUVECs. These results suggested that inactivation of ROS/p38 MAPK signaling was essential for vaccarin to protect HUVECs from inflammatory response induced by ox-LDL.
Figure 6.
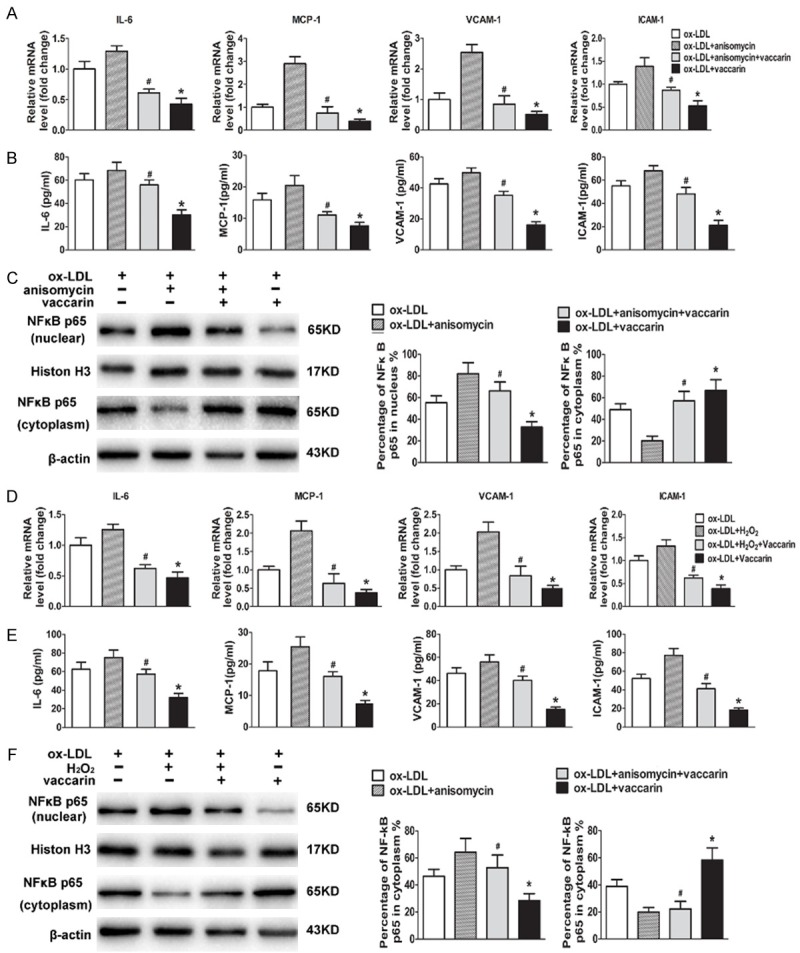
Anisomycin or H2O2 repressed ox-LDL and vaccarin treated HUVEC inflammation via suppression of ROS/p38 MAPK signaling pathway. HUVECs were pretreated with anisomycin (25 μg/mL) for 30 min prior to ox-LDL (100 μg/mL) and vaccarin (5 μM) treatment for 24 h. A. RT-PCR showing the intracellular mRNA levels of MCP-1, IL-6, VCAM-1 and ICAM-1. B. The concentrations of MCP-1, IL-6, VCAM-1 and ICAM-1 in HUVECs cultured supernatants measured by ELISA. C. Represented immunoblots showing the protein expression of NFκB p65 in nucleus and in cytoplasm. HUVECs were treated with H2O2 (100 μM/mL) for 30 min before ox-LDL (100 μg/mL) and vaccarin (5 μM) for 24 h. D. RT-PCR showing the relative intracellular mRNA levels. E. ELISA showing the relative concentrations of MCP-1, IL-6, VCAM-1 and ICAM-1 in HUVECs cultured supernatants. F. The protein expression of NFκB p65 in nucleus and in cytoplasm were detected by western blot. Values are mean ± S.D. *P < 0.05 vs ox-LDL, #P < 0.05 vs ox-LDL+ vaccarin, n = 3/group.
