Abstract
Background: The erythropoietin helix B surface peptide (HBSP) has been shown to have neuroprotective and repair-damaging myocardium effects similar to erythropoietin (EPO). However, the protective mechanism of HBSP on cardiomyocyte hypoxia-reoxygenation (H/R) injury is not clear. Methods: H9C2 cells were pretreated with HBSP and subjected to hypoxia/reoxygenation (H/R), changes in cell function, autophagy and apoptosis were assessed, respectively. Cells were transfected with miR-21 mimic and miR-NC, and the relative expression of miR-21 and Atg12 were detected by qRT-PCR. The target role of miR-21 and Atg12 was evaluated by dual-luciferase reporter. After transfected with si-Atg12 and si-NC, western blot was used to assess autophagy and apoptosis proteins, flow cytometry assay was used to detect apoptosis rate. Results: We found the expression of miR-21 was significantly down-regulated, accompanied by remarkably activated of autophagy and apoptosis in H9C2 cells during H/R injury. Pleasantly, HBSP pretreatment has a similar effect as transfection of miR-21 mimic, which is to evidently inhibit autophagy and apoptosis by up-regulating miR-21 expression. Moreover, Bioinformatics analysis and luciferase reporter assay revealed that Atg12 was directly bond to miR-21. To further understand whether Atg12 is involved in the process of miR-21 regulating autophagy, si-Atg12 and si-NC were transfected into H9C2 cell, the results showed that knockdown of Atg12 enhances the inhibition autophagy and apoptosis effect of HBSP. Conclusion: These results demonstrate that HBSP inhibits myocardial H/R injury induced by autophagy over-activation and apoptosis via miR-21/Atg12 axis.
Keywords: Erythropoietin helix B surface peptide, myocardial hypoxia reoxygenation injury, miR-21, Atg12
Introduction
Acute myocardial infraction (AMI) is one of the most important reasons of human death [1]. Early effective reperfusion therapy including thrombolysis and percutaneous coronary intervention can significantly reduce myocardial infraction area and improve cardiac function, and has become crucial treatment AMI [2,3]. Despite recovery of blood supply by reperfusion, severe arrhythmia, endothelial dysfunction, stunned myocardium, apoptosis and over-activation of autophagy abolish the benefits of reperfusion [4]. Altered autophagy has been observed in ischemic heart disease, cardiac hypertrophy and heart failure in response to pathological stimuli [5]. Although some studies indicate that cell death caused by excessive activation of autophagy is the important reason of reperfusion injury, there is no effect treatment for autophagy-related reperfusion injury [6].
Erythropoietin (EPO) is a glycoprotein hormone secreted by kidney that promotes hematopoiesis. Recent years, experiments have proved that EPO has protective effect in myocardial infraction, MIRI and heart failure models, but adverse reaction such as thrombosis and hypertension limit its clinical application [7]. Erythropoietin helix B surface peptide (HBSP) is a peptide derived from the waterborne surface of helix B in the tertiary structure of the EPO molecule, which preserves the tissue protective effect of EPO and removes the erythropoiesis effect [8]. Several studies have been demonstrated that HBSP significantly reduced myocardial infraction area and MIRI through anti-apoptosis, anti-inflammatory and anti-oxidative stress [9,10]. In mice liver I/R model, HBSP protected hepatic I/R injury via regulating autophagy by targeting mTOR [7].
microRNAs (miRNAs) are act as negative regulators of gene expression by pairing with sites in the 3’-UTR regions of mRNAs of protein-coding genes. Many studies have revealed that miRNAs are involved in cardiac muscle contraction, conducting electrical signals, heart growth and pathogenesis of MIRI [11]. miR-21 has many studies on the pathogenesis and progression of various tumors [12]. Evidence from Xing et al demonstrated that gastrodin exerts the protective effect of hypoxia-induced H9c2 cell injury through activating PTEN/PI3K/AKT and NF-κB pathways via up-regulating miR-21 [13]. However, it remains unclear whether miR-21 could influence autophagy during I/R in cardiomyocytes.
In this study, we constructed H/R cardiomyocytes model to mimic MIRI in vitro and observe the effect of HBSP on autophagy and apoptosis in H/R induced H9C2 cell. Meanwhile, we explored the possibly regulatory mechanism of HBSP through investigating the target relationship between miR-21 and Atg12.
