Abstract
Evidence has indicated the important roles of long non-coding RNAs (lncRNAs) in the human cancer biology, providing potential targets for cancer intervention. However, the expression profile and function of lncRNA TP73-AS1 in human epithelial ovarian cancer (EOC) remain to be investigated. In the EOC specimens and cell lines, TP73-AS1 was identified to be significantly up-regulated. EOC patients with high TP73-AS1 expression had poor survival rate. The gain and loss of functional assay uncovered that TP73-AS1 promoted the proliferation, invasion and reduced the apoptosis of EOC cells in vitro. And, the knockdown of TP73-AS1 inhibited the tumor growth in vivo. By using chromatin immunoprecipitation and luciferase reporter assay, we identified TP73-AS1 epigenetically repressed p21 via recruiting EZH2. In conclusion, this finding supports that TP73-AS1 promotes the EOC progression via epigenetically repressing p21, serving as a prognosis predictor for EOC patients.
Keywords: Epithelial ovarian cancer, TP73-AS1, p21, EZH2
Introduction
Worldwide, ovarian cancer has been the most frequent malignant gynecological tumors with more than 20,000 newly diagnosed patients every year, especially epithelial ovarian cancer (EOC) [1,2]. The mainstream therapeutic method for the EOC is surgical excision, and more subsequent therapies were replenished to prevent recurrence, such as chemotherapy and radiotherapy [3,4]. Even though these attempts, the clinical outcome is still not optimistic. Therefore, it is urgently required to unveil the deepgoing molecular mechanism underlying EOC to discover the tumorigenesis.
Long noncoding RNAs (lncRNAs) are a vital member in the noncoding RNA family accompanied by microRNA, 20-22 nt in length, and circular RNA, a covalent closed circular loop [5,6]. LncRNAs are a series of nucleotide sequence with over 200 nucleotides in length and amounting roles in human cancers. For example, lncRNA LINC00460 expression is upregulated in EOC tissues and cells compared with that in adjacent tissues and cells, and LINC00460 exerts the oncogenic roles in EOC through binding miR-338-3p [7]. LncRNA CASC2 is found to be downregulated in EOC, and it could be able to act as a tumor suppressor and is an independent risk factor for poor prognosis [8]. Thus, these literature could indicate the emerging role of lncRNA in the EOC.
The lncRNA TP73-AS1 is reported to be oncogene in multiple human cancers, such as osteosarcoma, bladder cancer and clear cell renal cell carcinoma [9-11]. In the EOC, we found that TP73-AS1 was up-regulated in the EOC tissue and cells, and then investigated its roles on the EOC tumor phenotype, showing its functions and playing vital functions in biological processes.
Materials and methods
Patients and tissue samples
EOC tumor tissues samples were obtained from the patients who underwent surgical resection therapy at the Shandong Provincial Hospital affiliated to Shandong University, which were immediately frozen at -80°C for further RNA isolation. And, the Ethics Committee has approved all these experimental process. Written informed consents were obtained from all patients.
Cell lines
Epithelial ovarian cancer cell lines (SKOV3, CAOV3, HO8910, OV420) and normal human ovarian epithelial cell line (HOSEPiC) were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI 1640 medium supplemented with 1% penicillin/streptomycin (Invitrogen, Carlsbad, USA) and 10% FBS (fetal bovine serum, Gibco, Life Technologies, USA).
The cultured atmosphere was set at 37°C with 5% CO2.
Silencing of RNA
siRNA for TP73-AS1 and p21 synthesized by GenePharma (Shanghai, China). EOC cells were transfected with TP73-AS1 or p21 siRNA using were transiently transfected using Lipofectamine RNAiMax (Invitrogen, USA) respectively according to the manufacturer’s protocol.
