Abstract
5-hydroxytryptamine receptors 2A and 1A (5-HT2A and 5-HT1A receptors) are most closely related to anxiety-like behavior in post-traumatic stress disorder. This study was aimed at determining how 5-HT2A and 5-HT1A receptors mediate stress-induced anxiety-like behavior. C57BL/6 mice were exposed to conditioned fear stress combined with single-prolonged stress and injected with corresponding antagonists of 5-HT2A or 5-HT1A receptors or DMSO. The established mouse model was used in conjunction with open-field test, freezing behavioral test and elevated plus maze test. Protein expression levels of 5-HT2A and 5-HT1A receptors, ERK1 and ERK2, pERK1, pERK2 and c-Myc in mice hippocampus were evaluated by Western blot analysis and immunofluorescence labeling. Relative mRNA expression levels of 5-HT2A and 5-HT1A receptors, ERK1, ERK2 and c-Myc were analyzed with RT-qRCR. 5-HT2A receptor plays a significant role in anxiety-like behavior by inhibiting 5-HT1A receptor expression. Effect of 5-HT2A and 5-HT1A receptors on stress-related anxiety-like behavior was elicited via ERK1 and ERK2 phosphorylation. On the basis of our experimental results, we hypothesize interaction between 5-HT2A and 5-HT1A receptors in mouse hippocampus to mediate anxiety-like behavior via ERK pathway.
Keywords: 5-HT2A and 5-HT1A receptors, anxiety-like behavior, ERK pathway, hippocampus, post-traumatic stress disorder
Introduction
Anxiety-like behavior in post-traumatic stress disorder (PTSD) is a debilitating condition induced in individuals exposed to severe traumatic events, such as natural disasters and wars. This behavior is characterized by re-experiencing trauma with intrusive memories (flashback), stimulus avoidance symptoms, distressing recollections and hyper-arousal symptoms [1-6]. 84.8% of survivors of the 512 Wenchuan earthquakes in China exhibited anxiety-like behavior between 1 and 2 months following the earthquake event [7]. In United States, lifetime prevalence of traumatic event induced anxiety-like behavior is 8% [7-9]. With increasing incidence of severe traumatic events such as natural and humanitarian disasters, anxiety-like behavior related traumatic stress have significant effect on mental health state of the general population [6,10,11]. Nonetheless, the underlying mechanism of PTSD remains unclear.
Previous studies have suggested serotonergic, GABAergic, glutamatergic and dopaminergic pathways to play a significant role in stress-related mental disorders such as anxiety-like behavior [4,12]. In serotonergic pathways, the 5-hydroxytryptamine (5-HT) receptor family can be classified into 7 major families of receptors (such as 5-HT1-5-HT7) and 14 different subtypes (such as 5-HT2A, 5-HT2B, and 5-HT2C) based on their pharmacological profile and signal transduction mechanism [13]. Whereas 5-HT2A activation promotes or increases anxiety-like behavior, 5-HT1A activation inhibits anxiety-like behavior [14,15].
Does serotonin receptors 2A and 1A have any connection to anxiety-like behavior? By what mechanism if a connection does exist? In this study, conditioned fear (CF) stress and single-prolonged stress (SPS) were implemented in mice; open-field, freezing behavior and elevated plus maze tests were carried out [5,16,17]. Protein expression levels of 5-HT2A and 5-HT1A receptors were evaluated through Western blot analysis and immunofluorescence labeling [18]. mRNA expression levels of 5-HT2A and 5-HT1A receptors, ERK1, ERK2 and c-Myc were examined through RT-qRCR [19].
Materials and methods
Animals
Pathogen-free 6-week old male C57BL/6 mice, provided by the Academy of Life of Medical Sciences in Zhejiang University, were used in this project. Animal feeding was in accordance with National Institutes of Health Guide for Care and Use of Laboratory Animals guidelines and approved by Ethics Committee for Use of Experimental Animals in Zhejiang University. C57BL/6 mice were housed under the following conditions: 12 hour/12 hour light/dark cycle, 21°C ± 1°C, 55% ± 5% humidity and free access to food and water in ventilated racks with plastic housing cages lined with chipped or shaved wood bedding, and with 5 C57BL/6 mice per cage [7,18,20].
Experimental groups and drug administration
Thirty-two C57BL/6 mice were randomly distributed into four groups, each group comprising of eight mice: DMSO (sham) group, CF stress combined with SPS (PTSD) group, CF+SPS+ketanserin (PTSD+K) group and CF+SPS+WAY100635 (PTSD+W) group. Mice were housed for 7 days after being exposed to stress.
5-HT2A-receptor antagonist (Ketanserin) and 5-HT1A-receptor antagonist (WAY10063), were both dissolved in DMSO (Selleck, Selleckchem, Houston, USA). Ketanserin was intraperitoneally injected at a dosage of 0.3 mg/kg [21], and WAY100635 was subcutaneously injected at a dosage of 3 mg/kg [22]. Mice in control group were treated with an equivalent dose of DMSO via same approach as in ketanserin. DMSO was diluted to 10% by 0.9% normal saline [19].
Mouse model establishment
Mouse model was established in accordance with CF+SPS model with slight modifications [5] (Figure 1). Following the respective injection of Ketanserin, WAY100635 and DMSO, mice in PTSD+K, PTSD+W and PTSD groups were exposed to CF. After 5 successive days of conditioned fear stress, mice were subjected to single-prolonged stress. Mice were exposed to foot electric shock on the first day. One mouse was placed in a foot electric shock chamber. After a 60-second adaptation period, bright light was turned on for 10 seconds in the chamber, with the mouse receiving 1 mA shock for 4 seconds. Injection and electric shock were repeated for 5 successive days. On 6th day, mice were subjected to SPS. First, they were individually immobilized for 2 hours in a 50-mL conical tube. Afterwards, mice were subjected to 20 minutes forced swimming arranged in a plastic bucket (40 cm D × 80 cm H, 25°C water temperature, water depth of 30 cm) with one mouse every time. After 15 minutes of rest, mice were anesthetized with isoflurane for 0.5 minutes. Following recuperation, they were housed under the previously described conditions. Sham group was not subjected to CF+SPS.
Figure 1.
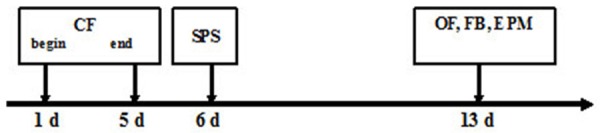
Experimental schedule for mouse model establishment and behavioral test. Following the respective injection of Ketanserin, WAY100635 and DMSO, the mice of PTSD+K group, PTSD+W group, and PTSD group were exposed to CF for 5 consecutive days (started on the 1st day and ended on the 5th day) and progressive single-prolonged stress (SPS) on the 6th day. After CF+SPS was completed, the mice in PTSD+K group, PTSD+W group, PTSD group and sham group were housed for 7 days and subjected to open field [63], freezing behavior (FB) and elevated plus maze (EPM) tests, n = 8 per group.
