Abstract
Osteoarthritis is a major global health burden. Joint destruction resulting from excessive degradation of type II collagen and aggrecan in the articular extracellular matrix by metalloproteinases and aggrecanases, respectively, is a major pathological hallmark of osteoarthritis. However, the exact mechanisms remain poorly understood. Currently, there are few non-invasive therapies capable of slowing or halting the progression of the disease. In the present study, we investigated the involvement of the P2Y11 purinergic protein and its receptor P2Y11R in TNF-α-mediated degradation of the extracellular matrix in SW1353 cell line chondrocytes using the novel P2Y11R antagonist NF157. To our knowledge, this is the first study to explore the effects of NF157 in OA. Our results indicate that P2Y11R may indeed play a role in TNF-α-induced degradation of extracellular matrix in OA as treatment with NF157 significantly reduced expression of metalloproteinase (MMP)-3, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and ADAMTS-5, and ameliorated degradation of type II collagen and aggrecan in SW1353 chondrocytes in a dose-dependent manner. Furthermore, we show that treatment with NF157 significantly reduced nuclear translocation of p65 and subsequent activation of nuclear factor κB (NF-κB).
Keywords: Osteoarthritis, P2Y11 receptor, degradation of extracellular matrix, type II collagen, aggrecan, NF-κB
Introduction
Osteoarthritis (OA) is recognized a major global health burden. Joint destruction due to OA is largely considered to be irreversible, and therefore, the advanced stages of the disease are highly debilitating. Although there are several risk factors for OA, including obesity, injury, excessive mechanical loading, and chronic inflammatory disease, the main risk factor for developing OA is age [1,2]. According to statistics from the Centres for Disease Control (CDC), there are currently more than 30 million people in the U.S. alone suffering with OA. Furthermore, along with the increasing age of the general population, this number is forecasted to rise to over 70 million by 2030 [3]. Numerous studies have investigated the mechanisms underlying the development and progression of OA. However, the processes involved are complicated and an in-depth understanding of the disease still eludes researchers.
It is widely accepted that degradation of type II collagen and aggrecan, the two main components of the articular extracellular matrix (ECM), is a pivotal event in the pathological progression of OA [4]. Metalloproteinases (MMPs) are a family of degradative enzymes that have been shown to play a role in cell migration, invasion, proliferation, and apoptosis. Additionally, these proteinases are involved in regulating developmental processes such as wound healing, branching morphogenesis, angiogenesis, and degradation of ECM [5]. Of these, MMP-3 (stromelysin) and MMP-13 (collagenase-3) are recognized as two of the main enzymes that degrade type II collagen [6,7]. Owing to its rigid nature, type II collagen forms the structural backbone of joint cartilage and has a very slow rate of synthesis. Thus, excessive degradation of type II collagen by MMPs is considered to be a major irreversible event in the pathogenesis of OA [8]. Aggrecan, also known as cartilage-specific proteoglycan core protein (CSPCP) or chondroitin sulfate proteoglycan 1, is the other main component of the ECM and complements type II collagen with its shock-absorptive function and capacity to withstand pressure [9]. Excessive degradation of aggrecan is another key event in the pathogenesis of OA. A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) comprise a large family of extracellular protease enzymes and are considered to play a key role in remodeling of the ECM [10]. It has been shown that the interglobular domain between amino acids 373 and 374 is a major site of enzymatic aggrecan cleavage in OA. Notably, ADAMTS-4 and ADAMTS-5 are recognized as the two aggrecanases with the highest capacity to cleave aggrecan at this site [11]. Another major factor in the development and progression of OA as well as numerous other inflammatory diseases is activation of the NF-κB signaling pathway. Under normal physiological conditions, the p50/65 heterodimer of NF-κB is stringently sequestered in the cytoplasm by IκB protein. However, under pathological conditions, such as exposure to cytokines including TNF-α, IκB is degraded and NF-κB translocates to the nucleus to activate transcription [12]. This triggers the inflammatory response.