Real-time PCR
For the RT-PCR, total RNA was isolated from EOC cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was reversely transcribed from total RNA using the PrimeScript RT reagent Kit (Takara, Japan). RNA or genes’ level were determined using a standard SYBR-Green on the ABI7500 Real-Time PCR System (Applied Biosystems, Waltham, MA). The relative data was normalized to that of GAPDH using 2-ΔΔCt method. The primer sequences were presented as in Table S1.
Western blotting
For western blot analysis, total protein was extracted from cells samples using the RIPA lysis buffer (Beyotime, Jiangsu, China) as previously described. Protein sample (60 μg) was transfered to 15% SDS-polyacrylamide gel (SDS-PAGE). After electrophoresis using the PVDF blotting membrane (Life Science), the member was probed with primary antibodies, including anti-p21, anti-EZH2 antibodies (Abcam Inc., USA), and anti-GAPDH as the internal control. The immunoreactivity was measured using the Odyssey infrared imaging system (LI-COR, Inc., Lincoln, NE, USA) for the band intensity.
Proliferation CCK-8 assay
EOC cells (SKOV3, CAOV3) were seeded into the 96 well plates at the destiny of 2 × 103 cells per well, and cultured for described times. After the transfection, cells were administrated with Cell Counting Kit-8 agent (CCK8, Dojindo, Tokyo, Japan) according to the manufacturer’s protocol.
Transwell invasion assay
Cells were coated with the Matrigel (BD Biosciences, San Jose, CA) for invasion assay using transwell inserts (8 μm pore size; Costar, Cambridge, MA). Cells (2 × 104) were seeded in the serum free medium in the upper chamber. The complete DMEM medium were added in the bottom chamber. After 24 hour culturing, the invaded cells in the lower surface of chamber member were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Lastly, the invaded cell numbers were counted using a light microscope.
Cellular apoptosis assay
After transfection of oligonucleotides for 48 h, cells were rinsed with ice-cold PBS and respectively stained with propidium iodide (PI, 50 μg/ml, Sigma) in the dark at room temperature for 10 min. The apoptosis rate was analyzed by flow cytometry (FACSCalibur, BD Biosciences).
Chromatin immunoprecipitation (ChIP) assay
To study the combination of EZH2 and the promoter of p21, the ChIP assays were performed using a EZ-Magna ChIP™ A/G Chromatin Immunoprecipitation Kit (Millipore) according to the manufacturer’s instructions. Cells were lysed and sonicated to be DNA fragments (200 to 1000 bp). DNA fragments were precipitated with non-specific IgG antibodies and anti-EZH2 antibody (Abcam). After the immunoprecipitation, DNA was eluted and measured using the PCR.
Animal experiment
The animal experiment was approved by the Ethics Committee of Shandong Provincial Hospital affiliated to Shandong University. The experimental procedure was performed according to the guidelines of the National Institutes of Health. TP73-AS1 silencing transfection or control SKOV3 cells were subcutaneously injected into the flank of BALB/c nude mice (4-week, 5 × 106 cells in 100 μl). The size of tumor was measured with a caliper every three days and calculated as (length × width2)/2.
Statistical analysis
The data analysis was expressed from SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) as the means ± SD. The analysis was carried out using the Student’s t-test or one way ANOVA. A value of p less 0.05 was considered as significant.
Results
LncRNA TP73-AS1 is over-expressed in the EOC tissue and cells
The expression level of lncRNA TP73-AS1 in the EOC tissue was measured using qRT-PCR. The level of TP73-AS1 in EOC tissue specimens illustrated that TP73-AS1 was drastically over-expressed in the tissue compared to adjacent normal specimens (Figure 1A). In accordance with the tissue results, the level of TP73-AS1 in EOC cells (SKOV3, CAOV3, HO8910, OV420) was also markedly increased in relation to non-tumor tissue (Figure 1B). Kaplan-Meier survival curve analysis based on the TCGA database (GEPIA, http://gepia.cancer-pku.cn/) suggested that the EOC patients with higher TP73-AS1 level was significantly correlated with the poorer prognosis (Figure 1C). Therefore, this data suggests that lncRNA TP73-AS1 is over-expressed in the EOC tissue and cells, besides, the overexpression was correlated with the poor clinical outcome.