Open field test
Following CF+SPS procedure, mice were subjected to behavior-sensitized fear test on 13th day. Mice were allowed to adapt in testing room for 1 hour prior to test. In the open-field test, a white acrylic plastic cubic chamber (45 cm × 45 cm × 45 cm) was placed in a soundproof room, which was dimly illuminated with a switchable bright light. During test, each mouse was initially placed in the center of open field arena. Time taken by each mouse to explore center or edges of arena was recorded for 5 minutes using an automatic analyzing system (VideoTrack, Viewpoint Inc., France). Each mouse was tested only once, with the next mouse examined after chamber had been thoroughly cleaned [5,6,14,23,24].
Freezing behavior test
Freezing behavior test was performed in the same set of apparatus as the open field test. Freezing behavior was defined as an immobility of all body movement including head with the exception of respiratory movement. Freezing behavior was scored by observing animals every 10 seconds for 5 minutes using an automatic analyzing system (VideoTrack, Viewpoint Inc., France). Total seconds spent in freezing behavior during each measurement period were recorded and evaluated as a percentage of total time. Each mouse was tested only once, with the next mouse examined after chamber had been thoroughly cleaned with 75% alcohol to avoid carry-over of olfactory cues [16,17,25-27].
Elevated plus maze test
Following completion of open-field test, mice were allowed to rest for 30 minutes and subsequently subjected to elevated plus maze test. Elevated plus maze apparatus consisted of two opposite-facing closed arms (30 cm × 5 cm × 15 cm), two opposite-facing open arms (30 cm × 5 cm) and a central area (5 cm × 5 cm), which were composed of gray plexiglas and raised 50 cm above ground by a base. During test, closed arm was adjoined to wall, with mouse arranged in central area facing open arms. Location was recorded for 5 minutes. Frequency and time a mouse entered or stayed in open and closed arms were recorded using an automatic analyzing system (ANY-maze, Stoelting Inc., USA). The following parameters were scored: number of entries into open arms or closed arms and time spent in open arms or closed arms. An entry was counted only when all paws of mouse entered same arm [5].
Western blot
Mice were killed via overdose of 10% chloral hydrate. Brains were quickly removed, placed immediately in a 10 mL Eppendorf tube at -20°C for 2 hours and stored at -80°C. Hippocampus from one hemisphere of all experimental groups was dissected and separately homogenized in RIPA buffer containing inhibitors (1 mM PMSF, 10 mg·mL-1 aprotinin, 10 mg·mL-1 leupetin, 10 mg·mL-1 pepstatin A, 10 mg·mL-1 antipain, 10 mg·mL-1 chymostatin, and 5 mg·mL-1 trypsin inhibitor; Beyotime Biotechnology, China) and centrifuged at 12,000 rpm 4°C for 20 minutes. Supernatant was preserved at -80°C. Protein concentration was measured by BCA kit (KeyGEN, Nanjing, China) and adjusted to 3 μg/μL prior to conducting Western blot. 10 μL boiled proteins per well with 5 × loading buffer (Beyotime Biotechnology, China) were separated in 12% SDS-PAGE at 70 V for 20 minutes and 100 V for 100 minutes. Samples were then transferred to PVDF membranes (Millipore, Bedford, MA, USA) at 350 mA for 105 minutes. Membranes were blocked with 5% skimmed milk 2 hours at room temperature and incubated with primary antibodies (anti-5-HT1A receptor, rabbit polyclonal antibody, Abcam, 5 μg/mL; anti-5-HT2A receptor, goat polyclonal antibody, Santa Cruz, 1:200; anti-ERK12, rabbit polyclonal antibody, CST, 1:100; anti-pERK12, rabbit polyclonal antibody, CST, 1:2000; anti-c-Myc, mouse polyclonal antibody, Santa Cruz, 1:100; anti-β-actin, rabbit polyclonal antibody, CST, 1:2000) overnight at 4°C, and secondary antibodies (HRP-labeled goat anti-rabbit IgG, HRP-labeled donkey anti-goat IgG, HRP-labeled rabbit anti-mouse IgG, Boster Biological Technology Ltd., 1:8000) for 2 hours at room temperature. Protein samples were visualized using enhanced chemiluminescence detection kits (ECL-Plus, Beyotime Biotechnology, China) in a gel image analysis system (Tanon 2500R, Shanghai, China) [18,19,28-30].
RT-qRCR
Total RNA was isolated using a Trizol kit (Invitrogen, USA). Total RNA was reversely transcribed into cDNA by using the Bestar qPCR RT kit (DBI Bioscience, Germany). Total RNA concentration was adjusted to 100 ng/μL, and reverse transcription procedure was as follows: 15 minutes at 37°C and 5 minutes at 98°C. Specific primers were obtained from Invitrogen (USA; Table 1). cDNA (1 μL) and specific primers (1 μL) were separately added to SYBR Green Mix (DBI Bioscience, Germany). RT Q-PCR (Bio-Rad CFX, USA) was performed for 5 minutes at 95°C, 45 cycles for 10 seconds at 95°C, and 10 seconds at 60°C, 10 seconds at 72°C. mRNA of β-actin was used as an internal control. Relative expression level of target gene was determined by 2-ΔΔCt method: relative expression level of target gene = 2-ΔΔCt, where ΔΔCt = (Ct, X-Ct, β-actin) sample - (Ct, X-Ct, β-actin) control, and X was the target gene [14,19,29,31].
Table 1.