Materials and methods
Cell culture and treatment
Rat ventricular H9c2 cell line purchased from Chinese Academy of Sciences were cultured in DMEM medium (Gibco) containing 10% fetal bovine serum (Hyclone), supplemented with 100 U/ml penicillin and 100 g/ml streptomycin (Hyclone), and incubation under an atmosphere of 5% CO2 and 95% air at 37°C. For cell H/R model, cells were incubated in a hypoxic plastic chamber filled with a mixture of 95% N2 and 5% CO2 and replaced the media with fresh 1.5 g/L glucose DMEM without serum for 6 h at 37°C. After that, cells were incubated in 95% air with normal media for 6 h reoxygenation [14]. HBSP was synthesized at Shanghai Ketai Biotechnology and dissolved in sterile saline to 1 μg/ml as mother solution. Before H/R treatment, cells were pretreated by HBSP (5 ng/ml) to observe its protective effects.
Cell transfection
The miR-21 negative control (NC), miR-21 mimic, si-Atg12 and si-NC were synthesized from GenePharma Co., Ltd (Shanghai, China). The sequences were as follow: miR-21 sense: 5’-CUUAGUGUUCAGACUAGGUGUUC-3’, miR-21 antisense: 5’-ACAGACUAGUCUGAACACUGUUGG-3’, miR-NC sense: 5’-UUCUCCGAACGUGUCACGUTT-3’, miR-NC antisense: 5’-ACGAUACACGUUCGGAGAATT-3’. The rat Atg12 RNAi target sequence is 5’-CCAAGCGCGTGTCTGAAAC-3’, a scramble form was used as a control (5’-AGCCGTGCACTGCACTAGC-3’). Then, the cells were transfected with Lipofectamine® 3000 (Invitrogen, Thermo Fisher Scientifc, Inc.) according to the manufacturer’s protocol. The medium was replaced for 6-8 h after transfection, and the cells were collected for further experiments after 48 h of transfection.
Cytotoxicity assays
Cell viability was assessed with cell count kit-8 (CCK-8, Dojindo, Japan) according to the manufacturer’s instructions. After treatment completed, 10 μl of CCK-8 solution were added in each well and incubated for 2 h. Absorbance values were obtained with an enzyme-mark-analyser (Bio-Rad, USA) at a wavelength of 450 nm. Cardiomyocyte cytotoxicity was evaluated by detecting the release of lactate dehydrogenase (LDH) from the cells. After cells exposure completed, cell supernatant was collected and LDH activity was determined by enzyme-mark-analyser (Bio-Rad, USA).
Flow cytometry
The apoptotic ratio of cells was performed by Annexin-V/PI double staining (Keygen, Nanjing, China). Cells were collected and washed twice by cold D-Hanks solution (Keygen, Nanjing, China) and then suspend with 100 μl binding buffer. 5 μl Annexin V-FITC solution and 10 μl PI solution were added and incubated 15 min at room temperature. Subsequently, 400 μl binding buffer was added and the apoptotic ratio of cells was detected using a flow cytometer (Becton, USA) with an excitation wavelength of 488 nm.
TUNEL assay
TUNEL cell death detection kit was purchased from Roche (Germany) to detect cell apoptosis death ratio. Briefly, cells were cultured in glass coverslips and fixed with 4% paraformaldehyde for 15 min. After washed by PBS, cells were permeabilized in 0.1% Triton X-100 solution and stained with reaction mixture at 37°C for 60 min. Next, cells were cultured with DAPI (Solarbio, Beijing) for 10 min. The images were captured by fluorescence microscope (Nikon, Japan) and apoptosis rate were calculated from five fields in each group.