Figure 1.
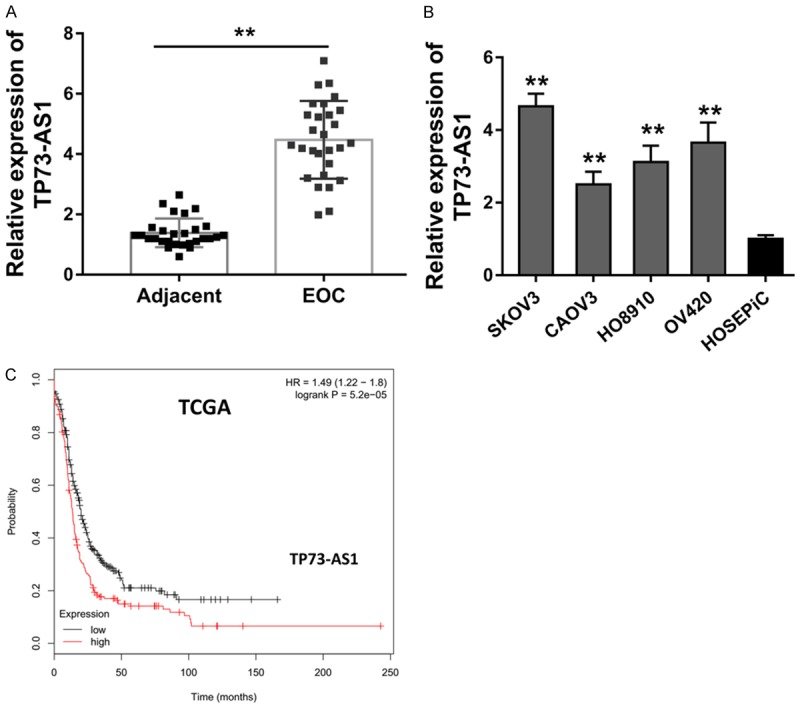
LncRNA TP73-AS1 is over-expressed in the EOC tissue and cells. A. qRT-PCR detected the level of TP73-AS1 in EOC tissue specimens compared to adjacent normal specimens. B. qRT-PCR detected the TP73-AS1 level in EOC cells (SKOV3, CAOV3, HO8910, OV420). C. Kaplan-Meier survival curve analysis based on the TCGA database suggested the prognosis of EOC patients with higher or lower TP73-AS1 level.
TP73-AS1 facilitates the EOC cells’ proliferation in vitro and in vivo
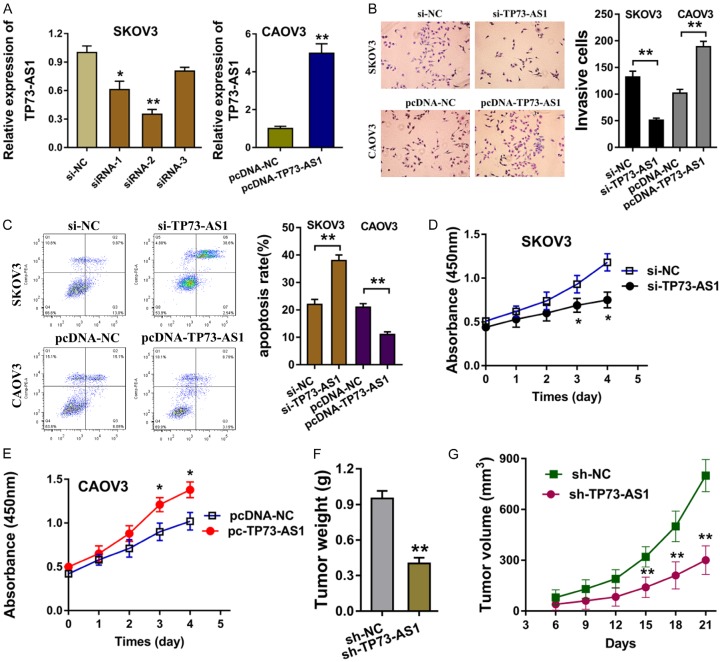
To explore the biological functions of TP73-AS1 on the EOC cells, the silenced expression siRNA and overexpression plasmid for TP73-AS1 were transfected into EOC cells (SKOV3, CAOV3) to knockdown or increase its expression (Figure 2A). Transwell invasion assay showed that the silenced TP73-AS1 expression inhibited the invaded cell quantity, while the enforced TP73-AS1 expression promoted the invaded cell quantity (Figure 2B). Flow cytometry analysis revealed that the silenced TP73-AS1 expression stimulated the apoptosis of EOC cells, while the enforced TP73-AS1 expression receded the apoptosis (Figure 2C). Proliferative CCK-8 assay showed that TP73-AS1 knockdown decreased the proliferation, while the TP73-AS1 enforced level promoted the proliferation (Figure 2D, 2E). In vivo xenograft assay illustrated that TP73-AS1 knockdown repressed the tumor weight and growth compared to the negative control (Figure 2F, 2G). Thus, this data suggests that TP73-AS1 facilitates the EOC cells’ proliferation in vitro and in vivo.
Figure 2.