Mouse primer sequences
| Name | Upstream Primer | Downstream Primer |
|---|---|---|
| 5-HTR1A | 5’-TCGCTCACTTGGCTCATTGGCTTT-3’ | 5’-TTCCAACTTCTTGACCGTCTTGCG-3’ |
| 5-HTR2A | 5’-CTGGACCGCTACGTGGCTAT-3’ | 5’-TATGGTCCACACCGCAATGA-3’ |
| ERK1 | 5’-TGGCTTTCTGACGGAGTATG-3’ | 5’-GGTCCAGGTAGTGCTTGC-3’ |
| ERK2 | 5’-CCTCAAGCCTTCCAACCTC-3’ | 5’-GCCCACAGACCAAATATCAATG-3’ |
| c-Myc | 5’-GCTTCCCACCCCGCCCCTGTC-3’ | 5’-CCACCGCCGCCGTCATCGTCTT-3’ |
| β-actin | 5’-GAGACCTTCAACACCCCAGC-3’ | 5’-ATGTCACGCACGATTTCCC-3’ |
Immunofluorescence labeling
Mice were anaesthetized with 10% chloral hydrate, perfused with 0.9% saline and 4% paraformaldehyde. Brains were removed and soaked in a 4% paraformaldehyde solution for 48 hours, transferred to 30% sucrose solution for 72 hours, embedded in O.C.T (Optimal Cutting Temperature) compound (Sakura Finetek, USA) and stored at -80°C. Slices were 16 μm thick. Sections were dried for 40 minutes at 37°C and blocked with 5% normal goat serum for 1 hour at room temperature. Primary antibodies (anti-5-HT1A receptor, rabbit polyclonal antibody, Abcam, 5 μg/mL; anti-5-HT2A receptor, goat polyclonal antibody, Santa Cruz, 1:100; anti-ERK12, rabbit polyclonal antibody, CST, 1:100) and negative control sections were incubated with 0.01 M PBS at 4°C overnight. The next day, slices were washed with 0.01 M PBS for five times and separately incubated with a second antibody (FITC-labeled goat anti-rabbit IgG, FITC-labeled rabbit anti-goat IgG, 1:500, Boster, wuhan, China) for 3 hours at room temperature. Sections were then washed six times, each wash lasting 5 minutes, and then examined through fluorescence detection. Slices were observed by using fluorescence microscope (Olympus BX51, NIKON, Japan) at excitation/emission wavelengths of 550/570 nm (Cy3, red), 492/520 nm (FITC, Green), and 360/460 nm (FITC, blue) [4,5,18,32].
Statistical analysis
Histograms were analyzed by using GraphPad Prism 5. Data are presented as mean ± SEM. Statistical significance was determined through one-way ANOVA with post-hoc Bonferroni’s tests in SPSS 17.0. P < 0.05 was considered statistically significant.
Results
Validation of PTSD mice model
Established animal model was examined by conducting open-field, freezing behavior and elevated plus maze tests. Times through the central grille and total distance of PTSD group significantly decreased compared to sham group (Figure 2A, 2B). Also, Middle dist/Total dist of PTSD group significantly increased compared to sham group (Figure 2C). Times through the central grille and total distance of PTSD+W and Middle dist/Total dist of PTSD+W and PTSD+K groups significantly decreased when compared to PTSD group (Figure 2A-C). These results indicated that anxiety-like behavior was increased after subjected to CF+SPS and blockage of 5-HT1AR.
Figure 2.
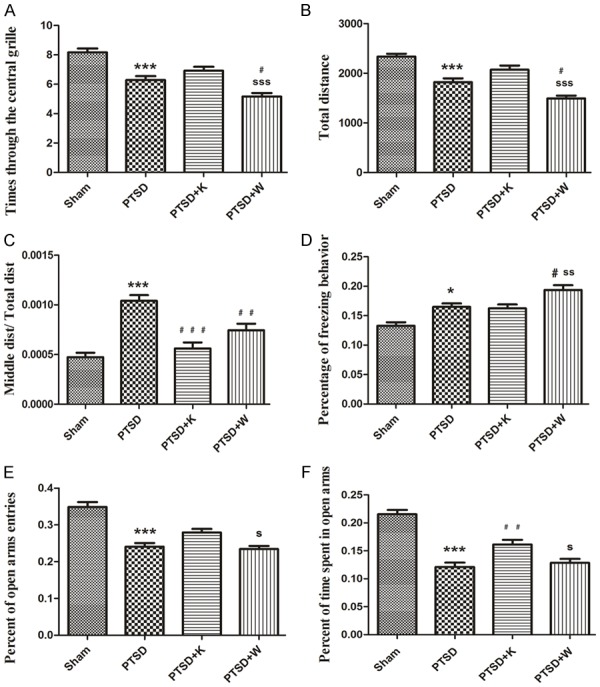
Behavioral experiments in the established mouse model. A. Times through the central grille of the Open-field test (OFT). B. Total distance traveled in the OFT. C. Middle dist/Total dist of the OFT. Middle dist = smldist/lardist, Total dist = smldist + lardist. Lardist denoted the total distance (in cm) covered by the animal in large movements. Smldist denoted the total distance covered by the animal in small movement. D. Freezing behavior test. The percentage of freezing behavior was the time spent in freezing behavior/the total time during each measurement period. E and F. Elevated plus maze test. “Percent of open arms entries” denoted the numbers of entries into the open arms/(the numbers of entries into the open arms + closed arms); “Percent of time spent in open arms” indicated the time spent in the open arms/(the time spent in the open arms + closed arms). Data were presented as mean ± SEM through ANOVA. Groups were compared by conducting Bonferroni’s test, n = 8. *P < 0.05, ***P < 0.001 vs. the sham group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. the PTSD group; sP < 0.05, ssP < 0.01, sssP < 0.001 vs. the PTSD+K group.
Percentage of freezing behavior for PTSD group was enhanced compared to sham group (Figure 2D). The freezing behavior of PTSD+W group was increased in comparison with PTSD group after antagonism of 5-HT1AR (Figure 2D). In elevated plus maze test, percentages of open arms entries and time spent in open arms of PTSD group were significantly decreased when compared to those of sham group (Figure 2E, 2F). Percent of open arms entries and time spent in open arms of PTSD+W group was notably lower than PTSD+K group (Figure 2E, 2F).
Protein expression levels
Western blot analysis was performed to quantify expressional levels of 5-HT2A and 5-HT1A receptors, ERK1, ERK2, pERK1, pERK2 and c-Myc (Figure 3A). Blots were quantified and normalized to corresponding internal control. After CF+SPS procedure, expressions of 5-HT2AR and 5-HT1AR both augmented in PTSD group (Figure 3B, 3C). 5-HT1AR antagonist WAY100635 promoted, whereas 5-HT2AR antagonist ketanserin decreased 5-HT2AR protein levels in mouse hippocampus (Figure 3B). As for 5-HT1AR, WAY100635 suppressed its expression although 5-HT2AR antagonist ketanserin had no significant effect on it (Figure 3C). 5-HT1AR antagonist WAY100635 remarkably increased expression of ERK1/2, pERK1/2 and c-Myc, which might suggest that the inhibited 5-HT1AR by its antagonist WAY100635 may somehow activated the 5-HT2AR and its downstream ERK and c-Myc pathway (Figure 3D-H). However, inhibition of 5-HT2AR did not affect the expressions of either ERK1/2 or pERK1/2 (Figure 3D-G).