The family of P2Y G protein-couple receptors are activated by extracellular nucleotides, of which P2Y11R is specifically activated by adenine nucleotides [13]. Numerous studies have explored the involvement of P2Y11R in a variety of diseases, however, there is still some debate regarding the ideal experimental method for use with P2Y11R as it is not expressed in murine models and thorough research exploring the effects of P2Y11-specific antagonists on other 5’-triphosphate-binding receptors has not been performed [14]. In the present study, we used P2Y11R-expressing SW1353 chondrocytic cell line cells and the P2Y11R-specific antagonist NF157 to investigate the involvement of this receptor in TNF-α-mediated degradation of the articular ECM. The selective P2Y11R antagonist NF157 has the chemical name 8,8’-[Carbonylbis [imino-3,1-phenylenecarbonylimino (4-fluoro-3,1-phenylene) carbonylimino]] bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt and is produced by Tocris Bioscience. As a newer agent, there has been little research on NF157, but it has shown high specificity for the P2Y11 and P2X1 receptors over the other members of the P2Y receptor family [15]. In the following experiments, we explored the effects of NF157 on TNF-α-mediated degradation of the articular ECM.
Materials and methods
Cell isolation and culture
Human primary chondrocytes (HPCs) were isolated from post-surgically discarded samples of human normal specimens (n=16) or osteoarthritis (n=6) of cartilage explants collected from the femoral heads of patients undergoing hip replacement. This approach was approved by the human subject protocols of our institution and followed the principles of the Declaration of Helsinki. All participants in the study have signed written informed consent. The tissue was mechanically minced into pieces (1-3 mm2) and then enzymatically treated in 0.15% type II collagenase (Worthington Biochemical Corporation, USA) at 37°C overnight. The isolated cells and human chondrocytic SW1353 cells were kept in DMEM containing 10% fetal calf serum (FBS) and 1% penicillin/streptomycin (P/S) in a humid incubator with 5% CO2 at 37°C. The medium was replaced completely every other day.
Real-time PCR analysis
Total RNA was isolated from chondrocytes using an RNA isolation kit (Norgen Biotek Corp., Canada) following the protocol from the manufacturer. The concentration of total RNA was quantified by Nanadrop nucleic acid quantification. cDNA was transcripted from 2 μg total RNA using a Transcriptor high-fidelity cDNA synthesis kit (Sigma Aldrich, USA) on a PCR machine following the instructions provided by the manufacturer. Then, the cDNA was mi4xed with different primers and universal SYBR Green Master Mix (Bio-Rad, USA) following manufacturer’s protocol before quantification. PCR was performed in triplicate on an ABI PCR machine (Thermo Fisher, USA). GAPDH was used as a house keeping gene to analyze the relative mRNA expression levels of target genes. Results were normalized using the highly efficient 2-ΔΔCT method. The following primers were used in this study: P2Y11R, forward, 5’-TTGGTGGCCAGTGGTGTG-3’; reverse, 5’-TGAGCACCCGCATGATGT-3’; MMP-3: forward, 5’-CCTCTATGGACCTCCCACAGAATC-3’; reverse, 5’-GGTGCTGACTGCATCGAAGGACAAA-3’; MMP-13: forward, 5’-CTGGCCTGCTGGCTCATGCTT-3’; reverse, 5’-CCTCAGAAAGAGCAGCATCGATATG-3’; ADAMTS-4: forward, 5’-ACACTGAGGACTGCCCAAC-3’; reverse, 5’-GGTGAGTTTGCACTGGTCCT-3’; ADAMTS-5: forward, 5’-GCAGAACATCGACCAACTCTACTC-3’; reverse, 5’-CCAGCAATGCCCACCGAAC-3’; GAPDH: forward, 5’-ACT GGCGTC TTCACCACCAT-3’; reverse, 5’-AAG GCC ATG CCA GTG AGC TT-3.
Western blot analysis
After the necessary treatment, paired control and experimental group cells were lysed with lysis buffer (Thermo Fisher Scientific, USA). Nuclear protein was obtained using a cytoplasmic and nuclear protein extraction kit (Fisher Scientific, USA) following the manufacturer’s instructions. A total of 20-50 μg of total protein was equally loaded into SDS-PAGE gel and resolved by electro-blotting. The separated protein was then transferred onto membranes (Thermo Fisher scientific, USA). Before incubation with primary antibody overnight in a cold room (4°C), membranes were blocked with 5% slim milk diluted with TBS-T buffer for 1 h at room temperature. The following day, cells were washed with TBS-T buffer 3 times for a total of 20 min and then immediately incubated with HRP-conjugated secondary antibodies for 1 h. After washing 3 times, blots were developed using enhanced chemiluminescence reagent (ECL) (Thermo Pierce, USA) and exposed to clear blue X-Ray film. Blots were scanned with a densitometer. The data was analyzed using Image J software. The following antibodies were used in this study: antibodies directed P2Y11R (1:600, #31254, SAB Signalway Antibody, USA); type II collagen (1:500, #ab21291, Abcam, USA); NF-κB p65 (1:2000, # sc-8008, Santa Cruz, USA); lamin B1 antibody (1:2000, #sc-377000, Santa Cruz, USA); β-actin (1:2000, #sc-1615, Santa Cruz, USA); aggrecan (1:500, #AF1220, R&D Systems, USA).