TP73-AS1 facilitates the EOC cells’ proliferation in vitro. A. The silenced expression siRNA and overexpression plasmid for TP73-AS1 were transfected into EOC cells (SKOV3, CAOV3). B. Transwell invasion assay showed the invaded cell quantity in the cells transfected with silenced TP73-AS1 expression and enforced TP73-AS1 expression. C. Flow cytometry analysis revealed the apoptosis of EOC cells transfected with silenced TP73-AS1 or enforced TP73-AS1 expression. D, E. Proliferative CCK-8 assay showed the proliferation of EOC cells. F, G. In vivo xenograft assay illustrated the tumor weight and growth. *P < 0.05, **< 0.01.
TP73-AS1 epigenetically inhibits p21 through binding with EZH2
The subcellular location of TP73-AS1 was found to be much more in the nuclear than the cytoplasm fraction (Figure 3A). The cellular location of TP73-AS1 suggests the transcriptional regulation for TP73-AS1 in the EOC cells. In the SKOV3 cells transfected with siRNA TP73-AS1, CDK inhibitors (CKIs) were measured using RT-PCR, including p15, p16, p21, p27, p57. The level of p21 was increased in the TP73-AS1 silencing (Figure 3B). Western blot revealed that p21 protein was enforced in the TP73-AS1 silencing transfection (Figure 3C). EZH2 is the core of subunit of polycomb repressive complex 2 (PRC2). RNA-binding protein immunoprecipitation (RIP) assay showed that TP73-AS1 could bind with EZH2 (Figure 3D). Chromatin immunoprecipitation (ChIP) revealed that EZH2 directly bound with p21 promoter region (Figure 3E). Kaplan-Meier survival curve analysis based on the TCGA database suggested the poor prognosis of EOC patients with higher than the lower group (Figure 3F). Therefore, the data shows that TP73-AS1 epigenetically inhibits p21 through binding with EZH2.
Figure 3.
