Abstract
Aims: Postoperative cognitive dysfunction (POCD) is a neurological disorder associated with neuroinflammation. Connexin 43 (Cx43), an essential component of gap junction, plays a crucial role in neuroinflammation. The present study was designed to investigate the role of Cx43 in the process of POCD. Methods: POCD model was established in aged mice with internal fixation of tibial fractures. Cognitive function was examined using the Morris water maze test. Hippocampus was collected for reverse transcription polymerase chain reaction (RT-PCR), western blotting, and immunofluorescence assays. Results: In the water maze test, mice undergoing surgery took longer time to reach target platform than the controls. IL-1β and TNF-α mRNA expressions in the hippocampus were significantly increased in surgery mice. Cx43 protein presence in the hippocampus was increased in the surgery group. Treatment of Gap26, a specific blocker of Cx43 hemichannel, reduced the Cx43 protein presence, decreased mRNA expressions of IL-1β and TNF-α, and improved cognitive score in the maze test. Conclusion: Internal fixation of tibial fractures in aged mice induces Cx43 hemichannels opening and enhances neuroinflammation in the hippocampus, leading to cognitive impairment. Administration of Gap26 reduces neuroinflammation in the hippocampus and improves postoperative cognitive function.
Keywords: Cognitive dysfunction, connexin 43, hemichannel, neuroinflammation, hippocampus
Introduction
Postoperative cognitive dysfunction (POCD) presents a decline in memory and learning ability after anesthesia and surgery, which often occurs in senile people. Chronic inflammation in the central nervous system is an important mechanism underlying cognitive impairment, including Alzheimer’s disease, stroke, and epilepsy [1,2]. Accumulating evidence indicates that microglia and astrocytes play a key role in the process of neuroinflammation.
Gap junctions are important for intercellular connections. Connexins (Cxs) are transmembrane channel proteins, which are assembled to juxtaposed gap junctions (GJs) or non-juxtaposed hemichannels (HCs) [3]. Under physiological condition, GJs play a dominant role in connecting two neighbor cells for transferring molecules, ions and electrical impulses. Under disease conditions, HCs act as a conduit between cytoplasm and extracellular space, leading to cell cytotoxicity. Connexin43 is widely expressed in astrocytes and microglia [4,5]. Local inflammation increases Cx43 expression and modifies gap junctions’ structure and functions in the central nervous system [6-9]. Inhibition of Cx43 HCs improves survival rates of neuron [8,10,11] and restores cognitive function in rats [12].
In the present study, POCD model was development by performing surgical fixation of tibial fractures in aged mice [13]. It was hypothesized that the surgery opens Cx43 HCs in glia in the hippocampus, leading to cognitive impairment. If so, inhibition Cx43 HCs improves cognitive function.
Materials and methods
Animals
Aged C57BL/6 male mice (16-18 months old, 33-35 g) were purchased from Shanghai Jiesijie Company (Shanghai, China) and housed separately in the Laboratory Animal Unit of Zhongshan Hospital (Shanghai, China). Mice were given free access to food and water. The experimental design was approved by the Animal Ethics Committee of Zhongshan Hospital, Fudan University.
POCD model
POCD model was set up by conducting internal fixation of tibial fractures in mice [13]. Aged mice were anaesthetised by 1% sodium pentobarbital (10 ml/kg, intraperitoneal injection). A 0.3-0.6 cm vertical incision was made near the tibial tubercle. The needle (#26) was inserted into the tibial tubercle. Gap26 (50 µg/kg) was administrated intraperitoneally one hour before the surgery [12]. After the surgery, mice were intraperitoneally given butorphanol (2 mg/kg) to relieve pain.
Morris water maze test
Morris water maze test was used to examine learning ability and memory. Spatial acquisition training was conducted for five continuous days. Escape latency was measured as time spent before mice climbed to the target platform. The exploratory experiment was performed on Day 6. Mice were gently placed into the pool from the contralateral quadrant of the target platform. Both swimming tracings and time spent on each quadrant were recorded.