ELISA assay
Part of the isolated protein was used for ELISA assay in accordance with the manufacturer’s instructions (R&D Systems, USA). The 96-well plates were coated with the oligonucleotide-specific protein binding agent. Bound protein was measured using its specific paired antibody. The following ELISA kits from R&D systems were used in this study: a human MMP-3 Quantikine ELISA Kit (#DMP300); a human MMP-13 Quantikine ELISA Kit (#DY511); a human ADAMTS-4 Quantikine ELISA Kit (#DY4307-05); a human ADAMTS-5 Quantikine ELISA Kit (#DY2198-05).
Determination of nuclear translocation of p65
The patterns of the nuclear translocation of p65 were measured to determine the transcriptional activity of NF-κB in human chondrocytic SW1353 cells. A commercial nuclear and cytoplasmic extraction kit (Thermo Fisher Scientific, USA) was used to extract nuclear protein from SW1353 cells. After indicated treatment, cells were lysed in a hypotonic buffer on ice for 15 min, followed by a brief centrifugation at 500 × g for 1 min to pellet nuclei. Pellets were then re-suspended in nuclear extract buffer and incubated for 15 min on ice followed by centrifugation at 14,000 × g for 10 min. Supernatants were collected for western blot analysis to assess nuclear translocation of p65.
NF-κB promoter-luciferase assay
The transcriptional activity of NF-κB was assessed by measuring the NF-κB promoter-luciferase activity. The NF-κB promoter-luciferase (Clontech, USA) and β-galactosidase plasmids were transfected into SW1353 cells with the Lipofectamine 2000. Human chondrocytic SW1353 cells were treated with 10 ng/ml TNF-α in the presence or absence of NF157 at the concentrations of 30 and 60 μM for 24 h. Cell lysates were prepared and used for determining luciferase activity and β-galactosidase activity using a Secrete-PairTM Dual luminescence assay kit (Gene Copoeia, MD) on a luminometer (Infinite F500 Multimode Reader). Luciferase activity was normalized to β-galactosidase activity.
Statistical analysis
Data are presented as means ± SD unless otherwise indicated. Statistical analysis were performed using SPSS (Version 19). ANOVA with Bonferroni correction was used for comparison between different groups. A p value less than 0.05 was considered significantly different.
Results
P2Y11R is increased in OA chondrocytes
First, we set out to confirm that P2Y11R is expressed on both HPCs and SW1353 chondrocytic cell line cells. Using β-actin and human huh-7 cells as controls, both cell types underwent real-time polymerase chain reaction (RT-PCR) and western blot analysis. As shown in Figure 1, our results indicate that P2Y11R is indeed expressed on the surface of both HPCs and SW1353 cells at both the mRNA and protein levels. Thus, SW1353 cells were deemed suitable for use in the following experiments. Next, we investigated P2Y11R expression in primary OA chondrocytes using β-actin as control. As shown in Figure 2, compared with normal chondrocytes (control), primary human OA chondrocytes showed significantly higher expression of P2Y11R at both the mRNA and protein levels. This indicates that P2Y11R may be a suitable target for the treatment of OA.
Figure 1.
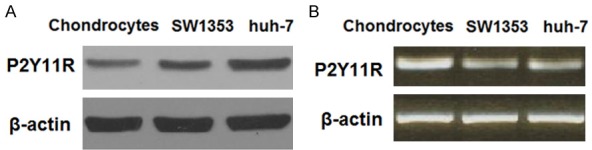
P2Y11R is expressed in primary human chondrocytes and chondrocytic cell line (SW1353) cells. Human huh-7 cells were used as a positive control as previously described [30]. A. RT-PCR of P2Y11R at the mRNA level; B. Western blot analysis of P2Y11R at the protein level.
Figure 2.