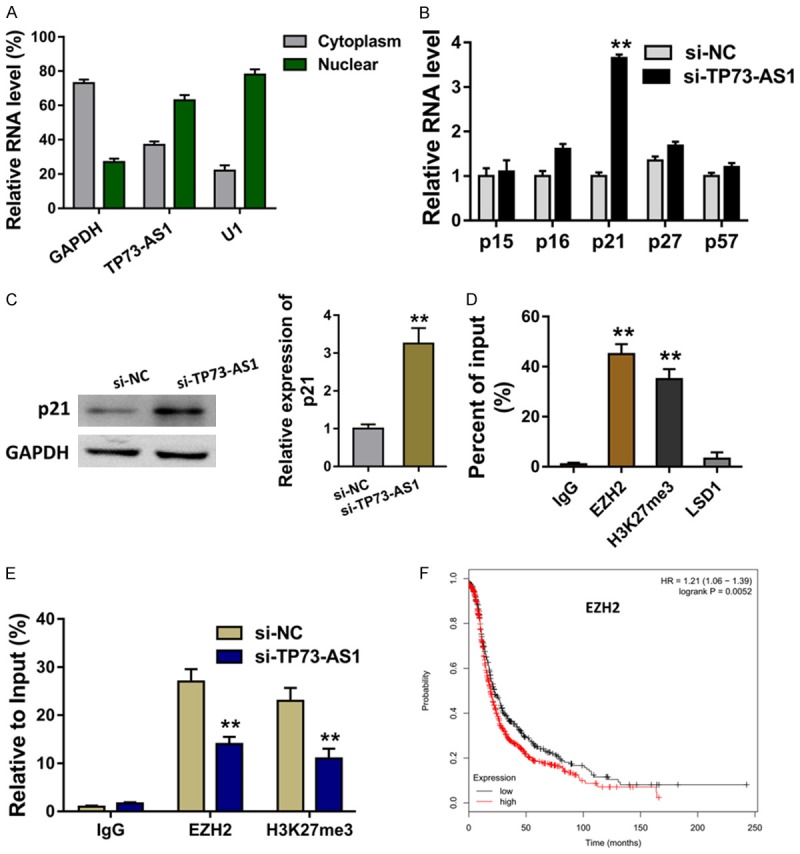
TP73-AS1 epigenetically inhibits p21 through binding with EZH2. A. The subcellular location of TP73-AS1 in the nuclear and the cytoplasm fraction. B. The level of CDK inhibitors (CKIs), including p15, p16, p21, p27, p57. C. Western blot revealed the p21 protein in the TP73-AS1 silencing transfection. D. RNA-binding protein immunoprecipitation (RIP) assay showed the binding of TP73-AS1 with EZH2. E. Chromatin immunoprecipitation (ChIP) revealed the direct bound of EZH2 with p21 promoter region. F. Kaplan-Meier survival curve analysis based on the TCGA database suggested the prognosis of EOC patients with higher or lower group. **< 0.01.
TP73-AS1 regulates the EOC progression through targeting p21
The interaction within TP73-AS1 and EZH2 was analyzed using the data based on the TCGA, showing the positive interaction of TP73-AS1 and EZH2 (Figure 4A). The p21 mRNA measured by the RT-PCR showed that p21 siRNA co-transfection could decrease the p21 mRNA level in the SKOV3 cells (Figure 4B). Transwell invasion and flow cytometry assay illustrated that p21 siRNA co-transfection up-regulated the invasive cells (Figure 4C), and reduced the apoptotic cells in the SKOV3 cells (Figure 4D). Meanwhile, the CCK-8 assay revealed that p21 siRNA co-transfection increased the proliferation (Figure 4E). Kaplan-Meier survival curve analysis based on the TCGA database suggested the poor prognosis of EOC patients with lower p21 level than the higher group (Figure 4F). Therefore, data shows that TP73-AS1 regulates the EOC progression through targeting p21.
Figure 4.