Vaccarin prevented ox-LDL-induced apoptosis via inactivation of ROS/p38 MAPK signaling
In ox-LDL-treated HUVECs, the increased apoptotic rate, upregulated Bax and cleaved-caspase-3 protein expressions, as well as decreased protein Bcl-2 protein were ameliorated by vaccarin, but these changes were eliminated with p38 MAPK activator anisomycin (Figure 7A and 7B). In parallel to the results from anisomycin, the negative effects of vaccarin on ox-LDL-induced changes in cell apoptosis and apoptosis-related protein expressions were also compromised by H2O2 treatment (Figure 7C and 7D). These results implied that pharmacological activation of ROS/p38 MAPK signaling pathway was sufficient to block the protective effects of vaccarin against ox-LDL-induced cell apoptosis.
Figure 7.
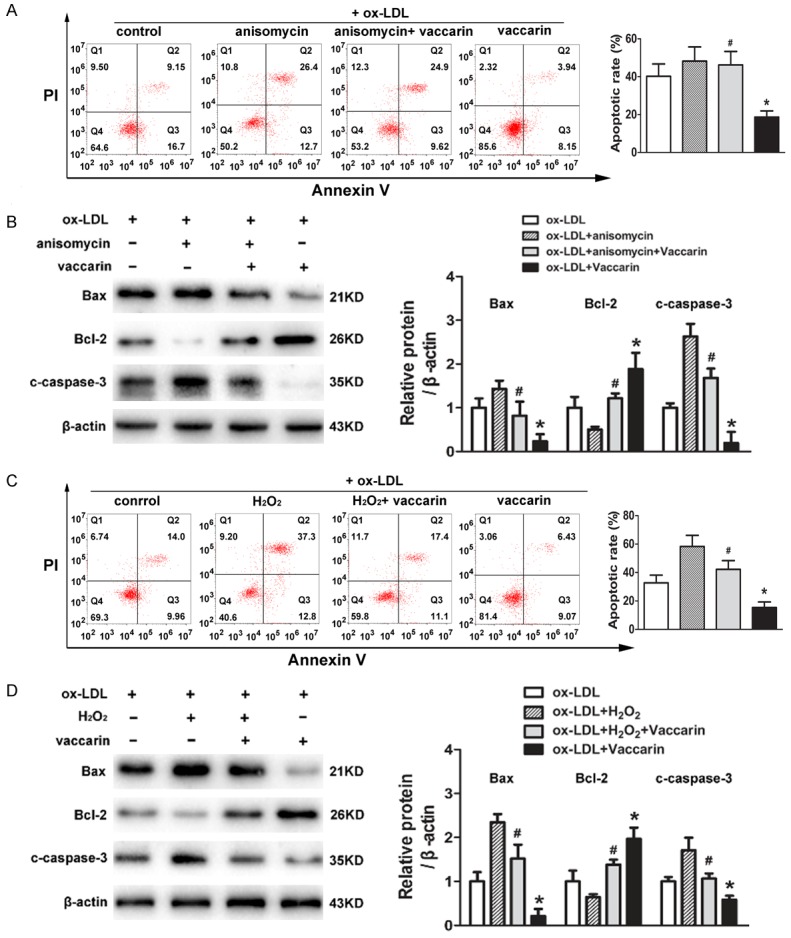
Vaccarin inhibited ox-LDL-induced HUVEC apoptosis by attenuation of ROS/p38 MAPK signaling pathway. HUVECs were pretreated with anisomycin (25 μg/mL) for 30 min followed by stimulation with ox-LDL (100 μg/mL) and vaccarin (5 μM) for 24 h. A. Flow cytometry showing the cell apoptosis, and the relative apoptosis rates by the ratio of Annexin-V-positive and Annexin-V/PI-double positive to the total cells. B. The protein levels of Bax, Bcl-2 and cleaved-caspase-3 detected by western blot. HUVECs were treated with H2O2 (100 μM/mL) for 30 min before ox-LDL (100 μg/mL) and vaccarin (5 μM) for 24 h. C. The cell apoptosis was analyzed by flow cytometry. D. Represented immunoblots showing the protein expressions of Bax, Bcl-2 and cleaved-caspase-3. Values are mean ± S.D. *P < 0.05 vs ox-LDL, #P < 0.05 vs ox-LDL+ vaccarin, n = 3/group.