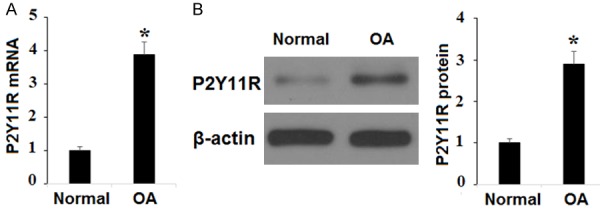
The expression of P2Y11R is higher in chondrocytes of osteoarthritis (OA) patients. A. Real-time PCR revealed that P2Y11R is higher in chondrocytes of osteoarthritis (OA) patients at the mRNA level; B. Western blot analysis revealed that P2Y11R is higher in chondrocytes of osteoarthritis (OA) patients at the protein level (*, P<0.01 vs. Normal group).
TNF-α upregulates expression of P2Y11R
TNF-α is known to be highly expressed in OA. We established an OA cell model by exposing SW1353 chondrocytes to various concentrations of TNF-α as previously described [16]. To investigate the mechanism behind the positive correlation between OA and P2Y11R expression in human chondrocytes, SW1353 cells were exposed to TNF-α at the concentrations of 5, 10, and 20 ng/ml for 24 h. As shown in Figure 3, TNF-α induced a linear increase in expression of P2Y11R at both the mRNA and protein levels in a dose-dependent manner. This indicates that the increase in expression of P2Y11R associated with OA may occur through the TNF-α signaling pathway.
Figure 3.
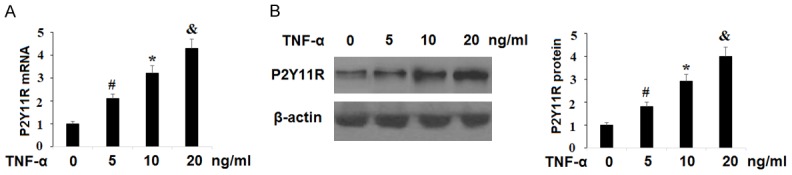
P2Y11R is upregulated in SW1353 cells in response to TNF-α in a dose-dependent manner. Human chondrocytic SW1353 cells were treated with TNF-α at the concentrations of 5, 10, 20 ng/ml for 24 h. A. mRNA of P2Y11R was determined by real time PCR analysis; B. Protein of P2Y11R was determined by western blot analysis (*, #, &, P<0.01 vs. vehicle group).
NF157 ameliorates TNF-α-induced degradation of type II collagen
Degradation of type II collagen in chondrocytes is a pivotal event in the progression of OA. In light of our finding that P2Y11R is upregulated in OA chondrocytes, we investigated whether P2Y11R plays a role in TNF-α-induced degradation of type II collagen. SW1353 cells were exposed to TNF-α at the concentration of 10 ng/ml in the presence or absence of 30 and 60 µM NF157, a highly selective P2Y11R inhibitor, for 24 h. As shown in Figure 4, blockage of P2Y11R using NF157 caused a significant reduction in degradation of type II collagen in a dose-dependent manner. Remarkably, a dose of 60 µM NF157 nearly completely rescued type II collagen from degradation induced by TNF-α.
Figure 4.
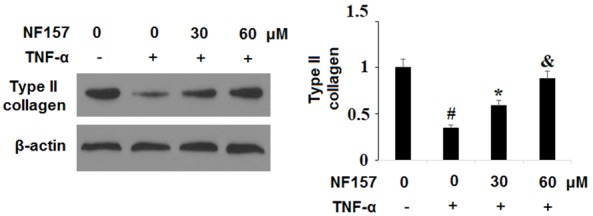
Blockage of P2Y11R using its specific antagonist NF157 ameliorated TNF-α-induced degradation of type II collagen. Human chondrocytic SW1353 cells were treated with 10 ng/ml TNF-α in the presence or absence of NF157 at the concentrations of 30 and 60 μM for 24 h. Expression of type II collagen was determined by western blot analysis (*, #, &, P<0.01 vs. previous column group).
NF157 reduces TNF-α-induced expression of MMP-3 and MMP-13
MMP-3 and MMP-13 are regarded as two of the main enzymes targeting type II collagen for degradation in OA. To determine whether MMP-3 and MMP-13 are involved in the protective effect of NF157 on degradation of type II collagen in human chondrocytes, we exposed SW1353 cells to 10 ng/ml TNF-α in the presence or absence of 30 and 60 µM NF157 for 24 h. As shown in Figure 5, the results of real-time PCR and ELISA indicate that NF157 significantly reduced expression of MMP-3 and MMP-13 at both the mRNA and protein levels in a dose-dependent manner. This suggests that expression of MMP-3 and MMP-13 may be regulated via the P2Y11R pathway. Thus, therapy with NF157 may be beneficial for the treatment of diseases involving overexpression of MMP-3 and MMP-13, including OA.