Figure 3.
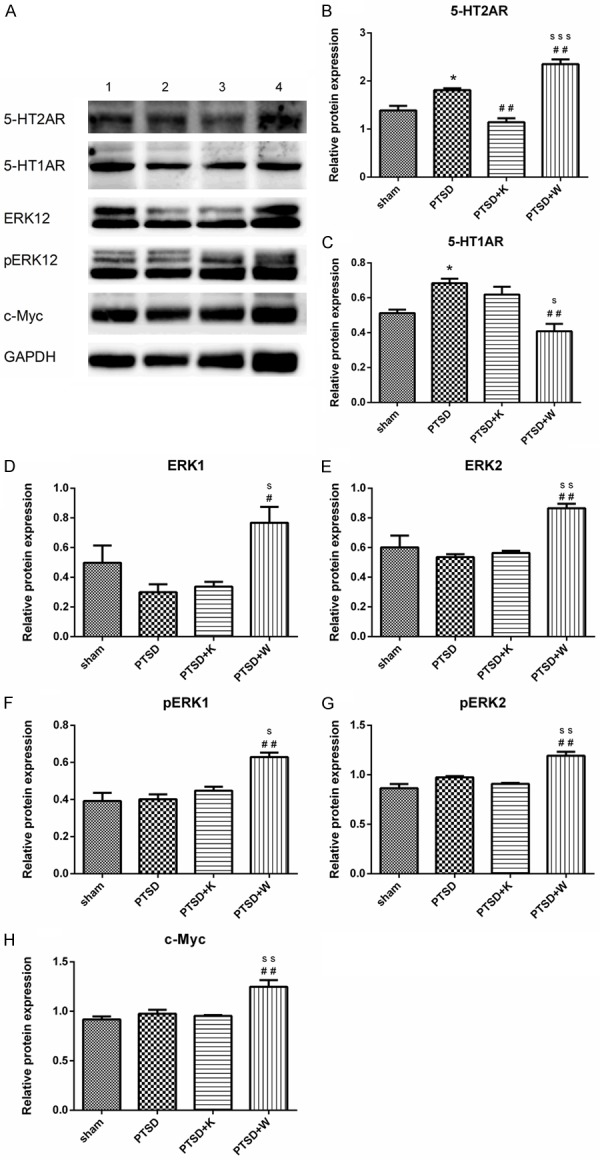
Western blot indicated the protein expression level in the mouse hippocampus. A. Lane 1, 2, 3, and 4 represent the protein expression levels in the sham group, PTSD group, PTSD+K group and PTSD+W group, respectively. B-H denotes the relative protein expression levels of 5-HT2A receptor, 5HT1A receptor, ERK1, ERK2, pERK1, pERK2, and c-Myc in the four experimental groups. Data were presented as mean ± SEM through ANOVA. Groups were compared by performing Bonferroni’s test, n = 4. *P < 0.05 vs. the sham group; #P < 0.05, ##P < 0.01 vs. the PTSD group; sP < 0.05, ssP < 0.01, sssP < 0.001 vs. the PTSD+K group.
mRNA expression levels
Relative mRNA expression levels of 5-HT2AR and 5-HT1AR, ERK1, ERK2, and c-Myc were analyzed through RT Q-PCR. In line with the Western blot results, the relative mRNA expression levels of 5-HT2A and 5-HT1A receptors were increased and decreased respectively by 5-HT1AR antagonist compared to PTSD group (Figure 4A, 4B). It is intriguing that 5-HT2AR antagonist ketanserin has a similar effect on the mRNA expression levels of 5-HT2A and 5-HT1A receptors as WAY100635 (Figure 4A, 4B). However, inconsistent results of protein and mRNA levels of 5-HT2AR in PTSD+K group may attribute to increased degradation (Figures 3B, 4A). As shown in Figure 4C and 4E, mRNA levels of ERK1 and c-Myc were notably enhanced by the stress procedure, while 5-HT2AR antagonist significantly reduced this effect. Also, mRNA levels of ERK1 and c-Myc were significantly higher in PTSD+W group compared with PTSD+K group (Figure 4C, 4E). There was no significant difference of ERK2 between these four groups in mRNA levels (Figure 4D).
Figure 4.
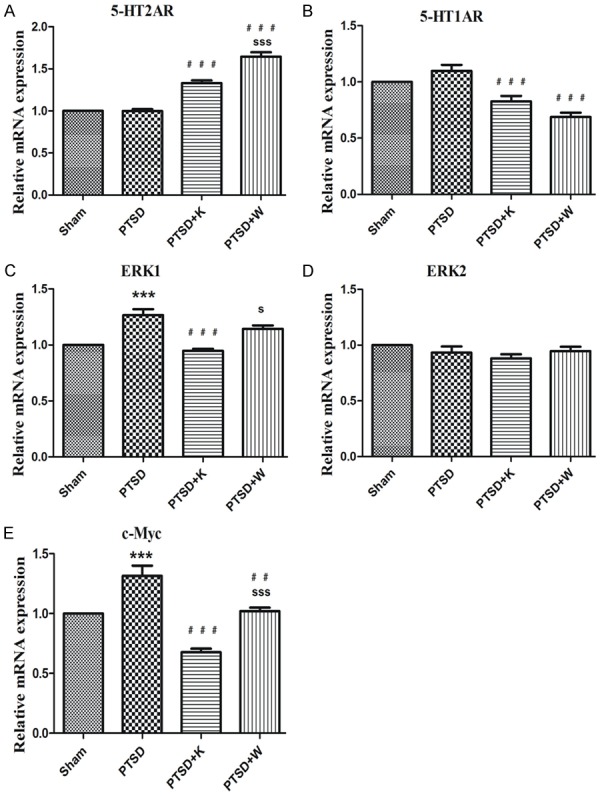
RT-qPCR showed the mouse hippocampus comparison regarding the relative mRNA expression levels of the 5-HT2A receptor (A), 5-HT1A receptor (B), ERK1 (C), ERK2 (D), and c-Myc (E) to those in the control group. Data were presented as mean ± SEM through ANOVA. Groups were compared by performing Bonferroni’s test, n = 4. ***P < 0.001 vs. the sham group; ##P < 0.01, ###P < 0.001 vs. the PTSD group; sP < 0.05, sssP < 0.001 vs. the PTSD+K group.