Fluorescence microscopy
H9C2 cell was transfected pcDNA3.1-GFP-LC3 plasmid and then treated as indicated. The plasmid pcDNA3.1-GFP-LC3 was extracted according to the instructions of the plasmid minipre2p purification kit (Beyotime, Jiangsu, China). Cells were washed with PBS and fixed with 4% paraformaldehyde. All cell images were visualized using a laser scanning microscope (Nikon, Japan). Cells with diffuse cytoplasmic GFP-LC3 staining were regarded as non-autophagic cells, those with more intense GFP-LC3 puncta were considered autophagic cells. A minimum of 50 cells were scored for each condition.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells. Quantitative real-time PCR was performed with an ABI 7500 Fast Real Time PCR system (Applied Biosystems, USA) using a reverse transcript kit (Takara, Dalian) and SYBR Green Master Mix kit (Takara, Dalian) according to the manufacturer’s instructions. Reverse transcription reaction conditions were processed at 42°C for 15 min, followed by 3 min at 95°C. The thermal cycling conditions were processed at 95°C pre-denaturation, followed by 40 cycles (95°C for 15 s; 60°C for 30 s; 72°C for 60 s). Relative quantification was determined by normalization to U6. The primers are shown in Table S1. All the qRT-PCR expression experiments were performed in triplicates to ensure the reproducibility. The relative gene expression was calculated using 2-ΔΔCt method.
Luciferase reporter assay
The wild type and mutant sequences of Agt12-3’UTR were synthesized and each sequence was ligated to a pmiR-report plasmid. 293T cell were seeded into 96-well plates with transfection mixture. The luciferase activity was detected by Dual-Glo Luciferase Assay System (Promega, Madison, WI, USA). The Renilla luciferase plasmid was used as an internal control for determining transfection efficiency.
Western blotting
Total protein was extracted using Lysis buffer (Solarbio, Beijing) and protein quantification was performed using BCA assay (Solarbio, Beijing). 10 μg proteins were loaded on SDS-PAGE and protein was transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 0.1% TBST with 5% defatted milk for 2 h at room temperature. The membranes were incubated with primary antibodies at 4°C overnight, then washed and incubated with secondary antibodies for 1.5 h at room temperature. Antibodies against LC3B (2775, 1:1000), P62 (39749, 1:1000), Atg5 (12994, 1:1000), Atg12 (4180, 1:1000), cleaved caspase 3 (9661, 1:1000) and GAPDH (5174, 1:1000) were purchased from Cell Signaling Technology, lnc (USA). The detection protein bands were performed by an enhanced chemi-luminescence (ECL) for western blotting kit (Millipore, USA). GAPDH was used as an internal control. Relative expression levels were calculated using Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed with SPSS 20.0 software (SPSS, Inc., Chicago, USA). All data are expressed as the mean ± SD. The data were analyzed using two independent sample t-tests or one-way analysis of variance. All P-values < 0.05 were considered statistically significant.
Results
HBSP pretreatment alleviates cardiomyocyte H/R injury
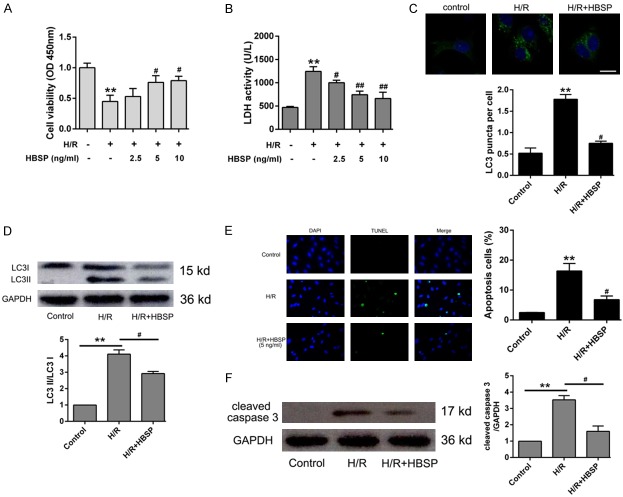
To investigate the protective effect of HBSP on cardiomyocytes H/R injury, the optimal dose of HBSP was screened using CCK-8 assay and LDH activity assay. As shown in Figure 1A and 1B, 5 ng/ml and 10 ng/ml HBSP pretreatment significantly reduced cell injuries, especially 5 ng/ml. To observe the formation of autophagosome, we transferred the constructed GFP-LC3 fusion fluorescent protein into H9C2 cells. The results showed that the fluorescent spots in the H/R group significantly increased, but decreased in the HBSP group, indicating that HBSP inhibits the accumulation of autophagosome induced by H/R (Figure 1C). Western blot analysis indicated that HBSP retreatment obviously decrease the expression of LC3II/LC3I ratio (Figure 1D). Moreover, TUNEL assay indicated that 5 ng/ml HBSP obviously inhibits apoptosis (Figure 1E). The elevated apoptosis protein cleaved caspase 3 was significantly reduced in H/R group when pretreated with 5 ng/ml HBSP (Figure 1F).