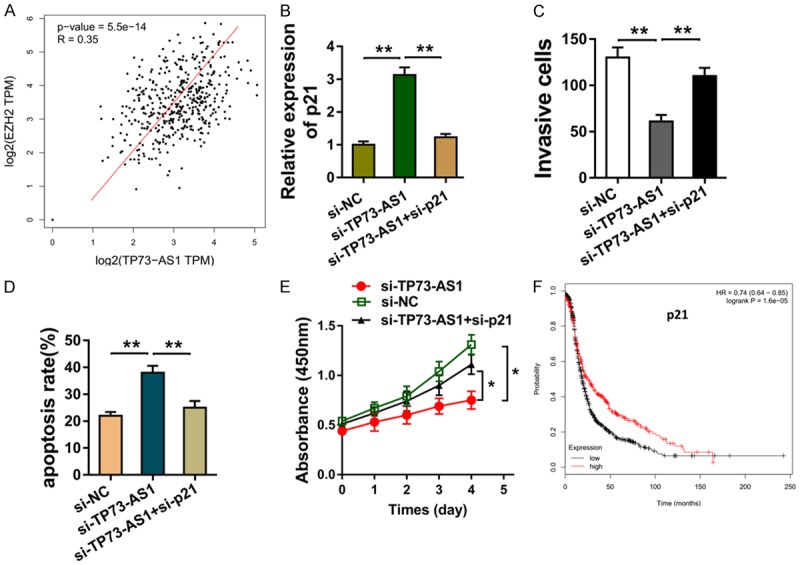
TP73-AS1 regulates the EOC progression through targeting p21. A. The interaction of TP73-AS1 and EZH2 was analyzed using the data based on the TCGA. B. p21 mRNA measured by the RT-PCR in the SKOV3 cells co-transfected with p21 siRNA and TP73-AS1 siRNA. C. Transwell invasion showed the invasive cells. D. Flow cytometry assay illustrated e apoptotic cells in the SKOV3 cells. E. CCK-8 assay revealed the proliferation. F. Kaplan-Meier survival curve analysis based on the TCGA database suggested the prognosis of EOC patients with lower p21 level or higher group. **< 0.01. *< 0.05.
Discussion
LncRNAs are a group of emerging regulator in the human ovarian cancer, especially epithelial ovarian cancer (EOC) [12,13]. In this study, we revealed that lncRNA TP73-AS1 is the key upstream effector for the oncogenic function of p21 in EOC carcinogenesis through EZH2, and the expressions exhibit a close correlation with the tumors phenotype.
There are many oncogenes or tumor suppressor in the EOC carcinogenesis and emerging evidence illustrates the vital role in this molecular progression [14]. The fields mediated by these elements contain transcriptional regulation and post-transcriptional regulation [15]. The tumorous phenotypes involving this regulation include invasion, metastasis, proliferation and metastasis [16]. For instance, lncRNA HOXD-AS1 is upregulated in EOC tissues and cell lines, which is closely correlated with lymph node metastasis, advanced FIGO stage and poor overall survival in EOC patients [17]. LncRNA ABHD11-AS1 is higher in EOC tissues than that in normal ovarian tissue, which is positively associated with the tumor stage and well/poorly/moderately differentiated group [18].
In this research, we discovered that TP73-AS1 is markedly up-regulated in the EOC tissue and cells. Moreover, this aberrant high level of TP73-AS1 is responsible for the poor clinical outcome and prognosis for the EOC patients. TP73-AS1 is also found to be oncogene in human cancers. In non-small cell lung cancer, TP73-AS1 is significantly increased and regulates NSCLC cell proliferation, tumor growth and cycle progression by targeting miR-449a-EZH2 axis [19]. In colorectal cancer, TP73-AS1 negatively regulates the miR-194 and then regulates the downstream target of transforming growth factor alpha (TGFα) [20].
The mechanical research revealed that the TP73-AS1 silencing could activate the expression of p21 among these cyclin kinase inhibitors (CDKIs). Further research showed that the TP73-AS1 epigenetically silenced the p21 protein through binding the EZH2, a key element for the polycomb protein inhibits complex 2 (PRC2). EZH2 is validated to be over-expressed in EOC and enhances angiogenesis, promotes cell proliferation and inhibits apoptosis by targeting both ovarian cancer cells and ovarian tumor microenvironment [21]. Overall, this data suggest the critical function of TP73-AS1 in the EOC tumorigenesis.