Figure 5.
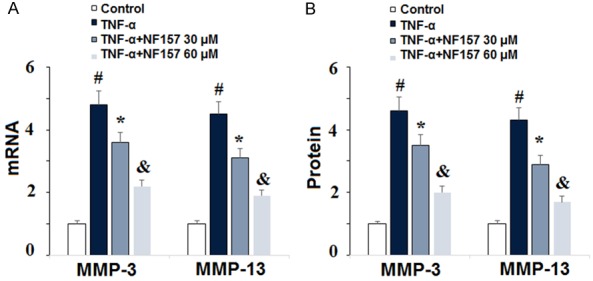
Inhibition of P2Y11R using its specific antagonist NF157 reduced TNF-α-induced expression of MMP-3 and MMP-13. Human chondrocytic SW1353 cells were treated with 10 ng/ml TNF-α in the presence or absence of NF157 at the concentrations of 30 and 60 μM for 24 h. A. mRNA of MMP-3 and MMP-13 as determined by real-time PCR; B. Protein of MMP-3 and MMP-13 as determined by ELISA (*, #, &, P<0.01 vs. previous column group).
NF157 ameliorates TNF-α-induced degradation of aggrecan
Aggrecan is a major component of the articular ECM and excessive degradation of aggrecan is recognized as a key event in the pathogenesis of OA. To explore the involvement of P2Y11R in TNF-α-induced degradation of aggrecan, SW1353 cells were exposed to 10 ng/ml TNF-α in the presence or absence of the specific P2Y11R antagonist NF157 at the concentrations of 30 and 60 µM for 24 h. As shown in Figure 6, blockage of P2Y11R using NF157 caused a significant reduction in degradation of aggrecan in a dose-dependent manner. Once again, a dose of 60 µM NF157 nearly completely rescued aggrecan from degradation induced by TNF-α.
Figure 6.
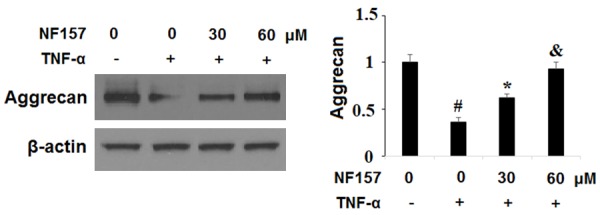
Blockage of P2Y11R using its specific antagonist NF157 ameliorated TNF-α-induced degradation of aggrecan. Human chondrocytic SW1353 cells were treated with 10 ng/ml TNF-α in the presence or absence of NF157 at the concentrations of 30 and 60 μM for 24 h. Expression of aggrecan was determined by western blot analysis (*, #, &, P<0.01 vs. previous column group).
NF157 reduces TNF-α-induced expression of ADAMTS-4 and ADAMTS-5
Degradation of aggrecan in OA is primarily mediated by the aggrecanases ADAMTS-4 and ADAMTS-5. To determine whether ADAMTS-4 and ADAMTS-5 are involved in the protective effect of NF157 on degradation of aggrecan in human chondrocytes, we exposed SW1353 cells to 10 ng/ml TNF-α in the presence or absence of 30 and 60 µM NF157 for 24 h. As shown in Figure 7, real-time PCR and ELISA demonstrate that NF157 significantly reduced expression of ADAMTS-4 and ADAMTS-5 at both the mRNA and protein levels in a dose-dependent manner. This suggests that expression of ADAMTS-4 and ADAMTS-5 may be regulated via the P2Y11R pathway.
Figure 7.