Immunofluorescence analysis
Immunofluorescence staining was applied to investigate expression patterns of 5-HT2A and 5-HT1A receptors as well as ERK1/2 in the subfields of the hippocampus. Fluorescence images of 5-HT2AR, 5-HT1AR, and ERK1/2 (green channel) are respectively shown in Figure 5A1, 5B1 and 5C1. Quantification of positive cell number was carried out in five random fields per slice using Image-Pro Plus image analysis software (Media Cybernetics) (Figure 5A2, 5B2, 5C2). The stress procedure markedly increased 5-HT2AR and 5-HT1AR expressions in PTSD group compared to sham group (Figure 5A2, 5B2). Both 5-HT1AR and 5-HT2AR antagonist significantly reversed the increase of 5-HT1AR caused by stress procedure (Figure 5A2). Also, 5-HT2AR expression in hippocampus was significantly increased by 5-HT1AR antagonist in comparison to PTSD and PTSD+K groups (Figure 5B2). PTSD can increase ERK1/2 expression compared to sham group, while inhibition of 5-HT2AR suppressed, whereas 5-HT1AR antagonist promoted, the expression of ERK1/2 (Figure 5C2).
Figure 5.
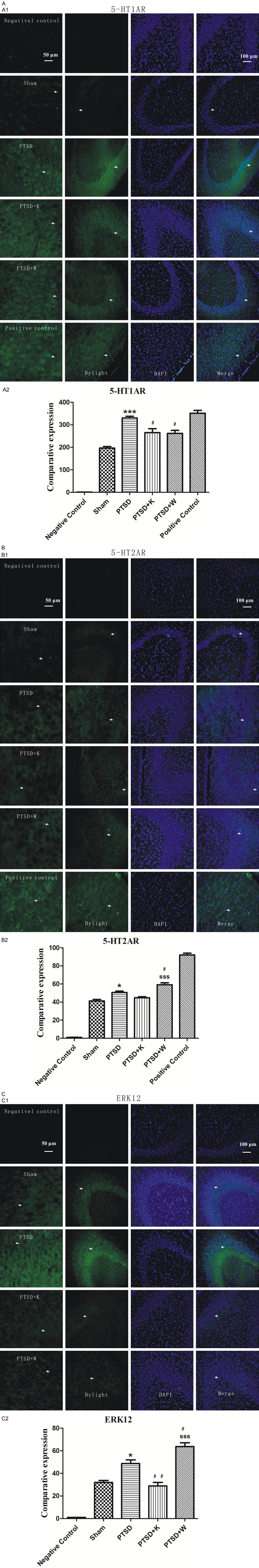
Immunofluorescence labeling revealed the comparative protein expression levels of 5-HT2A receptor, 5-HT1A receptor, ERK1, and ERK2 in the dentate gyrus of the mouse hippocampus. A1, B1, and C1 showed immunofluorescence images of 5-HT1AR, 5-HT2AR and ERK1/2, respectively. A2, B2, and C2 showed quantification analysis of 5-HT1AR, 5-HT2AR and ERK1/2 positive cells, respectively. Positive cells were indicated by white arrows. Bar = 50, 100 μm. Data were presented as mean ± SEM through ANOVA. Groups were compared by performing Bonferroni’s test. n = 4. *P < 0.05, ***P < 0.001 vs. the sham group; #P < 0.05, ##P < 0.01 vs. the PTSD group; sssP < 0.001 vs. the PTSD+K group.
Discussion
When exposed to severe traumatic stress, individuals are likely to display behavioral alterations, including anxiety-like behavior, which is related to serotonin (5-HT) [33-35]. 5-HT2A and 5-HT1A receptors involved in behavioral responses are expressed in the raphe nuclei, the frontal cortex and the hippocampus [34]. On the basis of the contribution of serotonin to emotional behavior, at least 13 different subtypes of serotonin receptors, including 5-HT2A and 5-HT1A receptors, which are closely associated with anxiety-like behaviors have been reported [36-39]. In PTSD-related animal models, 5-HT2A and 5-HT1A receptors are expressed in cerebral cortex, hippocampus, amygdala and brain stem. 5-HT2A and 5-HT1A receptors have been reported to be involved in the occurrence of stress-induced psychiatric symptoms such as anxiety-like and depressive behaviors [28,40]. In the hippocampus, 5-HT1A receptors play an important role in anti-depressant mechanism of individuals affected by traumatic stress [41-43]. In a study on lipopolysaccharide-induced shock in mice, 5-HT2A receptor elicited its effect via ERK pathway [44]. Paliperidone stimulates 5-HT2A receptor to induce ERK sensitization [45]. In non-neuronal cells, 5-HT1A receptors activate ERK through phosphorylation [46]. In this study, our results demonstrated that the 5-HT2A and 5-HT1A receptors in the mouse hippocampus were related to anxiety-like behavior via ERK pathway.
Post-traumatic stress disorder (PTSD) is a common psychosomatic disorder that is characterized by its delayed onset and persistence of symptoms after a traumatic experience. In order to better understand this disorder, several animal models have been proposed, including single-prolonged stress, foot shock, and social stress [47]. Although some symptoms of these animal models closely mimic those of PTSD, it is difficult to comprehensively assess the behavioral and physiological changes of PTSD. SPS can cause enhanced inhibition of the hypothalamic-pituitary-adrenal (HPA) axis, which can be observed in most PTSD patients [48]. However, it’s difficult to apply this model to study the exaggerated fear responses caused by persistent trauma [49]. By combining SPS with CF, we can comprehensively assess the psychological and behavioral changes of PTSD.
Thus, our animal model of PTSD was established by CF stress combined with SPS [5]. We applied two antagonists, ketanserin and WAY100635, to determine the effect of the 5-HT2A and 5-HT1A receptors on anxiety-like behavior [22,50]. After the CF+SPS procedure, the mice of PTSD group showed significantly increased anxiety-like behavior, indicated by decreased times through the central grille and total distance of open field test (Figure 2A, 2B), decreased open arms entries as well as time spent in open arms of elevated plus maze test (Figure 2E, 2F), increased freezing behavior (Figure 2D) and Middle dist/Total dist (Figure 2C). After inhibition of 5-HT1AR, the anxiety-like behavior notably increased in PTSD+W group compared with PTSD group (Figure 2A, 2B, and 2D). However, after inhibition of 5-HT2AR, the mice of PTSD+K group showed no statistical difference in most tests compared with PTSD group (Figure 2A, 2B, 2D and 2E). The elevated plus maze and open-field tests exhibit a different sensitivity on anxiety, as such, these tests fail to yield similar results [52]. On the basis of these results, we concluded that PTSD mouse model was successfully established via CF+SPS procedures, and the anxiety-like behavior was aggravated by 5-HT1AR antagonist WAY100635, but 5-HT2AR antagonist ketanserin was incapable of changing the anxiety-like behavior caused by CF+SPS.