The previous studies illustrated the potential of lncRNAs, playing key roles in regulation of the malignant phenotypes of cancer. For example, lncRNA DLEU1 is involved in the EOC genesis and accelerates the cell proliferation, migration, and invasion, and inhibited apoptosis [22]. A critical role of CCAT1 promotes the EOC via CCAT1-miR-152/miR-130b-ADAM17/WNT1/STAT3/ZEB1 regulatory network [23]. Our finding validates the novel role of TP73-AS1 in the EOC.
Taken together, our work reveals that the TP73-AS1 exerts a critical function in the EOC as an oncogene in the proliferation, invasion and tumor growth, through epigenetically silence p21 protein via the H3K27me3 induced by EZH2. These findings suggest a new insight into EOC origin and progression, and provodes novel potential therapeutic target for EOC diagnosis.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Moufarrij S, Dandapani M, Arthofer E, Gomez S, Srivastava A, Lopez-Acevedo M, Villagra A, Chiappinelli KB. Epigenetic therapy for ovarian cancer: promise and progress. Clin Epigenetics. 2019;11:7. doi: 10.1186/s13148-018-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomakos N, Malakasis A, Machairiotis N, Zarogoulidis P, Rodolakis A. Fertility sparing management in non-epithelial ovarian cancer. Which patients, what procedure and what outcome? J Cancer. 2018;9:4659–4664. doi: 10.7150/jca.26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrado G, Salutari V, Palluzzi E, Distefano MG, Scambia G, Ferrandina G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther. 2017;17:1147–1158. doi: 10.1080/14737140.2017.1398088. [DOI] [PubMed] [Google Scholar]
- 4.Marth C, Reimer D, Zeimet AG. Front-line therapy of advanced epithelial ovarian cancer: standard treatment. Ann Oncol. 2017;28:viii36–viii39. doi: 10.1093/annonc/mdx450. [DOI] [PubMed] [Google Scholar]
- 5.Shin H, Kim Y, Kim M, Lee Y. BC200 RNA: an emerging therapeutic target and diagnostic marker for human cancer. Dis Markers. 2018;41:993–999. doi: 10.14348/molcells.2018.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinelli C, Adnani L, Choi D, Rak J. Extracellular vesicles as conduits of non-coding RNA emission and intercellular transfer in brain tumors. Noncoding RNA. 2018;5 doi: 10.3390/ncrna5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Wen J, Wang H, Wang Y. Long non-coding RNA LINC00460 promotes epithelial ovarian cancer progression by regulating microRNA-338-3p. Biomed Pharmacother. 2018;108:1022–1028. doi: 10.1016/j.biopha.2018.09.103. [DOI] [PubMed] [Google Scholar]
- 8.Xue Z, Zhu X, Teng Y. Long noncoding RNA CASC2 inhibits progression and predicts favorable prognosis in epithelial ovarian cancer. Mol Med Rep. 2018;18:5173–5181. doi: 10.3892/mmr.2018.9550. [DOI] [PubMed] [Google Scholar]
- 9.Liu G, Zhao X, Zhou J, Cheng X, Ye Z, Ji Z. LncRNA TP73-AS1 promotes cell proliferation and inhibits cell apoptosis in clear cell renal cell carcinoma through repressing KISS1 expression and inactivation of PI3K/Akt/mTOR signaling pathway. Cell Physiol Biochem. 2018;48:371–384. doi: 10.1159/000491767. [DOI] [PubMed] [Google Scholar]
- 10.Tuo Z, Zhang J, Xue W. LncRNA TP73-AS1 predicts the prognosis of bladder cancer patients and functions as a suppressor for bladder cancer by EMT pathway. Biochem Biophys Res Commun. 2018;499:875–881. doi: 10.1016/j.bbrc.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Song R, Wang L, Wu X. Knockdown of long non-coding RNA TP73-AS1 inhibits osteosarcoma cell proliferation and invasion through sponging miR-142. Biomed Pharmacother. 2018;103:1238–1245. doi: 10.1016/j.biopha.2018.04.146. [DOI] [PubMed] [Google Scholar]
