Abstract
MicroRNAs (miRNAs) are often abnormally expressed in human cancers to act as either oncogenes or tumor suppressor genes. MiRNA-501 (miR-501) has been found to be abnormally expressed in certain types of cancer, but its expression and biological role in hemangioma remain to be fully elucidated. In this study, the expression of miR-501 in hemangioma cell lines was analyzed by quantitative real-time polymerase chain reaction (qRT-PCR). The TargetScan algorithm, luciferase activity reporter assay, and Western blot analysis were conducted to validate homeobox D10 (HOXD10) as a direct target of miR-501. The results revealed that miR-501 expression was upregulated in hemangioma cell lines. Downregulation of miR-501 inhibited hemangioma cell proliferation, cell cycle progression, colony formation, migration, and invasion in vitro. Bioinformatics analysis indicated that HOXD10 was a putative target of miR-501. In addition, in a luciferase reporter system, it was confirmed that HOXD10 is a direct target of miR-501. It was also demonstrated HOXD10 downregulation reversed the effects of the miR-501 inhibitor on hemangioma cell activities. These findings indicated that miR-501 targeted HOXD10 to promote hemangioma cell processes, suggesting that miR-501 has an oncogenic role in the pathogenesis of hemangioma.
Keywords: miR-501, HOXD10, hemangiomas, cell behaviors, oncogenic miRNA
Introduction
Hemangioma (HA) is one of the most common infantile tumors and is characterized as an abnormal blood vessel endothelial cell growth [1]. Approximately 10-15% of HAs can be life threatening, but most HAs do not require intervention [2,3]. The current treatment regimens for HAs, including chemotherapy and surgery, are not effective enough to prevent death from HA [4]. However, currently, little is known about the mechanisms underlying HAs progression.
MicroRNAs (miRNAs), a class of non-coding RNA molecules of 18-25 nucleotides in length, are closely associated with abnormal cell activities in human cancers, including proliferation, migration, invasion, apoptosis and drug resistance [5-7]. MiR-501 has been reported to be abnormally expressed in multiple malignancies, including hepatocellular carcinoma, cervical cancer, and clear cell renal cell carcinoma [8-11]. For instance, miR-501 expression was significantly enhanced in hepatitis B virus (HBV)-infected cell lines and tissues [8]. It was also found that downregulation of miR-501 significantly inhibited HBV replication by targeting the hepatitis B X-interacting protein [8]. In addition, a single nucleotide polymorphism (rs112489955, G>A) in the mature region of miR-501 was shown to disrupt the interaction with cylindromatosis (CYLD) and such variant genotype was associated with enhanced tumor growth [9]. Moreover, miR-501 was found upregulated, while CYLD was downregulated, in cervical cancer tissues [10]. The overexpression of miR-501 or downregulation of CYLD was a poorer indicator for malignancy of cervical cancer [10]. Additionally, upregulation of miR-501 promoted cervical cell proliferation, invasion, and migration through targeting CYLD [10]. On the other hand, miR-501 was shown to be downregulated in clear cell renal cell carcinoma and targeted various genes, including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), scavenger receptor class B member 1 (SCARB1) and heme oxygenase 1 (HMOX1), which correlated with cell renal cell carcinoma progression [11]. However, there has been no study examining the biological functions of miR-501 in HAs.
We conducted this study to investigate the expression levels of miR-501 in HA cell lines and evaluate the effects of miR-501 on cell proliferation, cell cycle, colony formation, migration, and invasion. We further examined whether homeobox D10 (HOXD10) was a functional target of miR-501.
Materials and methods
Cell lines and cell culture
The HDEC and EOMA cell lines were cultured in Dulbecco’s Modified Eagle’s medium (DMEM; Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.), 100 U/ml of penicillin and 100 μg/ml of streptomycin (Thermo Fisher Scientific, Inc.). Human umbilical vein endothelial cells (HUVECs) purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) were maintained in M200 medium (Thermo Fisher Scientific, Inc.) containing Low Serum Growth Supplement (LSGS; Thermo Fisher Scientific, Inc.). The cell lines were maintained at 37°C in a humidified incubator containing 5% of CO2.