Real-time PCR
Primers used in this study were designed and synthesized by Sangon. GAPDH (forward, 5’-GTT CAA CGG CAC AGT CAA G-3’; reverse, 5’-GCC AGT AGA CTC CAC GAC AT-3’); Cx43 (forward, 5’-GAG TTT GCC TAA GGC GCT C-3’; reverse, 5’-AGG AGT TCA ATC ACT TGG CG-3’), IL-1β (forward, 5’-GAA ATG CCA CCT TTT GAC AGT G-3’; reverse, 5’-TGG ATG CTC TCA TCA GGA CAG-3’); TNF-α (forward, 5’-CAG GCG GTG CCT ATG TCT C -3’; reverse, 5’-CGA TCA CCC CGA AGT TCA GTA G-3’). Total RNA was extracted with Trizol (TAKARA, Japan), and reverse transcribed into cDNA with the TAKARA reverse transcription kit. A two-step PCR reaction program was set as follows: degeneration at 95°C for 30 s (1 cycle), 95°C denaturation for 5 s, 60°C annealing for 30 s, and 70°C extension for 30 s, (40 cycles).
Western blotting
Mouse hippocampi were minced and lysed by RIPA buffer. Protein concentration was measured by bicinchoninic acid assay (Beyotime, China). A total of 75 μg protein was loaded for electrophoresis and transferred to polyvinylidene difluoride membrane. After blocking with skimmed milk, the membrane was incubated with primary antibodies (1:1000) overnight at 4°C. On the second day, the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (1:10000) for one hour at room temperature. Target protein was normalized with the housekeeping gene β-Tubulin and analyzed using software ImageJ.
Immunofluorescence assay
Frozen mouse hippocampus was cut into 4-µm thickness and rewarmed at room temperature for about 30 min. After blocking for 20 min at room temperature, samples were incubated with primary antibody against Cx43 (1:100) and glial fibrillary acidic protein (GFAP, 1:100) overnight at 4°C. On the second day, samples were incubated with secondary antibodies (1:300, Fluor 488-labeled anti-rabbit and Fluor 594-labeled anti-rat, respectively) for one hour. Hoechst was stained for nuclei. Images were taken by laser-scanning microscopy (Leica Microsystems, German).
Reagents
Gap26 was purchased from Shanghai Botai Company (Shanghai, PRC). Primers were designed and synthesised by Sangon (Shanghai, PRC). Trizol and reverse transcription kit were bought from TAKARA (TAKARA, Japan). Primary antibodies against Cx43 phosphorylated Cx43 and β-Tubulin, as well as secondary antibodies, were purchased from Cell Signaling Technology (CST, Danvers, MA). GFAP antibody was purchased from Servicebio Biotechnology (Hubei, PRC). Chemiluminescence reagent ECL was bought from Xinsaimei Biotechnology (Shanghai, PRC).
Statistical analysis
Data are presented as means ± SEM. The statistical analysis was done by one-way ANOVA followed by post hoc comparison using the Bonferroni test (GraphPad Prism 5 software San Diego, CA, USA). P < 0.05 was considered statistically significant.
Results
Mice undergoing surgery exhibit impaired learning ability and memory loss
Under basal condition, control and surgery mice had similar performances in swimming pool. Since Day 3, control mice spent less time to reach the target quadrant than surgery mice (Figure 1A). In the exploratory experiment (Day 6), control mice spent most of their exploring time in the target quadrant, while the surgery mice spent equal time in each quadrant (Figure 1B and 1C). There were no statistical differences in swimming speed between the two groups (Figure 1D).
Figure 1.