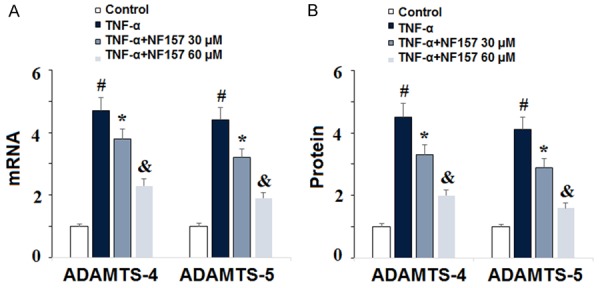
Inhibition of P2Y11R using its specific antagonist NF157 reduced TNF-α-induced expression of ADAMTS-4 and ADAMTS-5. Human chondrocytic SW1353 cells were treated with 10 ng/ml TNF-α in the presence or absence of NF157 at the concentrations of 30 and 60 μM for 24 h. A. mRNA of ADAMTS-4 and ADAMTS-5 as determined by real-time PCR; B. Protein of ADAMTS-4 and ADAMTS-5 as determined by ELISA (*, #, &, P<0.01 vs. previous column group).
NF157 reduces TNF-α-induced activation of NF-κB
Activation of the NF-κB pathway is a major driver of the inflammatory response. To explore the effects of blockage of P2Y11R on activation of the NF-κB pathway in OA, SW1353 cells were exposed to 10 ng/ml TNF-α in the presence or absence of 30 and 60 µM NF157 for 24 h. We investigated activation of NF-κB through quantification of nuclear translocation of p65 and by measuring the luciferase activity of NF-κB using a commercial kit. As shown in Figure 8, treatment with NF157 almost fully restored nuclear translocation of p65 triggered by TNF-α and significantly reduced the luciferase activity of NF-κB. This demonstrates that blockage of P2Y11R using its specific antagonist NF157 may be an effective strategy for regulating the NF-κB pro-inflammatory signaling pathway.
Figure 8.
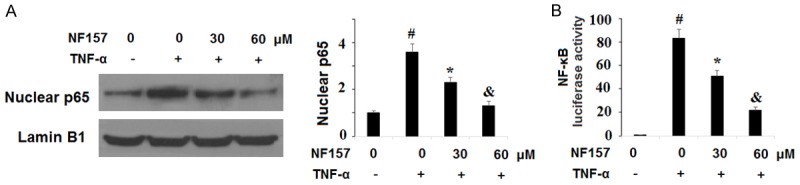
Inhibition of P2Y11R using its specific antagonist NF157 reduced TNF-α-induced activation of NF-κB. Human chondrocytic SW1353 cells were treated with 10 ng/ml TNF-α in the presence or absence of NF157 at the concentrations of 30 and 60 μM for 24 h. A. Nuclear level of p65; B. Promoter luciferase activity of NF-κB as determined by a commercial kit (*, #, &, P<0.01 vs. previous column group).
Discussion
As OA becomes an increasingly greater threat to human well-being, it is imperative that new strategies for the treatment and prevention of this painful disease are developed. Although OA has been under study for decades, the exact mechanisms driving the pathology of the disease remain unclear. Recently, the effects of new and existing drugs on degradation of type II collagen and aggrecan have been explored. It is assumed that blockage of one more upstream factors related to the expression of degradative enzymes that target type II collagen and aggrecan may be a safe and effective non-invasive therapeutic option. Presently, however, the preferred treatment for late-stage OA is arthroplasty, which creates a great burden for patients and their families due to its high cost, potential complications and possible long recovery times, the risks of which increase with age. Therefore, innovative and advanced research into the potential mechanisms of action of OA is of great and increasing clinical value. In the present study, we explored the involvement of the P2Y11 receptor in TNF-α-induced degradation of type II collagen and aggrecan using the novel P2Y11R antagonist NF157. Our findings indicate that blockage of P2Y11R with 30 and 60 µM NF157 potently inhibited TNF-α-induced expression of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5, subsequent degradation of type II collagen and aggrecan, and activation of NF-κB pro-inflammatory signaling in a dose-dependent manner.
Adenosine 5’-triphosphate (ATP), a damage-associated molecular pattern molecule (DAMP), is an important messenger molecule involved in intracellular communication. Various stimuli, such as inflammation, shear stress, apoptosis, etc., cause ATP to be released into the extracellular space where it triggers a signal cascade by binding to the ubiquitously expressed purinergic G protein-coupled P2Y receptors [17-19]. Of these, the P2Y11 receptor is coupled to both Gq/11 and Gs proteins [20]. Purinergic receptors including P2Y11 have been shown to be involved in the inflammatory response, and a recent study explored the role of P2Y11R in rheumatoid arthritis, but there has been little research on the role of P2Y11R in OA [21,22]. Furthermore, it has been demonstrated that the specific P2Y11 antagonist NF157 significantly suppresses elevation of TNF-α [23]. TNF-α is well-documented as an important cytokine in the pathogenesis of OA, which induces elevated expression of MMPs and ADAMTS, the two main enzymes involved in degradation of the articular ECM [24,25]. However, the exact mechanisms driving TNF-α-induced upregulation of collagenases (MMPs) and aggrecanases (ADAMTS) in OA remain to be fully elucidated. As demonstrated by the results of this study, blockage of P2Y11R by NF157 had a significant inhibitory effect on the expression of MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 in SW1353 chondrocytes at both the mRNA and protein levels in a dose-dependent manner. Furthermore, our results confirm that this inhibition of MMPs and ADAMTS results in significant rescue of degradation of type II collagen and aggrecan. These findings imply the P2Y11R may indeed play a role in regulating degradation of the articular ECM in OA.