Cell transfection
MiR-501 inhibitor (5’-AGAAUCCUUGCCCGGGUGCAUU-3’) and the corresponding negative control (NC-miR, 5’-GUCGGUUCGCAUACUCACUGGA-3’) were purchased from RiboBio (Guangzhou, China). Small interfering RNA targeting HOXD10 (si-HOXD10, 5’-CCGAACAGAUCUUGUCGAATT-3’) and NC-siRNA (5’-UUCUCCGAACGUGUCACGUTT-3’) were also purchased from RiboBio. All transfections were conducted using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Inc.) with cells grown to 80% confluence. After 48 h of transfection, cells were collected for further analyses.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
To determine the expression levels of miR-501, qRT-PCR analysis was performed. Total RNA was extracted with TRIzol (Beyotime, Haimen, Jiangsu, China) according to the manufacturer’s protocol. First-strand complementary deoxyribonucleic acid (cDNA) was synthesized using TaqMan microRNA Reverse Transcription Kit (Life Technologies, Thermo Fisher Scientific, Inc.). The qRT-PCR analysis was performed on an Applied Biosystems 7500 System (Applied Biosystems, Foster City, CA, USA) using a SYBR Green PCR kit (Takara, Dalian, China). The PCR conditions were as follows: 95°C for 10 min, then 45 cycles of 94°C for 20 s, 55°C for 30 s, and 70°C for 30 s. The sequences of the PCR primers used are as follows: miR-501 forward: 5’-CTGCTCTGCTCGTCCTCTCT-3’ and reverse 5’-CTCCTGTCCTCACATGAAGA-3’; U6 small nuclear RNA (U6 snRNA) forward: 5’-CTCGCTTCGGCAGCACA-3’ and reverse 5’-AACGCTTCACGAATTTGCGT-3’. Relative gene expression levels were calculated with the 2-ΔΔCt method using U6 snRNA as internal control.
Western blot analysis
Total protein was extracted from harvested cells using Radio Immunoprecipitation Assay lysis buffer (Beyotime). Equal amounts of proteins were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with fat-free milk, the membranes were incubated overnight at 4°C with the appropriate primary antibody (rabbit anti-HOXD10: ab138508, rabbit anti-matrix metalloprotein-9 (MMP-9): ab76003, rabbit anti-GAPDH: ab181602; Abcam, Cambridge, UK) according to the instructions provided by the manufacturer. Then, horseradish peroxidase-linked secondary antibody (ab6721; Abcam) was added and incubated at room temperature for 2 h. The bands were visualized using BeyoECL kit (Beyotime) according to the manufacturer’s instructions.
Cell proliferation assay
Cell proliferation was assessed by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. Cells were incubated at a density of 2 × 103 cells/well in 96-well-plates and incubated with 10 μl/well of MTT solution at the indicated time for 4 h. Then, 150 μl DMSO was added to each well to dissolve the crystals. Optical density was measured at 570 nm on an enzyme immunoassay analyzer (Bio-Rad, Hercules, CA, USA).
Cell cycle assay
Cell cycle distribution was determined by flow cytometry analysis. In brief, cells were harvested and fixed in 70% ethanol, and incubated with propidium iodide (PI)/RNase Staining Buffer (BD Biosciences, San Jose, CA, USA) for 15 min at room temperature and analyzed by flow cytometry.
Colony formation assay
A total 1 × 103 cells were seeded in 6-well plates and incubated for 14 days at the above-mentioned conditions. Colonies were stained with 0.1% crystal violet and counted under a microscope.
Wound healing assay
The wound healing assay was performed to measure cell migration. A 10-μl pipette tip was used to create scratches after the cells reached to 90% confluence. Images were captured at 0 or 24 h after wound creation under a microscope (Olympus Corporation, Tokyo, Japan).
Transwell invasion assay
A matrigel (BD Biosciences) coated transwell chamber with 8 μm pore size was used to measure cell invasion. A total of 2 × 104 cells were added to the upper chamber without FBS, and DMEM supplemented with FBS was added to the lower chamber. After 48 h of incubation, non-invading cells were removed by cotton swab. The invading cells were fixed with 100% ethanol and stained with 0.1% crystal violet for 10 min. Cell number was counted in five random fields and then the mean value was calculated.