Discussion
Vascular endothelial cell injury is the common pathological basis of various cardiovascular diseases associated with high mortality and disability [34,35]. Ox-LDL plays a pivotal role in early stages of lesion formation in atherosclerosis via accelerating endothelial cell EndMT, inflammation and apoptosis [36]. The primary novel findings in our study were that vaccarin protected HUVECs from ox-LDL-induced EndMT, inflammation and apoptosis via suppression of ROS/p38 MAPK signaling pathway. These results revealed that vaccarin may be considered to be as a new therapeutic drug for atherosclerosis.
Normal endothelial cells response to proinflammatory factors may undergo EndMT which were characterized by fibroblastoid morphology and cell-to-cell junction rearrangement [5]. EndMT may facilitate endothelial cells to acquire mesenchymal fate, as reflected by the shape and properties of mesenchymal cells [37]. Recent studies have identified that EndMT plays a pivotal role in the pathogenesis of cardiovascular diseases, and may represent a novel therapeutic target for cardiovascular disorders [38]. Disruption of baseline fibroblast growth factor (FGF) signal leads to EndMT via activation of transforming growth factor (TGF)-β signaling [39]. Fibroblast growth factor receptor 1 (FGFR1) is emerged as the key anti-EndMT molecule in human dermal microvascular endothelial cells [40]. These published papers have established that FGF signaling is a critical factor in maintenance of endothelial homeostasis. Intriguingly, our previous study has illustrated that FGF-2-mediated FGFR1 signaling in human microvascular endothelial cells is activated by vaccarin to promote angiogenesis [41]. Theoretically, we deduced that vaccarin can be used to combat the development of EndMT. As expected, our results showed that HUVECs exposed to ox-LDL underwent EndMT through downregulating endothelial marker CD31 and upregulating mesenchymal marker SM22α, which was dramatically inhibited by vaccarin treatment. These results indicated that vaccarin was able to antagonize the process of EndMT in ox-LDL-induced HUVECs.
Vascular endothelial cell inflammation and apoptosis participate in each stage of atherosclerosis [42]. Ox-LDL promotes the formation and development of atherosclerotic plaques by inducing vascular endothelial inflammation and apoptosis [23,43,44]. Moreover, ox-LDL accelerates the expressions of adhesion molecules such as MCP-1, IL-6, VCAM-1 and ICAM-1, NFκB activation and expression of apoptotic proteins in endothelial cells [45,46]. Thereafter, the new drugs can be used to treat atherosclerosis via targeting endothelial inflammation and apoptosis. Recently, our groups have shown that investigate that vaccarin promotes endothelial cell proliferation and inhibits apoptosis of high glucose-induced endothelial cells [19]. However, it remains to be clarified whether vaccarin had effect on ox-LDL-induced endothelial inflammation and apoptosis. In the present study, we found that vaccarin treatment restrained the expression of adhesion molecules MCP-1, IL-6, VCAM-1 and ICAM-1, and NFκB p65 nuclear translocation in ox-LDL-induced HUVECs. Furthermore, ox-LDL upregulated Bax, cleaved-caspase-3 protein levels, but downregulated downregulated Bcl-2 protein expression in HUVECs, which was mitigated by vaccarin. Consequently, these data revealed that vaccarin served as a therapeutic regulator for endothelial repair via inhibition of endothelial cell inflammation and apoptosis.
MAP kinases, including ERK1/2 (extracellular signalling-regulated kinase), JNK (c-Jun N-terminal kinase) and p38 MAPK, regulate the activity of many proteins, enzymes and transcription factors and thus are involved in a biological response of a cell to an external stimulus [47]. More and more studies have proposed that p38 MAPK is a key target for endothelial cell inflammation and apoptosis in the pathogenesis of atherosclerosis [48]. Reactive oxygen species (ROS) are well known for their role in modulating both physiological and pathophysiological signal transduction [49]. Substantial evidence suggests that increased oxidative stress plays a prominent role in the pathogenesis of vascular endothelial dysfunction along with endothelial cell EndMT, inflammation and apoptosis [50-52]. Polymerized porcine hemoglobin attenuates H2O2-induced endothelial cell injury via decreasing the ROS overproduction and subsequent phosphorylation of p38 MAPK [53]. In the present study, our data demonstrated that ox-LDL elicited increased p38 MAPK phosphorylation tremendous ROS generation in HUVECs, and these effects were reversed by vaccarin pretreatment. It is noted that both ROS and p38 MAPK activators suppressed vaccarin-mediated protective effects against ox-LDL-induced endothelial cell EndMT, inflammation and apoptosis. These results hinted that blockade of ROS/p38 MAPK signaling pathway was responsible for the protective actions of vaccarin in ox-LDL-mediated endothelial dysfunction.