Figure 1.
HBSP pretreatment alleviates cardiomyocytes H/R injury. A. Cells were treated with different concentration of HBSP and cell viability was detected using CCK-8 kit. B. Cells were cultured with HBSP and the supernatant was collected to detect LDH activity. C. The relative expression of LC3B performed by Western blot. D. Cells were pretreated with HBSP, and then infected with GFP-LC3. Representative photos were shown in the upward side and cells with GFP-LC3 puncta were qualified in the downward. E. Representative images and quantitative of apoptosis was performed by TUNEL assay. F. Western blot analysis of cleaved caspase 3 after treated with HBSP. Data represent the mean ± SD. *P < 0.05, **P < 0.01 vs. Control group. #P < 0.05, ##P < 0.01 vs. H/R group.
HBSP reduces autophagy and apoptosis via up-regulating miR-21
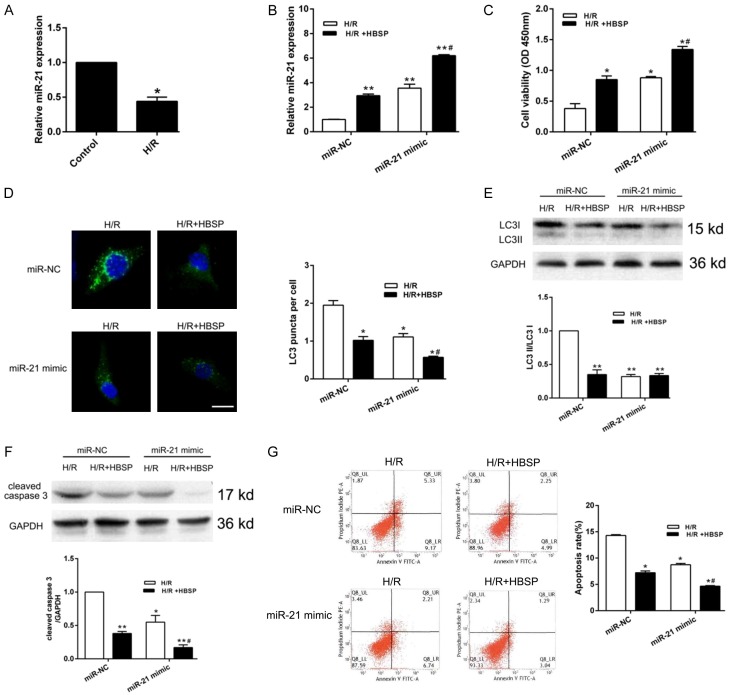
As shown in Figure 2A, qRT-PCR analysis indicated that miR-21 was lowly expressed in H/R group. However, miR-21 level was obviously elevated in HBSP pretreatment group and miR-21 mimic transfection group, suggesting that HBSP play a role in promoting miR-21 expression (Figure 2B). Then, CCK-8 assay was performed and the results indicated that the elevated of miR-21 induced by HBSP significantly recovered cell viability (Figure 2C). The accumulation of GFP-LC3 puncta is an important indicator of increased autophagy and autophagosome. The cumulative green fluorescent spots were observed in the H/R group (miR-NC), and fluorescent spots were significantly reduced after HBSP pretreatment (Figure 2D). In addition, HBSP reduced H/R induced up-regulation of LC3II/LC3I expression (Figure 2E) and enhanced the anti-apoptotic effect of miR-21 (Figure 2F, 2G), indicating an essential role of miR-21 in reducing the excessive activation of autophagy and apoptosis of cardiomyocytes by HBSP.
Figure 2.