Luciferase reporter assay
The wild-type (wt) and mutant (mt) 3’-untranslated region (3’-UTR) of HOXD10 were inserted into the pMIR-Report vector (Promega, Madison, WI, USA) and named as wt-HOXD10 and mt-HOXD10. Cells were co-transfected with 200 ng wt-HOXD10 and mt-HOXD10, and 50 μM of miR-501 inhibitor or NC-miR. Dual-luciferase reporter assay system (Promega) was used to measure luciferase activities after 48 h of transfection.
Statistical analysis
Data analysis was conducted using the SPSS 20.0 software (SPSS, IBM Corp., Armonk, NY, USA). Student’s t-test and analysis of variance (ANOVA) with the Tukey post-hoc test was used to analyze the statistical difference in two or above groups. A P<0.05 was regarded as statistically significant.
Results
miR-501 expression was upregulated and HOXD10 was downregulated in HA cell lines
To investigate the biological function of miR-501, we first analyzed miR-501 expression in HA cell lines by qRT-PCR. The analysis revealed significantly upregulated miR-501 levels in HDEC and EOM cells compared with the normal cells (Figure 1A). In addition, Western blot analysis was performed to determine the HOXD10 protein expression in HA cell lines. This analysis showed significantly downregulated HOXD10 protein expression in HA cell lines (Figure 1B).
Figure 1.
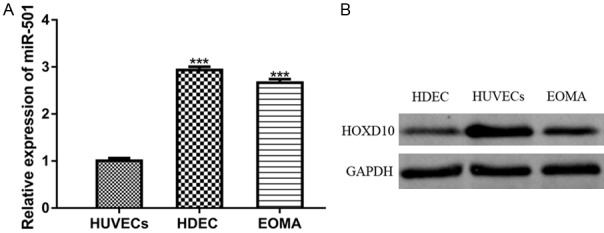
Expression of miR-501 was upregulated, while HOXD10 expression was downregulated in HA cell lines. (A) Expression of miR-501 in HA cell lines (HDEC and EOM) and HUVECs was analyzed by qRT-PCR analysis. (B) HOXD10 expression in HA cell lines (HDEC and EOM) and HUVECs was analyzed by Western blot analysis. (***P<0.001) miR-501: microRNA-501; HOXD10: homeobox D10; HAs: Hemangiomas; qRT-PCR: quantitative real-time polymerase chain reaction.
Downregulation of miR-501 suppressed cell proliferation, cell cycle progression and colony formation in vitro
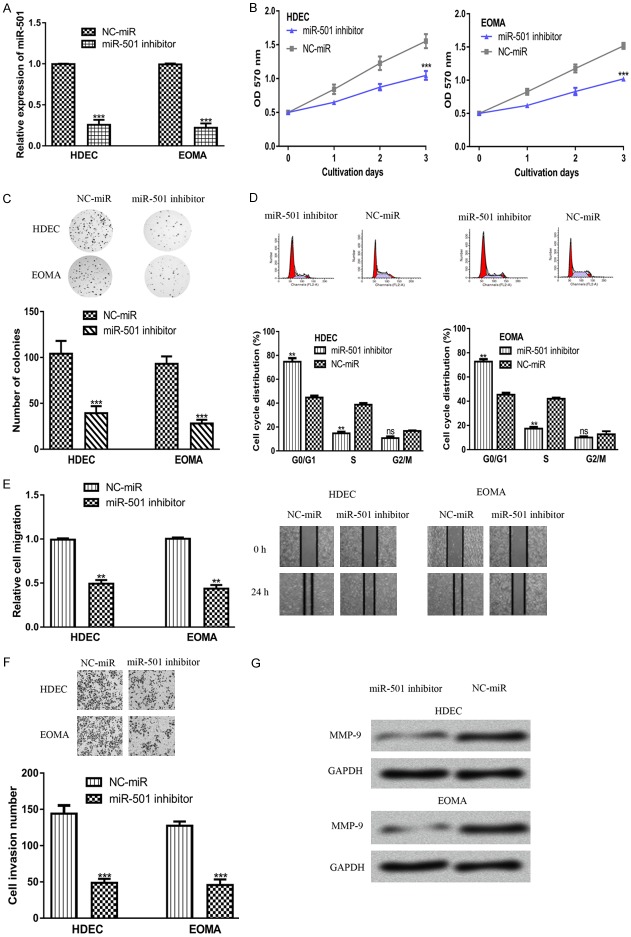
To investigate the biological functions of miR-501 in HAs, cells were transfected with miR-501 inhibitor or NC-miR and then subjected to cell proliferation and colony formation analyses. As shown in Figure 2A, transfection with the miR-501 inhibitor significantly decreased miR-501 expression levels in both cell lines. In addition, the MTT assay revealed that downregulation of miR-501 inhibited cell proliferation in both cell lines (Figure 2B). Also, the knockdown of miR-501 led to cell cycle arrest in the G0/G1 phase (Figure 2C). Additionally, the results of the colony formation assay revealed that the downregulation of miR-501 inhibited HA cell colony formation (Figure 2D). Together, these results indicated that downregulation of miR-501 inhibited cell proliferation and colony formation in vitro.