Collectively, our results demonstrated that vaccarin suppressed ox-LDL induced endothelial EndMT, inflammation and apoptosis via inhibiting ROS/p38 MAPK signaling pathway. Vaccarin may serve as a candidate for protection of vascular endothelium, thus providing a promising alternative for the treatment of atherosclerotic endothelial injury.
Acknowledgements
We thank the general support of experimental public platform, Wuxi School of Medicine, Jiangnan University. This work was supported by grants from Chinese National Natural Science Fund (81700364), Jiangsu Natural Science Foundation (BK20170179), Fundamental Research Funds for the Central Universities (JUSRP11754 and JUSRP11755), Project funded by China Postdoctoral Science Foundation (2017M611688), Public Health Research Center at Jiangnan University (JUPH201504) and Jiangnan University Youth Fund 2018 (K2050205).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Murciano C, Hor LI, Amaro C. Host-pathogen interactions in Vibrio vulnificus: responses of monocytes and vascular endothelial cells to live bacteria. Future Microbiol. 2015;10:471–487. doi: 10.2217/fmb.14.136. [DOI] [PubMed] [Google Scholar]
- 2.Wei J, Chen Q, James JL, Stone PR, Chamley LW. IL-1 beta but not the NALP3 inflammasome is an important determinant of endothelial cell responses to necrotic/dangerous trophoblastic debris. Placenta. 2015;36:1385–1392. doi: 10.1016/j.placenta.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Correia AC, Moonen JR, Brinker MG, Krenning G. FGF2 inhibits endothelial-mesenchymal transition through microRNA-20a-mediated repression of canonical TGF-beta signaling. J Cell Sci. 2016;129:569–579. doi: 10.1242/jcs.176248. [DOI] [PubMed] [Google Scholar]
- 4.Li A, Peng W, Xia X, Li R, Wang Y, Wei D. Endothelial-to-mesenchymal transition: a potential mechanism for atherosclerosis plaque progression and destabilization. DNA Cell Biol. 2017;36:883–891. doi: 10.1089/dna.2017.3779. [DOI] [PubMed] [Google Scholar]
- 5.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 6.Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC, Boehm M. TGF-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6:227ra234. doi: 10.1126/scitranslmed.3006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su G, Sun G, Liu H, Shu L, Zhang J, Guo L, Huang C, Xu J. Niacin suppresses progression of atherosclerosis by inhibiting vascular inflammation and apoptosis of vascular smooth muscle cells. Med Sci Monit. 2015;21:4081–4089. doi: 10.12659/MSM.895547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Y, Wang Y, Zhao Y, Peng K, Li W, Wang Y, Zhang J, Zhou S, Liu Q, Li X, Cai L, Liang G. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes. 2014;63:3497–3511. doi: 10.2337/db13-1577. [DOI] [PubMed] [Google Scholar]
- 10.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest. 2015;125:4514–4528. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin F, Pei L, Zhang Q, Han W, Jiang S, Lin Y, Dong B, Cui L, Li M. Ox-LDL induces endothelial cell apoptosis and macrophage migration by regulating caveolin-1 phosphorylation. J Cell Physiol. 2018;233:6683–6692. doi: 10.1002/jcp.26468. [DOI] [PubMed] [Google Scholar]