Western blot and immunofluorescence labeling results demonstrated a remarkable elevation in 5-HT2AR and 5-HT1AR protein expressions in PTSD group when compared to that of sham group (Figures 3A-C, 5A1, 5A2, 5B1, 5B2). However, the mRNA of 5-HT2AR and 5-HT1AR in PTSD group showed no statistical change compared with sham group (Figure 4A, 4B). These results indicated that the overexpression of 5-HT2AR and 5-HT1AR after stress procedure may be due to increased translation efficiency and mRNA stability. When compared to PTSD group, the inhibition of 5-HT1AR resulted in a significant decrease in 5-HT1AR and increase in 5-HT2AR in both protein and mRNA levels (Figures 3A-C, 4A, 4B, 5A1, 5B1, 5A2, 5B2). Nevertheless, inhibition of 5-HT2AR only significantly decreased its protein level but didn’t influence the expression of 5-HT1AR (Figure 3A-C). The inconsistent results of western blot and immunofluorescence might be due to the following reason: the fluorescence images were taken from the dentate gyrus of hippocampus, while the protein was isolated from the whole hippocampus. Thus, ketanserin might have different effects on 5-HT1AR expression of different hippocampus regions (Figures 3C, 5A2). These results confirmed that 5-HT2A receptor plays a significant role in anxiety-like behavior by inhibiting 5-HT1A receptor expression. Similar findings have been described by Leonard [33].
Previous studies have demonstrated in different cell lines that ERK is an important downstream molecular of 5-HT2AR [57-59]. Also, the phosphorylation of ERK1/2 is essential for 5-HT2AR downstream signal transduction both in vivo and in vitro [44,57,60,61]. In our study, both western blot and immunofluorescence results revealed dramatically increased expressions of ERK1/2, pERK1/2 and c-Myc in PTSD+W group compared to PTSD group (Figures 3D-H, 5C2). These results evinced that 5-HT2A receptor affected stress-related anxiety-like behavior by activating the ERK-cMyc pathway via the phosphorylation of both ERK1 and ERK2.
On the basis of our experiments, we hypothesize that 5-HT1AR antagonist (WAY100635) can increase the anxiety-like behavior of PTSD mice. The function of WAY100635 is mediated by inhibiting 5-HT1AR expression and promoting 5-HT2AR expression. Increase of 5-HT2AR further mediates the phosphorylation of ERK and activates ERK-cMyc pathway. Further studies are however needed as our study had some limitations. For instance, the role of 5-HT2AR antagonist in PTSD mouse model is still not clear. Moreover, 5-HT2A and 5-HT1A receptors can potentially be involved in other signaling pathways that can affect anxiety-like behavior. To elucidate the temporal effect of the related protein levels, we discussed in another paper that experiments was conducted 21 days after the model was established [62]. We do intend to probe the mRNA and protein expression levels of Bax, Bcl-2, Caspase-3, Beclin-1, and LC-3 and hope to determine other mechanisms underlying 5-HT2A and 5-HT1A receptors mediated anxiety-like behaviors.
Acknowledgements
The authors wish to thank Professor Xiaodong Wang and Professor Shucai Ling for their insightful comments on the paper, Henry Davies, from Zhejiang University School of Medicine, for his assistance in the language editing as well as Sanhua Fang and Daohui zhang from the Core Facilities of Zhejiang University Institute of Neuroscience for their technical assistance.
Disclosure of conflict of interest
None.
References
- 1.Rabe-Jablonska J, Bienkiewicz W. [Anxiety disorders in the fourth edition of the classification of mental disorders prepared by the American Psychiatric Association: diagnostic and statistical manual of mental disorders (DMS-IV -- options book] . Psychiatr Pol. 1994;28:255–268. [PubMed] [Google Scholar]
- 2.Harvey BH, Naciti C, Brand L, Stein DJ. Endocrine, cognitive and hippocampal/cortical 5HT1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res. 2003;983:97–107. doi: 10.1016/s0006-8993(03)03033-6. [DOI] [PubMed] [Google Scholar]
- 3.Clapp JD, Beck JG, Palyo SA, Grant DM. An examination of the synergy of pain and PTSD on quality of life: additive or multiplicative effects? Pain. 2008;138:301–309. doi: 10.1016/j.pain.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Wang HT, Han F, Shi YX. Activity of 5-HT1A receptor is involved in neuronal apoptosis of the amygdala in a rat model of post-traumatic stress disorder. Mol Med Rep. 2011;4:291–295. doi: 10.3892/mmr.2011.415. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Zuo D, He B, Qiao F, Zhao M, Wu Y. Conditioned fear stress combined with single-prolonged stress: a new PTSD mouse model. Neurosci Res. 2012;73:142–152. doi: 10.1016/j.neures.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Peng Z, Zhang R, Wang H, Chen Y, Xue F, Wang L, Yang F, Liu L, Kuang F, Tan Q. Ziprasidone ameliorates anxiety-like behaviors in a rat model of PTSD and up-regulates neurogenesis in the hippocampus and hippocampus-derived neural stem cells. Behav Brain Res. 2013;244:1–8. doi: 10.1016/j.bbr.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Xie H, Han F, Shi X. Single-prolonged stress induce changes of CaM/CaMKIIalpha in the rats of dorsal raphe nucleus. Neurochem Res. 2012;37:1043–1049. doi: 10.1007/s11064-012-0705-5. [DOI] [PubMed] [Google Scholar]
- 8.Vieweg WV, Julius DA, Fernandez A, Beatty-Brooks M, Hettema JM, Pandurangi AK. Posttraumatic stress disorder: clinical features, pathophysiology, and treatment. Am J Med. 2006;119:383–390. doi: 10.1016/j.amjmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Ma X, Liu X, Hu X, Qiu C, Wang Y, Huang Y, Wang Q, Zhang W, Li T. Risk indicators for post-traumatic stress disorder in adolescents exposed to the 5.12 Wenchuan earthquake in China. Psychiatry Res. 2011;189:385–391. doi: 10.1016/j.psychres.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Trickey D, Siddaway AP, Meiser-Stedman R, Serpell L, Field AP. A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clin Psychol Rev. 2012;32:122–138. doi: 10.1016/j.cpr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto Y, Morinobu S, Yamamoto S, Matsumoto T, Takei S, Fujita Y, Yamawaki S. Vorinostat ameliorates impaired fear extinction possibly via the hippocampal NMDA-CaMKII pathway in an animal model of posttraumatic stress disorder. Psychopharmacology (Berl) 2013;229:51–62. doi: 10.1007/s00213-013-3078-9. [DOI] [PubMed] [Google Scholar]