Figure 2.
MiR-501 regulates HA cell proliferation, colony formation, migration, and invasion. (A) miR-501, (B) cell proliferation, (C) cell cycle distribution, (D) colony formation, (E) cell migration, and (F) cell invasion, and (G) MMP9 expression in cells transfected with the miR-501 inhibitor or NC-miR. (***P<0.001, **P<0.01) miR-501: microRNA-501; HAs: Hemangiomas; NC-miR: negative control miRNA; MMP9: matrix metalloprotein 9.
Downregulation of miR-501 suppressed cell migration and invasion in vitro
The wound-healing assay revealed that the transfection of miR-501 inhibitor significantly decreased cell migration in both cell lines compared with NC-miR (Figure 2E). In addition, the result of the transwell invasion assay showed that cells transfected with the miR-501 inhibitor showed decreased cell invasion ability compared with those transfected with NC-miR (Figure 2F). Additionally, we also found that MMP9 expression was downregulated by the miR-501 inhibitor (Figure 2G). Therefore, miR-501 was able to inhibit HA cell migration and invasion in vitro.
Identification of HOXD10 as a target gene of miR-501
To investigate the molecular mechanism of miR-501 in HAs, TargetScan was used to determine the putative target of miR-501. The result revealed that HOXD10 was a potential target of miR-501 (Figure 3A). Additionally, the results of the luciferase activity reporter assay indicated that transfection with the miR-501 inhibitor significantly increased the luciferase activity in cells transfected with wt-HOXD10 but not in those with mt-HOXD10 (Figure 3B). Western blot analysis revealed that HOXD10 expression can be elevated by the miR-501 inhibitor (Figure 3C). Thus, these results demonstrated that HOXD10 is a direct target of miR-501.
Figure 3.
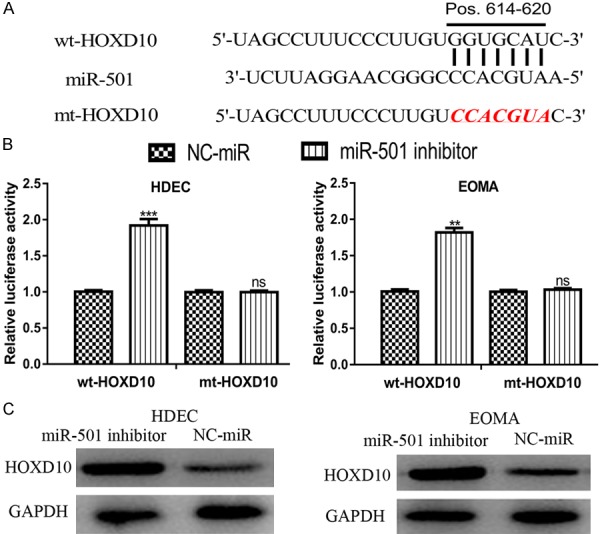
HOXD10 was a direct target of miR-501. (A) Binding site of miR-501 and the 3’-UTR of HOXD10. (B) Luciferase activity in cells transfected with the miR-501 inhibitor or NC-miR and wt-HOXD10 or mt-HOXD10. (C) HOXD10 expression in cells transfected with miR-501 inhibitor or NC-miR was analyzed by Western blot analysis. (***P<0.001, ns: not significant) miR-501: microRNA-501; HOXD10: homeobox D10; UTR: untranslated region; wt: wild-type; mt: mutant; NC-miR: negative control miRNA.