- 12.Fang F, Yang Y, Yuan Z, Gao Y, Zhou J, Chen Q, Xu Y. Myocardin-related transcription factor a mediates OxLDL-induced endothelial injury. Circ Res. 2011;108:797–807. doi: 10.1161/CIRCRESAHA.111.240655. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Mu Q, Zhou Z, Song H, Zhang Y, Wu F, Jiang M, Wang F, Zhang W, Li L, Shao L, Wang X, Li S, Yang L, Wu Q, Zhang M, Tang D. Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One. 2016;11:e0158038. doi: 10.1371/journal.pone.0158038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeva RT, Karryev MO, Litvinenko VI, Abubakirov NK. Glycosides of Vaccaria segetalis V. Vaccarin. Chemistry of Natural Compounds. 1974;10:182–186. [Google Scholar]
- 15.Xie F, Feng L, Cai W, Qiu Y, Liu Y, Li Y, Du B, Qiu L. Vaccarin promotes endothelial cell proliferation in association with neovascularization in vitro and in vivo. Mol Med Rep. 2015;12:1131–1136. doi: 10.3892/mmr.2015.3503. [DOI] [PubMed] [Google Scholar]
- 16.Sun HJ, Cai WW, Gong LL, Wang X, Zhu XX, Wan MY, Wang PY, Qiu LY. FGF-2-mediated FGFR1 signaling in human microvascular endothelial cells is activated by vaccarin to promote angiogenesis. Biomed Pharmacother. 2017;95:144–152. doi: 10.1016/j.biopha.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 17.Xie F, Cai W, Liu Y, Li Y, Du B, Feng L, Qiu L. Vaccarin attenuates the human EA.hy926 endothelial cell oxidative stress injury through inhibition of Notch signaling. Int J Mol Med. 2015;35:135–142. doi: 10.3892/ijmm.2014.1977. [DOI] [PubMed] [Google Scholar]
- 18.Qiu Y, Du B, Xie F, Cai W, Liu Y, Li Y, Feng L, Qiu L. Vaccarin attenuates high glucose-induced human EA*hy926 endothelial cell injury through inhibition of Notch signaling. Mol Med Rep. 2016;13:2143–2150. doi: 10.3892/mmr.2016.4801. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Lei Y, Tan F, Gong L, Gong H, Yang W, Chen T, Zhang Z, Cai W, Hou B, Wang X, Sun H, Zhou Y, Qiu L. Vaccarin protects human microvascular endothelial cells from apoptosis via attenuation of HDAC1 and oxidative stress. Eur J Pharmacol. 2018;818:371–380. doi: 10.1016/j.ejphar.2017.09.052. [DOI] [PubMed] [Google Scholar]
- 20.Qiu Y, Qiu L, Cui J, Wei Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater Sci Eng C Mater Biol Appl. 2016;59:303–309. doi: 10.1016/j.msec.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Zhou Z, Zhang Q, Cai W, Zhou Y, Sun H, Qiu L. Vaccarin administration ameliorates hypertension and cardiovascular remodeling in renovascular hypertensive rats. J Cell Biochem. 2018;119:926–937. doi: 10.1002/jcb.26258. [DOI] [PubMed] [Google Scholar]
- 22.Cai W, Zhang Z, Huang Y, Sun H, Qiu L. Vaccarin alleviates hypertension and nephropathy in renovascular hypertensive rats. Exp Ther Med. 2018;15:924–932. doi: 10.3892/etm.2017.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H, Zhu X, Zhou Y, Cai W, Qiu L. C1q/TNF-related protein-9 ameliorates Ox-LDL-induced endothelial dysfunction via PGC-1alpha/AMPK-mediated antioxidant enzyme induction. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan F, Liu L, Lei Y, Hu Y. MiRNA-142-3p increases radiosensitivity in human umbilical cord blood mononuclear cells by inhibiting the expression of CD133. Sci Rep. 2018;8:5674. doi: 10.1038/s41598-018-23968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang GL, Xia XL, Li XL, He FH, Li JL. Identification and expression analysis of the MSP130-related-2 gene from Hyriopsis cumingii. Genet Mol Res. 2015;14:4903–4913. doi: 10.4238/2015.May.11.23. [DOI] [PubMed] [Google Scholar]
- 26.Wu DB, Chen JF, Xu Q, Lin JQ, Liao JQ, Wu W. Exogenous hydrogen sulfide inhibits high-glucose-induced injuries via regulating leptin/leptin receptor signaling pathway in human umbilical vein endothelial cells. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:1055–1061. [PubMed] [Google Scholar]