NF-κB is well-recognized as a key pro-inflammatory transcription factor that promotes the development and progression of OA as well as many other inflammatory diseases. A recent study from Japan demonstrated that activation of the P2Y11 receptor via γ-radiation-induced release of ATP activated NF-κB through the p38/mitogen-activated protein kinase (MAPK) pathway [26]. In a related study, the authors showed that treatment with NF157 inhibited activation of NF-κB [27]. Another study demonstrated that ATP selectively targeted p65 to activate NF-κB through the P2Z purinoceptor, which like P2Y11R, has a high affinity for ATP [28,29]. In the present study, we showed that TNF-α-induced nuclear translocation of p65 could be suppressed by blockage of P2Y11R with 30 and 60 µM NF157 in a dose-dependent manner. Additionally, we confirmed that antagonism of P2Y11R with NF157 significantly decreased NF-κB luciferase activity in a dose-dependent manner. The findings of our study demonstrate for the first time that blockage of the P2Y11 purinoceptor using the selective P2Y11 antagonist NF157 can significantly prevent degradation of the components of the articular ECM by downregulating TNF-α-induced expression of MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5, subsequent degradation of type II collagen and aggrecan, and activation of NF-κB. These positive results suggest that NF157 may have potential as a targeted therapeutic agent for the treatment and prevention of excessive degradation of type II collagen and aggrecan in OA. Further study is required to better understand the complex mechanisms through which these results were achieved.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (No. 81572174, 81272040, and 81772384).
Disclosure of conflict of interest
None.
References
- 1.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pap G, Eberhardt R, Stürmer I, Machner A, Schwarzberg H, Roessner A, Neumann W. Development of osteoarthritis in the knee joints of Wistar rats after strenuous running exercise in a running wheel by intracranial self-stimulation. Pathol Res Pract. 1998;194:41–47. doi: 10.1016/S0344-0338(98)80010-1. [DOI] [PubMed] [Google Scholar]
- 3.Osteoarthritis [Online] Centers for Disease Control and Prevention Centers for Disease Control and Prevention: 2018 [Google Scholar]
- 4.Qu H, Li J, Wu L, Chen W. Trichostatin a increases the TIMP-1/MMP ratio to protect against osteoarthritis in an animal model of the disease. Mol Med Rep. 2016;14:2423–2430. doi: 10.3892/mmr.2016.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshihara Y, Nakamura H, Obata KI, Yamada H, Hayakawa T, Fujikawa K, Okada Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann Rheum Dis. 2000;59:455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang W, Ao P, Li J, Wu T, Xu L, Deng Z, Chen W, Yin C, Cheng X. Autophagy protects advanced glycation end product-induced apoptosis and expression of MMP-3 and MMP-13 in rat chondrocytes. Biomed Res Int. 2017;2017:6341919. doi: 10.1155/2017/6341919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmoud RK, El-Ansary AK, El-Eishi HH, Kamal HM, El-Saeed NH. Matrix metalloproteinases MMP-3 and MMP-1 levels in sera and synovial fluids in patients with rheumatoid arthritis and osteoarthritis. Ital J Biochem. 2005;54:248–257. [PubMed] [Google Scholar]
- 8.Huang CY, Lai KY, Hung LF, Wu WL, Liu FC, Ho LJ. Advanced glycation end products cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription 3 pathway in porcine chondrocytes. Rheumatology. 2011;50:1379–1389. doi: 10.1093/rheumatology/ker134. [DOI] [PubMed] [Google Scholar]
- 9.Doege KJ, Sasaki M, Kimura T, Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991;266:894–902. [PubMed] [Google Scholar]