miR-501 regulates HAs cell events through targeting HOXD10
To investigate whether HOXD10 serves as a functional mediator for miR-501, the si-HOXD10 and miR-501 inhibitor were co-transfected into HA cell lines. It was found that transfection with si-HOXD10 decreased HOXD10 expression in HA cell lines (Figure 4A). Moreover, the stimulatory effect of the miR-501 inhibitor on HOXD10 expression can be partially abolished by si-HOXD10 (Figure 4A). Also, the MTT assay, flow cytometry analysis and colony formation assay revealed that si-HOXD10 transfection promoted HA cell proliferation (Figure 4B-D). The wound-healing assay and transwell invasion assay showed that downregulation of HOXD10 expression in HA cell lines promoted cell migration and invasion (Figure 4E and 4F). Moreover, we found that the transfection of si-HOXD10 partially reversed the inhibitory effects of the miR-501 inhibitor on HA cell processes (Figure 4B-F). In addition, we also showed that si-HOXD10 can increase the expression of MMP9 (Figure 4G).
Figure 4.
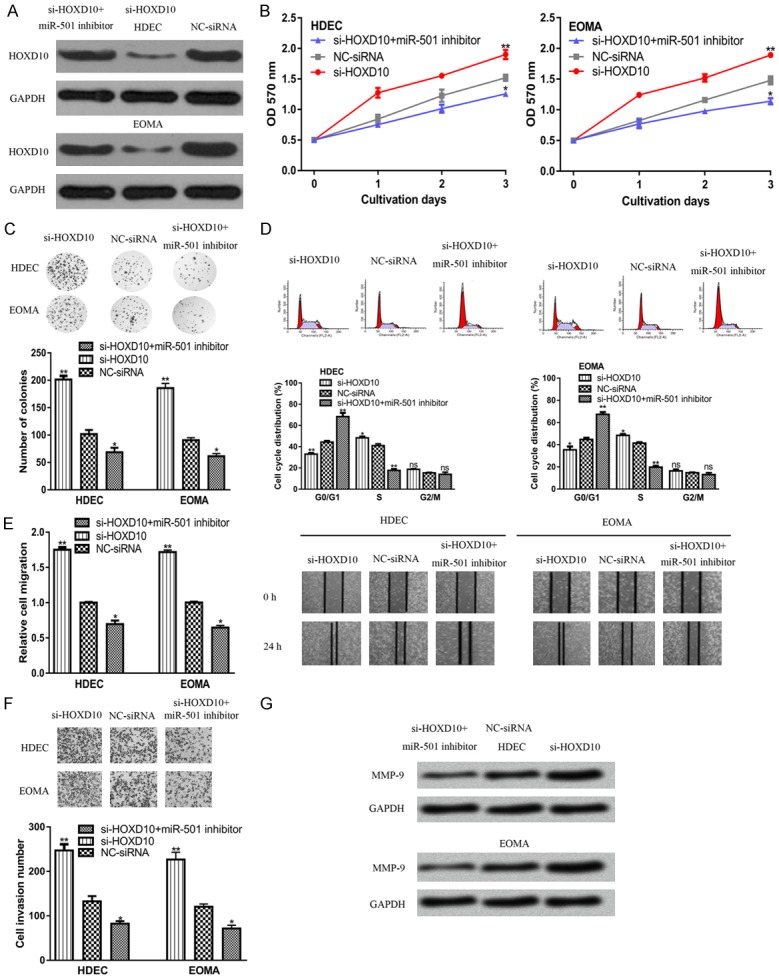
MiR-501 regulates HA cell processes by targeting HOXD10. (A) HOXD10, (B) cell proliferation, (C) cell cycle distribution, (D) colony formation, (E) cell migration, (F) cell invasion, and (G) MMP9 expression in cells transfected with si-HOXD10, NC-siRNA, or si-HOXD10 and miR-501 inhibitor. (**P<0.01, *P<0.05) miR-501: microRNA-501; HOXD10: homeobox D10; HAs: Hemangiomas; NC-siRNA: negative control siRNA; si-HOXD10: siRNA targeting HOXD10; MMP9: matrix metalloprotein 9.