- 27.Janabi M, Yamashita S, Hirano K, Sakai N, Hiraoka H, Matsumoto K, Zhang Z, Nozaki S, Matsuzawa Y. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler Thromb Vasc Biol. 2000;20:1953–1960. doi: 10.1161/01.atv.20.8.1953. [DOI] [PubMed] [Google Scholar]
- 28.Sun HJ, Zhao MX, Ren XS, Liu TY, Chen Q, Li YH, Kang YM, Wang JJ, Zhu GQ. Salusin-beta promotes vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via ROS/NFkappaB/MMP-9 pathway. Antioxid Redox Signal. 2016;24:1045–1057. doi: 10.1089/ars.2015.6475. [DOI] [PubMed] [Google Scholar]
- 29.Sun HJ, Xu DY, Sun YX, Xue T, Zhang CX, Zhang ZX, Lin W, Li KX. CO-releasing molecules-2 attenuates ox-LDL-induced injury in HUVECs by ameliorating mitochondrial function and inhibiting Wnt/beta-catenin pathway. Biochem Biophys Res Commun. 2017;490:629–635. doi: 10.1016/j.bbrc.2017.06.089. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Jia YH, Zhao XS, Zhou FH, Pan YY, Wan Q, Cui XB, Sun XG, Chen YY, Zhang Y, Cheng SB. Trichosanatine alleviates oxidized low-density lipoprotein induced endothelial cells injury via inhibiting the LOX-1/p38 MAPK pathway. Am J Transl Res. 2016;8:5455–5464. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu BB, Wang H, Chi YF, Wang YM, Yao XM, Liu S, Qiu H, Fang J, Yin PH, Zhang XM, Peng W. Protective effects of probucol on Ox-LDL-induced epithelial-mesenchymal transition in human renal proximal tubular epithelial cells via LOX1/ROS/MAPK signaling. Mol Med Rep. 2018;17:1289–1296. doi: 10.3892/mmr.2017.7935. [DOI] [PubMed] [Google Scholar]
- 32.Olejarz W, Bryk D, Zapolska-Downar D, Malecki M, Stachurska A, Sitkiewicz D. Mycophenolic acid attenuates the tumour necrosis factor-alpha-mediated proinflammatory response in endothelial cells by blocking the MAPK/NF-kappaB and ROS pathways. Eur J Clin Invest. 2014;44:54–64. doi: 10.1111/eci.12191. [DOI] [PubMed] [Google Scholar]
- 33.Bao MH, Zhang YW, Zhou HH. Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-kappaB pathway. J Ethnopharmacol. 2013;146:543–551. doi: 10.1016/j.jep.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Sun HJ, Hou B, Wang X, Zhu XX, Li KX, Qiu LY. Endothelial dysfunction and cardiometabolic diseases: role of long non-coding RNAs. Life Sci. 2016;167:6–11. doi: 10.1016/j.lfs.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Sun HJ, Zhu XX, Cai WW, Qiu LY. Functional roles of exosomes in cardiovascular disorders: a systematic review. Eur Rev Med Pharmacol Sci. 2017;21:5197–5206. doi: 10.26355/eurrev_201711_13840. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Mohamed AS, Zhou SH. Oxidized low density lipoprotein, stem cells, and atherosclerosis. Lipids Health Dis. 2012;11:85. doi: 10.1186/1476-511X-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen PY, Simons M. Future targets in endothelial biology: endothelial cell to mesenchymal transition. Curr Drug Targets. 2016;17:1707–1713. doi: 10.2174/1389450117666160502151308. [DOI] [PubMed] [Google Scholar]