- 13.Derangeon M, Bozon V, Defamie N, Peineau N, Bourmeyster N, Sarrouilhe D, Argibay JA, Herve JC. 5-HT4 and 5-HT2 receptors antagonistically influence gap junctional coupling between rat auricular myocytes. J Mol Cell Cardiol. 2010;48:220–229. doi: 10.1016/j.yjmcc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. Decreased expression of serotonin 1A receptor in the dentate gyrus in association with chronic mild stress: a rat model of post-stroke depression. Psychiatry Res. 2009;170:245–251. doi: 10.1016/j.psychres.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Ursano RJ, Zhang L, Li H, Johnson L, Carlton J, Fullerton CS, Benedek DM. PTSD and traumatic stress from gene to community and bench to bedside. Brain Res. 2009;1293:2–12. doi: 10.1016/j.brainres.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Li XM, Su F, Ji MH, Zhang GF, Qiu LL, Jia M, Gao J, Xie Z, Yang JJ. Disruption of hippocampal neuregulin 1-ErbB4 signaling contributes to the hippocampus-dependent cognitive impairment induced by isoflurane in aged mice. Anesthesiology. 2014;121:79–88. doi: 10.1097/ALN.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen H, Kaplan Z, Koresh O, Matar MA, Geva AB, Zohar J. Early post-stressor intervention with propranolol is ineffective in preventing posttraumatic stress responses in an animal model for PTSD. Eur Neuropsychopharmacol. 2011;21:230–240. doi: 10.1016/j.euroneuro.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Gao K, Hu Z, Li W, Davies H, Ling S, Rudd JA, Fang M. Autophagy upregulation and apoptosis downregulation in DAHP and triptolide treated cerebral ischemia. Mediators Inflamm. 2015;2015:120198. doi: 10.1155/2015/120198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lairez O, Calise D, Bianchi P, Ordener C, Spreux-Varoquaux O, Guilbeau-Frugier C, Escourrou G, Seif I, Roncalli J, Pizzinat N, Galinier M, Parini A, Mialet-Perez J. Genetic deletion of MAO-A promotes serotonin-dependent ventricular hypertrophy by pressure overload. J Mol Cell Cardiol. 2009;46:587–595. doi: 10.1016/j.yjmcc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Zhang GF, Song SW, Cai GJ, Liu WH, Miao CY, Su DF. Effects of ketanserin on endotoxic shock and baroreflex function in rodents. J Infect Dis. 2011;204:1605–1612. doi: 10.1093/infdis/jir609. [DOI] [PubMed] [Google Scholar]
- 22.Dill MJ, Shaw J, Cramer J, Sindelar DK. 5-HT1A receptor antagonists reduce food intake and body weight by reducing total meals with no conditioned taste aversion. Pharmacol Biochem Behav. 2013;112:1–8. doi: 10.1016/j.pbb.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Cunha JM, Masur J. Evaluation of psychotropic drugs with a modified open field test. Pharmacology. 1978;16:259–267. doi: 10.1159/000136777. [DOI] [PubMed] [Google Scholar]
- 24.Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S, Preisig M, Otowa T, Kendler KS, Chen X, Hettema JM, van den Oord EJ, Rubio JP, Guarente L. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ter Horst JP, Carobrez AP, van der Mark MH, de Kloet ER, Oitzl MS. Sex differences in fear memory and extinction of mice with forebrain-specific disruption of the mineralocorticoid receptor. Eur J Neurosci. 2012;36:3096–3102. doi: 10.1111/j.1460-9568.2012.08237.x. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima A, Saigusa D, Tetsu N, Yamakuni T, Tomioka Y, Hishinuma T. Neurobehavioral effects of tetrabromobisphenol a, a brominated flame retardant, in mice. Toxicol Lett. 2009;189:78–83. doi: 10.1016/j.toxlet.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Verma M, Bali A, Singh N, Jaggi AS. Investigating the role of nisoldipine in foot-shock-induced post-traumatic stress disorder in mice. Fundam Clin Pharmacol. 2016;30:128–136. doi: 10.1111/fcp.12174. [DOI] [PubMed] [Google Scholar]
- 28.Pilar-Cuellar F, Vidal R, Pazos A. Subchronic treatment with fluoxetine and ketanserin increases hippocampal brain-derived neurotrophic factor, beta-catenin and antidepressant-like effects. Br J Pharmacol. 2012;165:1046–1057. doi: 10.1111/j.1476-5381.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HT, Han F, Shi YX. Activity of the 5-HT1A receptor is involved in the alteration of glucocorticoid receptor in hippocampus and corticotropin-releasing factor in hypothalamus in SPS rats. Int J Mol Med. 2009;24:227–31. doi: 10.3892/ijmm_00000225. [DOI] [PubMed] [Google Scholar]
- 30.Xiang M, Zhou W, Gao D, Fang X, Liu Q. Inhibitor of apoptosis protein-like protein-2 as a novel serological biomarker for breast cancer. Int J Mol Sci. 2012;13:16737–16750. doi: 10.3390/ijms131216737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu D, Xiao B, Han F, Luo F, Wang E, Shi Y. Changes in 5-HT1A receptor expression in the oculomotor nucleus in a rat model of post-traumatic stress disorder. J Mol Neurosci. 2013;49:360–368. doi: 10.1007/s12031-012-9874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing S, Zhang Y, Li J, Zhang J, Li Y, Dang C, Li C, Fan Y, Yu J, Pei Z, Zeng J. Beclin 1 knockdown inhibits autophagic activation and prevents the secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy. 2012;8:63–76. doi: 10.4161/auto.8.1.18217. [DOI] [PubMed] [Google Scholar]
- 33.Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20(Suppl 3):S302–6. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 34.Bordukalo-Niksic T, Mokrovic G, Stefulj J, Zivin M, Jernej B, Cicin-Sain L. 5HT-1A receptors and anxiety-like behaviours: studies in rats with constitutionally upregulated/downregulated serotonin transporter. Behav Brain Res. 2010;213:238–245. doi: 10.1016/j.bbr.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Blazevic S, Colic L, Culig L, Hranilovic D. Anxiety-like behavior and cognitive flexibility in adult rats perinatally exposed to increased serotonin concentrations. Behav Brain Res. 2012;230:175–181. doi: 10.1016/j.bbr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 37.Deakin J. The role of serotonin in depression and anxiety. Eur Psychiatry. 1998;13(Suppl 2):57s–63s. doi: 10.1016/S0924-9338(98)80015-1. [DOI] [PubMed] [Google Scholar]