Discussion
Accumulating evidence has suggested that miRNAs play crucial roles in the pathogenesis of HAs [12-15]. Overexpression of miR-143 was shown to promote HA cell proliferation, cell cycle arrest and cell apoptosis by inducing the expression of CDKN1A and TP53, while reducing the expression of cyclin D1, cyclin dependent kinase 2, cyclin dependent kinase 4, and BCL2 apoptosis regulator (BCL2) [12]. Epigenetic silencing of miR-130a plays a tumor-suppressive role in HA cell proliferation and angiogenesis by targeting tissue factor pathway inhibitor 2 (TFPI2) through FAK/PI3K/Rac1/mdm2 signaling [13]. In addition, miR-199a-5p overexpression was found to suppress HAs cell proliferation and induce cell apoptosis by targeting hypoxia inducible factor 1 subunit alpha (HIF1A) [14]. Moreover, miR-424 overexpression was shown to inhibit HA cell growth and at the same time promote cell apoptosis by targeting vascular endothelial growth factor receptor 2 to impact the AKT serine/threonine kinase (AKT) and extracellular signal-regulated kinase (ERK) signaling pathway [15].
MiR-501 has been found to be dysregulated in several human cancers [8-11]. However, little is known about the biological roles of miR-501 in HAs. In this study, we found that miR-501 expression was significantly increased in HA cell lines compared with normal cells. It has been previously demonstrated that miR-501 can affect cancer cell behaviors [10]. Accordingly, we also investigated the effect of miR-501 expression on HA cell proliferation, cell cycle distribution, colony formation, cell migration, and cell invasion. The expression of miR-501 was manipulated with synthetic miRNAs. It was found that miR-501 expression level was reduced by the miR-501 inhibitor. We also found that downregulation of miR-501 inhibited HA cell proliferation, cell cycle progression, colony formation, cell migration, and cell invasion in vitro. These findings indicated that miR-501 functions as an oncogene in HAs, which is similar to its reported role in cervical cancer [10].
Additionally, the underlying molecular regulatory mechanisms of miR-501 in HA cell processes were investigated. Through bioinformatic analysis, we found that HOXD10, whose expression levels were decreased in HA cell lines compared with normal cells, was a putative target of miR-501. HOXD10, a member of the HOX gene family, encodes a sequence-specific transcription factor [16]. HOXD10 expression was shown to be regulated by miR-10b to regulate the progression of ovarian cancer and gastric cancer [17,18]. Furthermore, miR-224 was found to enhance non-small cell lung cancer cell invasion and metastasis by targeting HOXD10 [19]. In this study, using luciferase activity reporter assay and Western blot analysis, we proved that HOXD10 was a direct target of miR-501. Importantly, inhibition of HOXD10 expression promoted HA cell proliferation, cell cycle progression, colony formation, cell migration, and cell invasion. Rescue experiments showed that downregulation of HOXD10 expression partially reversed the effects of the miR-501 inhibitor on HA cell activities.
Conclusion
In summary, in this study, to the best of our knowledge, we provided the first evidence that miR-501 expression is elevated in HA cell lines and its downregulation inhibited proliferation, cell cycle distribution, colony formation, cell migration, and cell invasion by targeting the expression of HOXD10. Understanding the role of miR-501 in HA progression will provide to the basis for the use of miR-501 as potential therapeutic target for HAs.
Disclosure of conflict of interest
None.