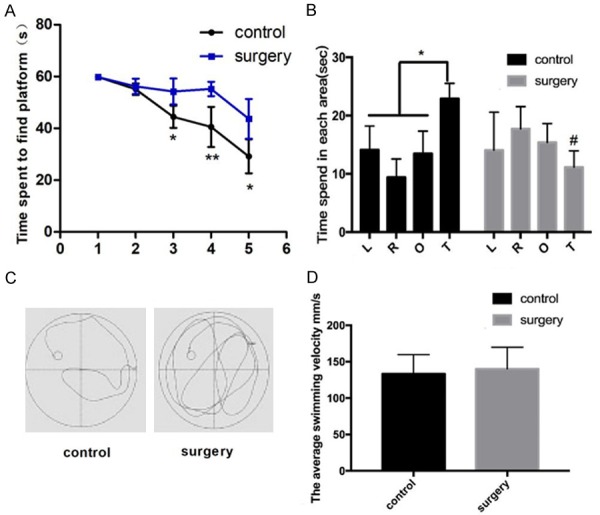
Surgery of tibial fractures fixation impairs learning ability and induces memory loss in aged mice. A. The latency time during acquired training. B. Time spent in each quadrant in the exploratory experiment. C. Mouse swimming trajectories in control (left) and surgery (right) groups. D. Mouse swimming speed in control and surgery groups. L; left, R: right, O: opposite, and T: target quadrant of the swimming pool. N=8 *P < 0.05 compared with the controls, #P < 0.05 compared with the control group on the target quadrant.
Surgical fixation of tibial fractures increases expressions of inflammatory factors in the hippocampus
Neuroinflammation plays a crucial role in the development of POCD. After the surgery, mRNA expressions of IL-1β and TNF-α were significantly increased in the hippocampus on Day 1 and 3, compared with those in control mice (Figure 2).
Figure 2.
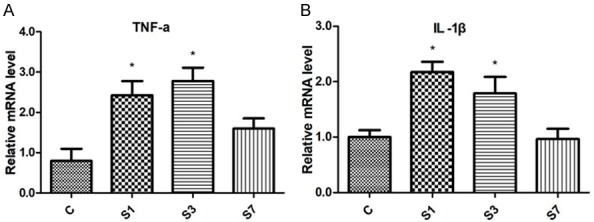
Surgery of tibial fractures fixation increases mRNA expressions of TNF-α (A) and IL-1β (B) in the hippocampus. N=6. c: control; s: surgery. *P < 0.05 versus the controls.
Surgical fixation of tibial fractures increases Cx43 expressions on astrocytes in the hippocampus
Cx43 protein was mainly expressed on astrocytes in the hippocampus since most Cx43 signals were co-localized with GFAP, a linage mark of astrocytes. Immunofluorescent signal of Cx43 was significantly increased on Day 1 when compared with control mice (Figure 3).
Figure 3.
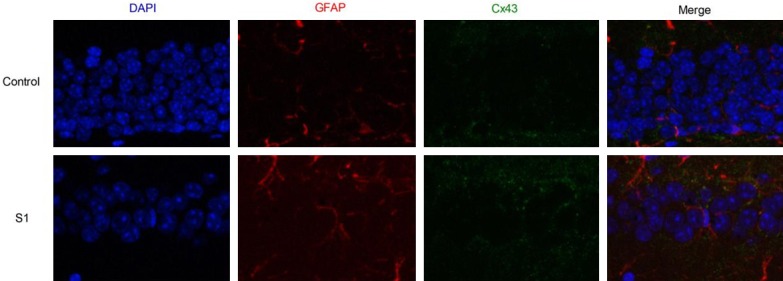
Surgery of tibial fractures fixation upregulates Cx43 protein in the mouse hippocampus before (upper panel) and after the surgery (lower panel). In immunofluorescence images, the nuclei was stained with DAPI (blue), the astrocyte was stained with GFAP (red), and Cx43 was stained in green, respectively.
Cx43 mRNA expression in the surgery group was increased on Day 1 and Day 3, compared with the control group (Figure 4A). Consistently, western blotting showed that the presence of total Cx43 protein was significantly increased in surgery mice (Figure 4B and 4C). On Day 1, phosphorylated level of Cx43 on s368 residue was significantly increased in the surgery group when normalized with the total Cx43 protein (Figure 4B and 4D).
Figure 4.