HBSP affects cell autophagy and apoptosis by regulating miR-21. A. Relative expression of miR-21 in H9C2 cell was detected by qRT-PCR. B. Cells were transfected with miR-NC or miR-21 mimic and the expression of miR-21 was detected by qRT-PCR. C. CCK-8 was performed when cells transfected with miR-NC or miR-21 mimic. D. HBSP pretreatment and miR-21 mimic transfection inhibits fluorescence puncta accumulations of GFP-LC3. E, F. Representative Western blot and quantitative evaluation of LC3B and cleaved caspase 3. G. Representative images and quantitative of induction of apoptosis performed by flow cytometry analysis after HBSP pretreatment and miR-21 mimic transfection. Data represent the mean ± SD. *P < 0.05, **P < 0.01 vs. Control or H/R (miR-NC). #P < 0.05 vs. H/R+HBSP (miR-NC).
miR-21 directly targets Atg12
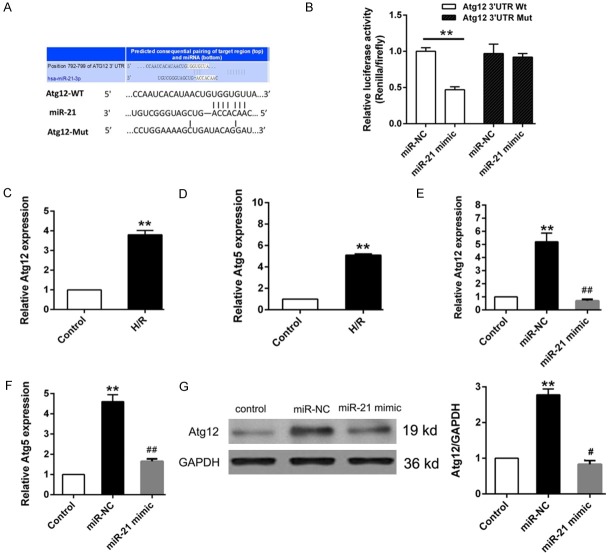
Based on the effect of miR-21 mimic on reducing LC3 aggregation, we used bioinformatics tool to screen the candidate autophagy related targets. Atg12 was stand out for its essential role in autophagosome membrane formation by forming a complex with Atg5 [15]. Bioinformatics analysis (TargetScan, http://www.targetscan.org/vert_71/) showed that the 3’-UTR of Atg12 contains putative binding sites for miR-21 (Figure 3A). The Dual luciferase reporter verified that the transcriptional activity of wild-type Atg12 3’-UTR was inhibited by miR-21, indicating the direct regulation of miR-21 on Atg12 (Figure 3B). In addition, qRT-PCR results indicated that Atg12 and Atg5 mRNAs were significantly elevated in H/R group (Figure 3C, 3D), but their expression were obviously depressed after transfected with miR-21 mimic (Figure 3E, 3F). Western blot further confirmed that miR-21 was directly targets Atg12 (Figure 3G).
Figure 3.
miR-21 directly targets Atg12. A. Bioinformatics analysis was performed to predict the putative binding site of between Atg12 and miR-21. B. The target relationship between miR-21 and Atg12 was confirmed by Dual luciferase reporter assay. C, D. Relative expression of Atg12 and Atg5 was determined by qRT-PCR. E, F. Cells were transfected with miR-NC or miR-21 mimic, the expression of Atg12 and Atg5 were detected by qRT-PCR. G. Western blot analysis of Atg12 expression. Data represent the mean ± SD. *P < 0.05, **P < 0.01 vs. Control, #P < 0.05 vs. miR-NC.