- 38.Jackson AO, Zhang J, Jiang Z, Yin K. Endothelial-to-mesenchymal transition: a novel therapeutic target for cardiovascular diseases. Trends Cardiovasc Med. 2017;27:383–393. doi: 10.1016/j.tcm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, Anderson DG, Kotelianski V, Wang F, Tellides G, Simons M. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012;2:1684–1696. doi: 10.1016/j.celrep.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Shi S, Srivastava SP, Kitada M, Nagai T, Nitta K, Kohno M, Kanasaki K, Koya D. FGFR1 is critical for the anti-endothelial mesenchymal transition effect of N-acetyl-seryl-aspartyl-lysyl-proline via induction of the MAP4K4 pathway. Cell Death Dis. 2017;8:e2965. doi: 10.1038/cddis.2017.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun HJ, Cai WW, Gong LL, Wang X, Zhu XX, Wan MY, Wang PY, Qiu LY. FGF-2-mediated FGFR1 signaling in human microvascular endothelial cells is activated by vaccarin to promote angiogenesis. Biomed Pharmacother. 2017;95:144–152. doi: 10.1016/j.biopha.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 42.Onat D, Brillon D, Colombo PC, Schmidt AM. Human vascular endothelial cells: a model system for studying vascular inflammation in diabetes and atherosclerosis. Curr Diab Rep. 2011;11:193–202. doi: 10.1007/s11892-011-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Izani Othman M, Gieseg SP. GW24-e3979 The role of calcium in oxLDL mediated cell death in human monocytes: possible mechanism of atherosclerotic plaque progression. Heart. 2013;99:A89–A90. [Google Scholar]
- 44.Cho S, Hazama M, Urata Y, Goto S, Horiuchi S, Sumikawa K, Kondo T. Protective role of glutathione synthesis in response to oxidized low density lipoprotein in human vascular endothelial cells. Free Radic Biol Med. 1999;26:589–602. doi: 10.1016/s0891-5849(98)00232-9. [DOI] [PubMed] [Google Scholar]
- 45.Riou S, Esposito B, Stengel D, Merval R, Ninio E, Tedgui A, Lehoux S. High pressure promotes monocyte adhesion to the vascular wall through induction of VCAM-1, MCP-1 and IL-6. Vascul Pharmacol. 2006;45:195. doi: 10.1161/01.RES.0000265231.59354.2c. [DOI] [PubMed] [Google Scholar]
- 46.Wu CY, Tang ZH, Jiang L, Li XF, Jiang ZS, Liu LS. PCSK9 siRNA inhibits HUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase3 pathway. Mol Cell Biochem. 2012;359:347–358. doi: 10.1007/s11010-011-1028-6. [DOI] [PubMed] [Google Scholar]
- 47.Bryk D, Olejarz W, Zapolska-Downar D. Mitogen-activated protein kinases in atherosclerosis. Postepy Hig Med Dosw (Online) 2014;68:10–22. doi: 10.5604/17322693.1085463. [DOI] [PubMed] [Google Scholar]
- 48.Jiang DJ, Jia SJ, Dai Z, Li YJ. Asymmetric dimethylarginine induces apoptosis via p38 MAPK/caspase-3-dependent signaling pathway in endothelial cells. J Mol Cell Cardiol. 2006;40:529–539. doi: 10.1016/j.yjmcc.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 49.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wing LY, Chen YC, Shih YY, Cheng JC, Lin YJ, Jiang MJ. Effects of oral estrogen on aortic ROS-generating and -scavenging enzymes and atherosclerosis in apoE-deficient mice. Exp Biol Med (Maywood) 2009;234:1037–1046. doi: 10.3181/0811-RM-332. [DOI] [PubMed] [Google Scholar]
- 51.Forstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 52.Taleb A, Ahmad KA, Ihsan AU, Qu J, Lin N, Hezam K, Koju N, Hui L, Qilong D. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed Pharmacother. 2018;102:689–698. doi: 10.1016/j.biopha.2018.03.140. [DOI] [PubMed] [Google Scholar]
- 53.Xue H, Yan K, Zhao X, Zhu W, Liu L, Xie Z, Zhu H, Chen C. Pretreatment with pPolyHb attenuates H2O2-induced endothelial cell injury through inhibition of JNK/p38 MAPK pathway by upregulation of heme oxygenase-1. Artif Cells Nanomed Biotechnol. 2015;43:163–173. doi: 10.3109/21691401.2014.1001494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.