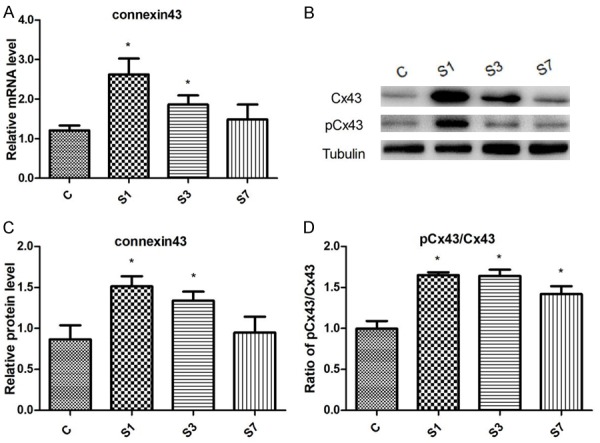
Changes of Cx43 protein in the hippocampus following surgical fixation of tibial fractures. A. Cx43 mRNA expression B. Representative Western blots of Cx43 protein. C. Densitometric quantification of Cx43 protein. D. Relative expression of phosphorylated Cx43. N=6. c: control; s: surgery. *P < 0.05 versus the controls.
Gap26 treatment reduces Cx43 expression in the hippocampus
A specific blocker of Cx43 hemichannel Gap26 [10,18,19], was administrated one hour before the surgery.
Gap26 treatment did not alter Cx43 presences, either phosphorylated level or total protein, in control mice (Data not shown).
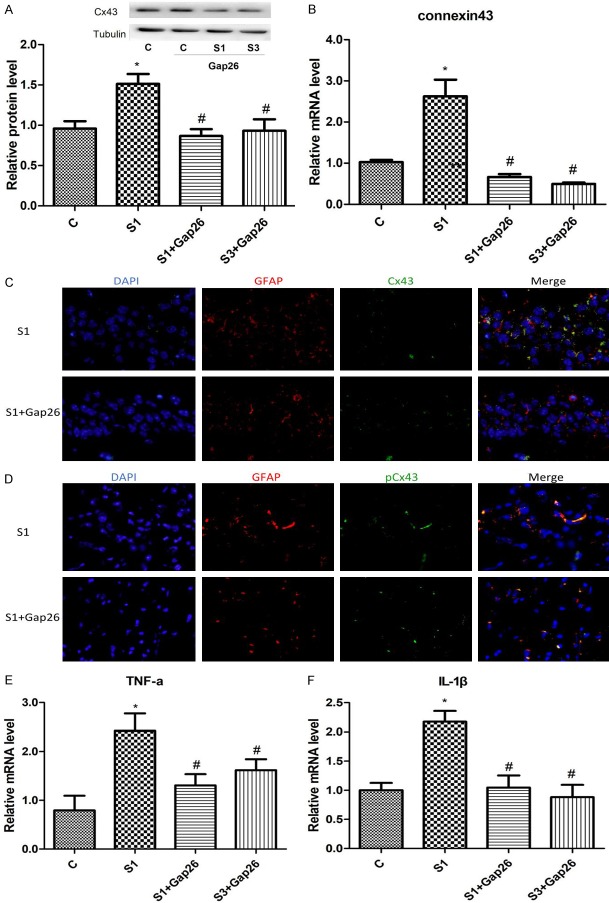
Gap26 treatment significantly reduced mRNA expressions of TNF-α and IL-1β on Day 1 in the surgery group (Figure 5E and 5F). Gap26 treatment profoundly reduced the protein expression of total Cx43 on Day1 and Day 3 as well (Figure 5A-C).
Figure 5.