HBSP attenuates accumulation of autophagy and apoptosis by regulating miR-21/Atg12 axis
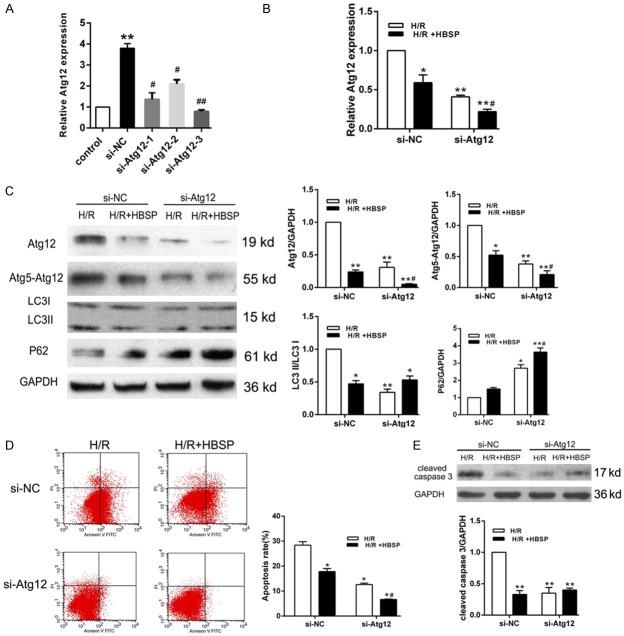
Next, we wondered whether Atg12 is the responder of HBSP regulating miR-21 on autophagy and apoptosis. We transfected H9C2 cell with siRNA against Atg12 (si-Atg12-1, si-Atg12-2 and si-Atg12-3) and detect expression levels of Atg12 using qRT-PCR (Figure 4A). According the results, Atg12-3 was selected for subsequent experiments due to higher transfection efficiency. As shown in Figure 4B, HBSP pretreatment strengthened the silence effect of Atg12. Besides, the pretreatment of HBSP significantly reduced Atg12 expression, Atg5-Atg12 complex formation, LC3II/LC3I ratio and P62 expression, further confirming the inhibited autophagy in H9C2 cells (Figure 4C). The results of flow cytometry assay and western blot also demonstrated that knockdown of Atg12 suppresses apoptosis, and HBSP treatment enhances the anti-apoptotic effect of si-Atg12 (Figure 4D). The above results indicate that HBSP pretreatment attenuates autophagy and apoptosis induced by cardiomyocyte H/R injury via regulating miR-21/Atg12 axis.
Figure 4.
Knockdown of Atg12 enhances the inhibition autophagy and apoptosis effect of HBSP. A. The transfection efficiency of Atg12 was determined by qRT-PCR. B. Cells were transfected with si-NC or si-Atg12 to detect the mRNA level of Atg12. C. HBSP pretreatment enhances the effect of knockout Atg12 on the expression of autophagy proteins Atg12, Atg5-Atg12, LC3B and P62. D. Cell apoptosis was assessed by flow cytometry assay and the experiment was repeated three times for the calculation of apoptosis rate. E. Cleaved caspase 3 was detected using Western blot. Data represent the mean ± SD. *P < 0.05, **P < 0.01 vs. Control or H/R (si-NC). #P < 0.05 vs. si-NC or H/R+HBSP (si-NC).
Discussion
The verification of the interaction between therapeutic drugs and miRNAs provides new sight for the mechanism of action of potential drugs. As a newly developed derivative of EPO, HBSP preserves the tissue protective effect of EPO with none of its adverse reaction. Liu et al [9] demonstrated that HBSP preconditioning significantly reducing infract size and myocardial apoptosis and improving cardiac function after MIRI. In mice hepatic I/R model, HBSP was confirmed enhance autophagy and reduce apoptosis against hepatic I/R injury [7]. However, whether HBSP can regulate miRNA expression to affect the fate of cardiomyocytes has not been reported. Our results firstly reported the protective effect of erythropoietin derivative HBSP against myocardial H/R injury by regulating miR-21/Atg12 axis.
Current study indicates that autophagy can be a double-edged sword in the process of I/R [16]. Although ATP generation and cellular homeostasis can mitigate I/R injury in ischemia phase, the underlying mechanism of autophagy caused cell death during reperfusion is still unclear. In this study, H9C2 cell was treated with H/R to simulate I/R injury, and the results showed that the cell survival rate was significantly decreased, the ratio of LC3II/LC3I and apoptosis rate were increased, indicating that the autophagy was activated to cause cell damage.
MiRNA-mediated autophagy-related gene expression and signaling pathways play important role in the pathophysiological process of MIRI [17]. Hu et al showed that Fibroblast growth factor 21 protects myocardial I/R injury by inhibiting autophagy related gene Angpt2 expression and up-regulating miR-145 level [17]. Overexpression of miR-204 promoted the protective effect against myocardial H/R injury via regulating Atg5-mediated autophagy [18]. miR-21 is highly expressed in the adult heart and plays a crucial role in regulating the biological function of myocardial tissue, including apoptosis and autophagy [19]. Overexpression of miR-21 inhibits autophagic activity and decreases apoptosis in H9C2 cell during H/R injury, and these function are mediated by Akt/mTOR pathway [20]. We showed the expression of miR-21 in H/R group was significantly down-regulated, accompanied by the accumulation of autophagosome and the increase of apoptosis, whereas cells transfected with miR-21 mimic significantly inhibited autophagosome formation and apoptosis. These observations are consistent with the reported role of miR-21 in MIRI.