- 38.Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl) 2010;212:13–23. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- 39.Hu X, Li Y, Hu Z, Rudd JA, Ling S, Jiang F, Davies H, Fang M. The alteration of 5-HT(2A) and 5-HT(2C) receptors is involved in neuronal apoptosis of goldfish cerebellum following traumatic experience. Neurochem Int. 2012;61:207–218. doi: 10.1016/j.neuint.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, Madronal N, Donaldson ZR, Drew LJ, Dranovsky A, Gross CT, Tanaka KF, Hen R. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci. 2015;18:1606–1616. doi: 10.1038/nn.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci U S A. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shishkina GT, Kalinina TS, Dygalo NN. Effects of swim stress and fluoxetine on 5-HT1A receptor gene expression and monoamine metabolism in the rat brain regions. Cell Mol Neurobiol. 2012;32:787–794. doi: 10.1007/s10571-012-9828-0. [DOI] [PubMed] [Google Scholar]
- 43.Burke TF, Advani T, Adachi M, Monteggia LM, Hensler JG. Sensitivity of hippocampal 5-HT1A receptors to mild stress in BDNF-deficient mice. Int J Neuropsychopharmacol. 2013;16:631–645. doi: 10.1017/S1461145712000466. [DOI] [PubMed] [Google Scholar]
- 44.Liu C, Zhang X, Zhou JX, Wei W, Liu DH, Ke P, Zhang GF, Cai GJ, Su DF. The protective action of ketanserin against lipopolysaccharide-induced shock in mice is mediated by inhibiting inducible no synthase expression via the MEK/ERK pathway. Free Radic Biol Med. 2013;65:658–666. doi: 10.1016/j.freeradbiomed.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, An S, Liu Y, Guo XX, Gao L, Wei JF, Xu TR. Novel serotonin receptor 2 (5-HT2R) agonists and antagonists: a patent review (2004-2014) Expert Opin Ther Pat. 2016;26:89–106. doi: 10.1517/13543776.2016.1113257. [DOI] [PubMed] [Google Scholar]
- 46.Lacivita E, Di Pilato P, De Giorgio P, Colabufo NA, Berardi F, Perrone R, Leopoldo M. The therapeutic potential of 5-HT1A receptors: a patent review. Expert Opin Ther Pat. 2012;22:887–902. doi: 10.1517/13543776.2012.703654. [DOI] [PubMed] [Google Scholar]
- 47.Borghans B, Homberg JR. Animal models for posttraumatic stress disorder: an overview of what is used in research. World J Psychiatry. 2015;5:387–396. doi: 10.5498/wjp.v5.i4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohda K, Harada K, Kato K, Hoshino A, Motohashi J, Yamaji T, Morinobu S, Matsuoka N, Kato N. Glucocorticoid receptor activation is involved in producing abnormal phenotypes of single-prolonged stress rats: a putative post-traumatic stress disorder model. Neuroscience. 2007;148:22–33. doi: 10.1016/j.neuroscience.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Zuo D, He B, Qiao F, Zhao M, Wu Y. Conditioned fear stress combined with single-prolonged stress: a new PTSD mouse model. Neurosci Res. 2012;73:142–152. doi: 10.1016/j.neures.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Chen L, Chen G, Guo Y, Liu L, Xiao L, Fan W, Shi B, Qian Y. Ketanserin, a serotonin 2A receptor antagonist, alleviates ischemia-related biliary fibrosis following donation after cardiac death liver transplantation in rats. Liver Transpl. 2014;20:1317–1326. doi: 10.1002/lt.23947. [DOI] [PubMed] [Google Scholar]
- 51.Zhang LM, Zhou WW, Ji YJ, Li Y, Zhao N, Chen HX, Xue R, Mei XG, Zhang YZ, Wang HL, Li YF. Anxiolytic effects of ketamine in animal models of posttraumatic stress disorder. Psychopharmacology (Berl) 2015;232:663–672. doi: 10.1007/s00213-014-3697-9. [DOI] [PubMed] [Google Scholar]
- 52.Ennaceur A, Chazot PL. Preclinical animal anxiety research-flaws and prejudices. Pharmacol Res Perspect. 2016;4:e00223. doi: 10.1002/prp2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Garcia AL, Meng Q, Richardson-Jones J, Dranovsky A, Leonardo ED. Disruption of 5-HT1A function in adolescence but not early adulthood leads to sustained increases of anxiety. Neuroscience. 2016;321:210–221. doi: 10.1016/j.neuroscience.2015.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of n-methyl-D-aspartate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem. 2008;283:17194–17204. doi: 10.1074/jbc.M801713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Paula BB, Leite-Panissi CR. Distinct effect of 5-HT1A and 5-HT2A receptors in the medial nucleus of the amygdala on tonic immobility behavior. Brain Res. 2016;1643:152–8. doi: 10.1016/j.brainres.2016.04.073. [DOI] [PubMed] [Google Scholar]
- 57.Kurrasch-Orbaugh DM, Parrish JC, Watts VJ, Nichols DE. A complex signaling cascade links the serotonin 2A receptor to phospholipase A2 activation: the involvement of MAP kinases. J Neurochem. 2003;86:980–991. doi: 10.1046/j.1471-4159.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- 58.Oufkir T, Vaillancourt C. Phosphorylation of JAK2 by serotonin 5-HT (2A) receptor activates both STAT3 and ERK1/2 pathways and increases growth of JEG-3 human placental choriocarcinoma cell. Placenta. 2011;32:1033–1040. doi: 10.1016/j.placenta.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Marinova Z, Walitza S, Grunblatt E. 5-HT2A serotonin receptor agonist DOI alleviates cytotoxicity in neuroblastoma cells: role of the ERK pathway. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:64–72. doi: 10.1016/j.pnpbp.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 60.Gooz M, Gooz P, Luttrell LM, Raymond JR. 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J Biol Chem. 2006;281:21004–21012. doi: 10.1074/jbc.M512096200. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y, Tian H, Yan X, Fan F, Wang W, Han J. Serotonin inhibits apoptosis of pulmonary artery smooth muscle cells through 5-HT2A receptors involved in the pulmonary artery remodeling of pulmonary artery hypertension. Exp Lung Res. 2013;39:70–79. doi: 10.3109/01902148.2012.758191. [DOI] [PubMed] [Google Scholar]
- 62.Xiang M, Jiang Y, Hu Z, Yang Y, Botchway BO, Fang M. Stimulation of anxiety-like behavior via ERK pathway by competitive serotonin receptors 2A and 1A in post-traumatic stress disordered mice. Neurosignals. 2017;25:39–53. doi: 10.1159/000481791. [DOI] [PubMed] [Google Scholar]
- 63.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]