References
- 1.Xie F, Bao X, Yu J, Chen W, Wang L, Zhang Z, Xu Q. Disruption and inactivation of the PP2A complex promotes the proliferation and angiogenesis of hemangioma endothelial cells through activating AKT and ERK. Oncotarget. 2015;6:25660–25676. doi: 10.18632/oncotarget.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng CE, Friedlander SF. Infantile hemangiomas, complications and treatments. Semin Cutan Med Surg. 2016;35:108–116. doi: 10.12788/j.sder.2016.050. [DOI] [PubMed] [Google Scholar]
- 3.Maguiness SM, Frieden IJ. Current management of infantile hemangiomas. Semin Cutan Med Surg. 2010;29:106–114. doi: 10.1016/j.sder.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Grzesik P, Wu JK. Current perspectives on the optimal management of infantile hemangioma. Pediatric Health Med Ther. 2017;8:107–116. doi: 10.2147/PHMT.S115528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D, Zhao X, Zhang L, Wang Z, Wang C. Identification of hub genes to regulate breast cancer metastasis to brain by bioinformatics analyses. J Cell Biochem. 2019;120:9522–9531. doi: 10.1002/jcb.28228. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Sun Y. miR-29a-3p inhibits growth, proliferation, and invasion of papillary thyroid carcinoma by suppressing NF-κB signaling via direct targeting of OTUB2. Cancer Manag Res. 2018;11:13–23. doi: 10.2147/CMAR.S184781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Xu M, Lu P, Zhou F. microRNA-769 is downregulated in colorectal cancer and inhibits cancer progression by directly targeting cyclin-dependent kinase 1. Onco Targets Ther. 2018;11:9013–9025. doi: 10.2147/OTT.S183847. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Jin J, Tang S, Xia L, Du R, Xie H, Song J, Fan R, Bi Q, Chen Z, Yang G, Liu J, Shi Y, Fan D. MicroRNA-501 promotes HBV replication by targeting HBXIP. Biochem Biophys Res Commun. 2013;430:1228–1233. doi: 10.1016/j.bbrc.2012.12.071. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Chai Y, Zhang J, Tang J. A function variant at miR-501 alters susceptibility to hepatocellular carcinoma in a Chinese Han Population. Cell Physiol Biochem. 2016;38:2500–2508. doi: 10.1159/000445600. [DOI] [PubMed] [Google Scholar]
- 10.Sanches JGP, Xu Y, Yabasin IB, Li M, Lu Y, Xiu X, Wang L, Mao L, Shen J, Wang B, Hou L, Ju J, Zhao J, Song B. miR-501 is upregulated in cervical cancer and promotes cell proliferation, migration and invasion by targeting CYLD. Chem Biol Interact. 2018;285:85–95. doi: 10.1016/j.cbi.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Liu B, Guo Y, Chen Z, Sun W, Gao W, Wu H, Wang Y. Key miRNAs and target genes played roles in the development of clear cell renal cell carcinoma. Cancer Biomark. 2018;23:279–290. doi: 10.3233/CBM-181558. [DOI] [PubMed] [Google Scholar]
- 12.Huang C, Huang J, Ma P, Yu G. microRNA-143 acts as a suppressor of hemangioma growth by targeting Bcl-2. Gene. 2017;628:211–217. doi: 10.1016/j.gene.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 13.Gao F, Wang FG, Liu RR, Xue F, Zhang J, Xu GQ, Bi JH, Meng Z, Huo R. Epigenetic silencing of miR-130a ameliorates hemangioma by targeting tissue factor pathway inhibitor 2 through FAK/PI3K/Rac1/mdm2 signaling. Int J Oncol. 2017;50:1821–1831. doi: 10.3892/ijo.2017.3943. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Dai YX, Wang SQ, Qiu MK, Quan ZW, Liu YB, Ou JM. miR-199a-5p inhibits proliferation and induces apoptosis in hemangioma cells through targeting HIF1A. Int J Immunopathol Pharmacol. 2018;31:394632017749357. doi: 10.1177/0394632017749357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei Z, Qiu M, Qi X, Dai Y, Wang S, Quan Z, Liu Y, Ou J. MicroRNA-424 suppresses the proliferation of hemangioma-derived endothelial cells by targeting VEGFR-2. Mol Med Rep. 2018;18:4065–4071. doi: 10.3892/mmr.2018.9409. [DOI] [PubMed] [Google Scholar]
- 16.Hedlund E, Karsten SL, Kudo L, Geschwind DH, Carpenter EM. Identification of a Hoxd10-regulated transcriptional network and combinatorial interactions with Hoxa10 during spinal cord development. J Neurosci Res. 2004;75:307–319. doi: 10.1002/jnr.10844. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama I, Shibazaki M, Yashima-Abo A, Miura F, Sugiyama T, Masuda T, Maesawa C. Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int J Oncol. 2013;43:63–71. doi: 10.3892/ijo.2013.1935. [DOI] [PubMed] [Google Scholar]
- 18.Wang YY, Li L, Ye ZY, Zhao ZS, Yan ZL. MicroRNA-10b promotes migration and invasion through Hoxd10 in human gastric cancer. World J Surg Oncol. 2015;13:259. doi: 10.1186/s12957-015-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Zhang J, Zhao Y, Wang F, Chen Y, Fei X. miR-224 enhances invasion and metastasis by targeting HOXD10 in non-small cell lung cancer cells. Oncol Lett. 2018;15:7069–7075. doi: 10.3892/ol.2018.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]