The Atg12 ubiquitin-like coupled binding system is one of two unique ubiquitin-like systems for autophagy. Atg12 and Atg5 bind by covalent bond under the action of E1-Atg7 and E2-Atg10, respectively, and then bind to the N-terminus of Atg16L to form the large protein complex [21,22]. Atg12 and Atg5 are essential proteins that form autophagosome and are involved in the interaction crosstalk of autophagy and apoptosis. We used bioinformatics analysis to predict that Atg12 is a target gene for miR-21. Moreover, qRT-PCR and western blot and luciferase reporter assay verified that miR-21 inhibits the expression of Atg12 by pairing with the 3’UTR region of the Atg12. To further understand whether Atg12 is involved in the process of miR-21 regulating autophagy, we transfected si-Atg12 into H9C2 cell and the results showed that the expression of autophagy related proteins and apoptosis rate were all reduced. It was found that Atg12 is not only required for caspase activation under a series of different apoptotic stimuli, but also promotes apoptosis by binding BCL-2, a BH3-like domain of Mcl-1 [23]. In this study, we found that the elevated expression of Atg12 in H/R group is accompanied by a significantly increased of cleaved caspase 3, which may be one of the mechanisms of increased autophagy and apoptosis induced by I/R injury.
In summary, our study demonstrated that HBSP pretreatment significantly attenuates myocardial H/R-induced autophagy activation and apoptosis increased by modulating the miR-21/Atg12 axis, providing a therapeutic basis for HBSP treatment of MIRI.
Acknowledgements
This work was supported by Shantou medical science and technology project NO.20180323262-02 and Shantou medical science and technology project.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 2.Guedeney P, Claessen BE, Mehran R, Kandzari DE, Aquino M, Davis S, Tamis L, Wang JC, Othman I, Gigliotti OS, Haghighat A, Singh S, Lopez M, Giugliano G, Horwitz PA, Sorrentino S, Underwood P, Allocco D, Meredith IT, Batchelor W. Small-vessel PCI outcomes in men, women, and minorities following platinum chromium everolimus-eluting stents: insights from the pooled PLATINUM diversity and PROMUS element plus post-approval studies. Catheter Cardiovasc Interv. 2019 doi: 10.1002/ccd.28071. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Steele L, Palmer J, Lloyd A, Fotheringham J, Iqbal J, Grech ED. The impact of smoking on mortality after acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: a retrospective cohort outcome study at 3 years. J Thromb Thrombolysis. 2019 doi: 10.1007/s11239-019-01812-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Binder A, Ali A, Chawla R, Aziz HA, Abbate A, Jovin IS. Myocardial protection from ischemia-reperfusion injury post coronary revascularization. Expert Rev Cardiovasc Ther. 2015;13:1045–1057. doi: 10.1586/14779072.2015.1070669. [DOI] [PubMed] [Google Scholar]
- 5.Xie M, Morales CR, Lavandero S, Hill JA. Tuning flux: autophagy as a target of heart disease therapy. Curr Opin Cardiol. 2011;26:216–222. doi: 10.1097/HCO.0b013e328345980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CY, Zhang YH, Li RB, Zhou LY, An T, Zhang RC, Zhai M, Huang Y, Yan KW, Dong YH, Ponnusamy M, Shan C, Xu S, Wang Q, Zhang YH, Zhang J, Wang K. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53-mediated myocardin transcription. Nat Commun. 2018;9:29. doi: 10.1038/s41467-017-02280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan R, Tian H, Yang B, Zhang B, Dai C, Han Z, Wang M, Li Y, Wei L, Chen D, Wang G, Yang H, He F, Chen Z. Autophagy and Akt in the protective effect of erythropoietin helix B surface peptide against hepatic ischaemia/reperfusion injury in mice. Sci Rep. 2018;8:14703. doi: 10.1038/s41598-018-33028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin C, Zhang M, Zhang Y, Yang K, Hu J, Si R, Zhang G, Gao B, Li X, Xu C, Li C, Hao Q, Guo W. Helix B surface peptide attenuates diabetic cardiomyopathy via AMPK-dependent autophagy. Biochem Biophys Res Commun. 2017;482:665–671. doi: 10.1016/j.bbrc.2016.11.091. [DOI] [PubMed] [Google Scholar]