- 12.Liang H, Zhao X, Wang C, Sun J, Chen Y, Wang G, Fang L, Yang R, Yu M, Gu Y. Systematic analyses reveal long non-coding RNA (PTAF)-mediated promotion of EMT and invasion-metastasis in serous ovarian cancer. Mol Cancer. 2018;17:96. doi: 10.1186/s12943-018-0844-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Shan H, Pang Y, Hou X, Yang C, Liu Y, Jiang G. Advances on chimeric antigen receptor-modified t-cell therapy for oncotherapy. Mol Cancer. 2018;17:91. doi: 10.1186/s12943-018-0840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Li Z, Xu L, Li Y, Li Y, Zhang X, Wang Y, Liu D. LIMD2 targeted by miR34a promotes the proliferation and invasion of nonsmall cell lung cancer cells. Mol Med Rep. 2018;18:4760–4766. doi: 10.3892/mmr.2018.9464. [DOI] [PubMed] [Google Scholar]
- 15.Qin W, Xiong Y, Chen J, Huang Y, Liu T. DC-CIK cells derived from ovarian cancer patient menstrual blood activate the TNFR1-ASK1-AIP1 pathway to kill autologous ovarian cancer stem cells. J Cell Mol Med. 2018;22:3364–3376. doi: 10.1111/jcmm.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao L, Wang X, Guo S, Xiao L, Liang C, Wang Z, Li Y, Liu Y, Yao R, Liu Y, Zhang Y. LncRNA HOTAIR functions as a competing endogenous RNA to upregulate SIRT1 by sponging miR-34a in diabetic cardiomyopathy. J Cell Physiol. 2019;234:4944–4958. doi: 10.1002/jcp.27296. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Dun Y, Zhou S, Huang XH. LncRNA HOXD-AS1 promotes epithelial ovarian cancer cells proliferation and invasion by targeting miR-133a-3p and activating wnt/beta-catenin signaling pathway. Biomed Pharmacother. 2017;96:1216–1221. doi: 10.1016/j.biopha.2017.11.096. [DOI] [PubMed] [Google Scholar]
- 18.Wu DD, Chen X, Sun KX, Wang LL, Chen S, Zhao Y. Role of the lncRNA ABHD11-AS1 in the tumorigenesis and progression of epithelial ovarian cancer through targeted regulation of RhoC. Mol Cancer. 2017;16:138. doi: 10.1186/s12943-017-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Fang F, He X. Long noncoding RNA TP73-AS1 promotes non-small cell lung cancer progression by competitively sponging miR-449a/EZH2. Biomed Pharmacother. 2018;104:705–711. doi: 10.1016/j.biopha.2018.05.089. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y, Yan P, Zhang G, Yang W, Wang H, Cheng X. Long non-coding RNA TP73-AS1 sponges miR-194 to promote colorectal cancer cell proliferation, migration and invasion via up-regulating TGFalpha. Cancer Biomark. 2018;23:145–156. doi: 10.3233/CBM-181503. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zhang R. Role of EZH2 in epithelial ovarian cancer: from biological insights to therapeutic target. Front Oncol. 2013;3:47. doi: 10.3389/fonc.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang LL, Sun KX, Wu DD, Xiu YL, Chen X, Chen S, Zong ZH, Sang XB, Liu Y, Zhao Y. DLEU1 contributes to ovarian carcinoma tumourigenesis and development by interacting with miR-490-3p and altering CDK1 expression. J Cell Mol Med. 2017;21:3055–3065. doi: 10.1111/jcmm.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y, Shi H, Ren F, Jia Y, Zhang R. Long non-coding RNA CCAT1 promotes metastasis and poor prognosis in epithelial ovarian cancer. Exp Cell Res. 2017;359:185–194. doi: 10.1016/j.yexcr.2017.07.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.