- 10.Sarah P, Ian MC, Lara K, Dylan RE. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, Flannery CR, Kanki K, Wang E, Peluso D, Yang Z, Majumdar MK, Morris EA. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–2558. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- 12.Krappmann D, Emmerich F, Kordes U, Scharschmidt E, DoÈrken B, Scheidereit C. Molecular mechanisms of constitutive NF-κB/Rel activation in Hodgkin/Reed-Sternberg cells. Oncogene. 1999;18:943. doi: 10.1038/sj.onc.1202351. [DOI] [PubMed] [Google Scholar]
- 13.Communi D, Robaye B, Boeynaems JM. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreisig K, Kornum BR. A critical look at the function of the P2Y11 receptor. Purinergic Signal. 2016;12:427–447. doi: 10.1007/s11302-016-9514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tocris Bioscience. NF157 Product Data Sheet. 2016 [Google Scholar]
- 16.Gebauer M, Saas J, Sohler F, Haag J, Söder S, Pieper M, Bartnik E, Beninga J, Zimmer R, Aigner T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1β. Osteoarthritis Cartilage. 2005;13:697–708. doi: 10.1016/j.joca.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 18.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Burnstock G. Purinergic signalling: past, present and future. Braz J Med Biol Res. 2009;42:3–8. doi: 10.1590/s0100-879x2008005000037. [DOI] [PubMed] [Google Scholar]
- 20.Greig AV, Linge C, Terenghi G, McGrouther DA, Burnstock G. Purinergic receptors are part of a functional signaling system for proliferation and differentiation of human epidermal keratinocytes. J Invest Dermatol. 2003;120:1007–1115. doi: 10.1046/j.1523-1747.2003.12261.x. [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, Hosoi J, Denda M. Extracellular ATP has stimulatory effects on the expression and release of IL-6 via purinergic receptors in normal human epidermal keratinocytes. J Invest Dermatol. 2007;127:362–371. doi: 10.1038/sj.jid.5700526. [DOI] [PubMed] [Google Scholar]
- 22.Tulapurkar ME, Schäfer R, Hanck T, Flores RV, Weisman GA, González FA, Reiser G. Endocytosis mechanism of P2Y2 nucleotide receptor tagged with green fluorescent protein: clathrin and actin cytoskeleton dependence. Cell Mol Life Sci. 2005;62:1388–1399. doi: 10.1007/s00018-005-5052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaki H, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS One. 2013;8:e59778. doi: 10.1371/journal.pone.0059778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motifs (ADAMTS)-5 mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–49. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedbom E, Häuselmann HJ. Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell Mol Life Sci. 2002;59:45–53. doi: 10.1007/s00018-002-8404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohsaki A, Miyano Y, Tanaka R, Tanuma SI, Kojima S, Tsukimoto M. A novel mechanism of γ-irradiation-induced IL-6 production mediated by P2Y11 receptor in epidermal keratinocytes. Biol Pharm Bull. 2018;41:925–936. doi: 10.1248/bpb.b18-00075. [DOI] [PubMed] [Google Scholar]
- 27.Ohsaki A, Tanuma SI, Tsukimoto M. TRPV4 channel-regulated ATP release contributes to γ-irradiation-induced production of IL-6 and IL-8 in epidermal keratinocytes. Biol Pharm Bull. 2018;41:1620–1626. doi: 10.1248/bpb.b18-00361. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-κB through the P2Z purinoreceptor by selectively targeting NF-κB p65 (RelA) J Cell Biol. 1997;139:1635–43. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadet S, Ivanes F, Benoist L, Salmon-Gandonnière C, Guibon R, Velge-Roussel F, Babuty D, Baron C, Roger S, Angoulvant D. Hypoxia/reoxygenation inhibits P2Y11 receptor expression and its immunosuppressive activity in human dendritic cells. J Immunol. 2015;195:651–60. doi: 10.4049/jimmunol.1500197. [DOI] [PubMed] [Google Scholar]
- 30.Khalid M, Brisson L, Tariq M, Hao Y, Guibon R, Fromont G, Mortadza SAS, Mousawi F, Manzoor S, Roger S, Jiang LH. Carcinoma-specific expression of P2Y11 receptor and its contribution in ATP-induced purinergic signalling and cell migration in human hepatocellular carcinoma cells. Oncotarget. 2017;8:37278–37290. doi: 10.18632/oncotarget.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]