- 9.Liu P, You W, Lin L, Lin Y, Tang X, Liu Y, Miao F. Helix B surface peptide protects against acute myocardial ischemia-reperfusion injury via the RISK and SAFE pathways in a mouse model. Cardiology. 2016;134:109–117. doi: 10.1159/000443680. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Lin Y, Tang X, Zhang P, Liu B, Liu Y, Miao F. Helix B surface peptide protects cardiomyocytes against hypoxia/reoxygenation-induced apoptosis through mitochondrial pathways. J Cardiovasc Pharmacol. 2016;67:418–426. doi: 10.1097/FJC.0000000000000367. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Jia Z, Zhang C, Sun M, Wang W, Chen P, Ma K, Zhang Y, Li X, Zhou C. miR-499 protects cardiomyocytes from H 2O 2-induced apoptosis via its effects on Pdcd4 and Pacs2. RNA Biol. 2014;11:339–350. [Google Scholar]
- 12.Ulivi P, Petracci E, Marisi G, Baglivo S. Prognostic role of circulating miRNAs in early-stage non-small cell lung cancer. J Clin Med. 2019;8 doi: 10.3390/jcm8020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Y, Li L. Gastrodin protects rat cardiomyocytes H9c2 from hypoxia-induced injury by up-regulation of microRNA-21. Int J Biochem Cell Biol. 2019;109:8–16. doi: 10.1016/j.biocel.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Wu QF, Qian C, Zhao N, Dong Q, Li J, Wang BB, Chen L, Yu L, Han B, Du YM, Liao YH. Activation of transient receptor potential vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomyocytes. Cell Death Dis. 2017;8:e2828. doi: 10.1038/cddis.2017.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otomo C, Metlagel Z, Takaesu G, Otomo T. Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat Struct Mol Biol. 2013;20:59–66. doi: 10.1038/nsmb.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiersma M, Meijering RAM, Qi XY, Zhang D, Liu T, Hoogstra-Berends F, Sibon OCM, Henning RH, Nattel S, Brundel B. Endoplasmic reticulum stress is associated with autophagy and cardiomyocyte remodeling in experimental and human atrial fibrillation. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu S, Cao S, Tong Z, Liu J. FGF21 protects myocardial ischemia-reperfusion injury through reduction of miR-145-mediated autophagy. Am J Transl Res. 2018;10:3677–3688. [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu R, Li W, Liu Y. MicroRNA-204 protects H9C2 cells against hypoxia/reoxygenation-induced injury through regulating SIRT1-mediated autophagy. Biomed Pharmacother. 2018;100:15–19. doi: 10.1016/j.biopha.2018.01.165. [DOI] [PubMed] [Google Scholar]
- 19.Fu X, He Y, Wang X, Peng D, Chen X, Li X, Wang Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther. 2017;8:187. doi: 10.1186/s13287-017-0641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, Wu S, Kong F, Cai X, Ye B, Shan P, Huang W. MicroRNA-21 protects against cardiac hypoxia/reoxygenation injury by inhibiting excessive autophagy in H9c2 cells via the Akt/mTOR pathway. J Cell Mol Med. 2017;21:467–474. doi: 10.1111/jcmm.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng Y, Song Y, Li Z, Wang Y, Lin H, Cheng H, Zhou R. RAB37 interacts directly with ATG5 and promotes autophagosome formation via regulating ATG5-12-16 complex assembly. Cell Death Differ. 2018;25:918–934. doi: 10.1038/s41418-017-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walczak M, Martens S. Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy. 2013;9:424–425. doi: 10.4161/auto.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubinstein AD, Eisenstein M, Ber Y, Bialik S, Kimchi A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol Cell. 2011;44:698–709. doi: 10.1016/j.molcel.2011.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.