Gap26 treatment downregulates Cx43 presences and decreases mRNA levels of TNF-α and IL-1β in the hippocampus of surgery mice. A. Representative western blots and densitometric quantification of Cx43 protein. B. Quantitative real-time PCR of Cx43 mRNA expression. C. Protein presence Cx43 in the hippocampus on day 1 treated with (lower panel) or without (upper panel) Gap26. In immunofluorescence images, the nuclei was stained with DAPI-stained (blue), the astrocyte was stained with GFAP (red), and Cx43 was stained in red, respectively. D. Protein presence phosphorylated Cx43 s368 in the hippocampus on day 1 treated with (lower panel) or without (upper panel) Gap26. In immunofluorescence images, the nuclei was stained with DAPI-stained (blue), the astrocyte was stained with GFAP (red), and Cx43 was stained in red, respectively. E. TNF-α mRNA expression in the hippocampus of surgery mice. F. IL-1β mRNA expression in the hippocampus of surgery mice. c: control; s: surgery. N=6. *P < 0.05 versus the controls. #P < 0.05 versus S1.
Gap 26 treatment improves the cognitive function of aged mice after surgery
Gap 26 treatment improved mice cognitive function in the surgery group (Figure 6A). Surgery mice treated with Gap26 spent most of their exploring time in the target quadrant as control mice (Figure 6B and 6C). There were no statistical differences in swimming speed (Figure 6D).
Figure 6.
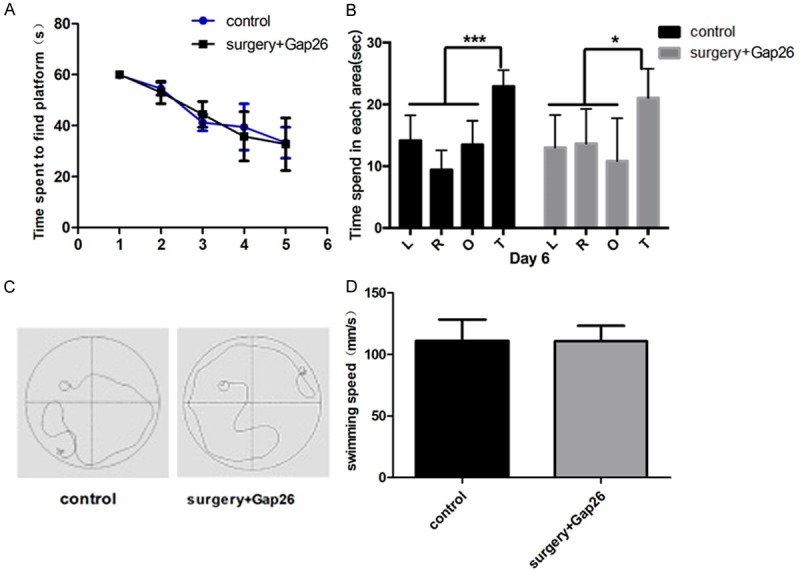
Gap26 treatment improves cognitive function in surgery mice. A. The latency time during the water maze test. B. Time spent in the exploratory experiment. C. Mouse swimming trajectories in control (left) and Gap26 (right) groups. D. Mouse swimming speed in control and treated groups. L; left, R: right, O: opposite, and T: target quadrants of the pool. N=6. *P < 0.05 versus the controls.
Discussion
The present study reports that surgery of tibial fractures fixation [13] increases the phosphorylated level of Cx43 s368 residue and elevates inflammatory responses in the hippocampus, resulting in cognitive dysfunction in 15-month old mice. Blockade of Cx43 HJs significantly improves cognitive function in mice.
Chronic neuroinflammation is a hallmark of a neurological disorder, including stroke, Parkinson’s disease, Huntington’s disease and Alzheimer’s disease. It is characterised by the activation of astrocytes and microglia in the central nervous system [18,20,21]. In the present study, enhanced mRNA expressions of IL-1β and TNF-α indicated that inflammatory responses took place in the central nervous system [13]. Taken together with the cognitive impairment in these mice, it suggests that neuroinflammation plays a vital role in the occurrence of POCD [22].
Connexins are transmembrane channel proteins, which are assembled to form gap junctions or hemichannels. In the central nervous system, Cx43 protein is mainly expressed on astrocytes and plays a crucial role in channel gating, ion conduction [23], synaptic transmission and plasticity [19], as well as blood-brain barrier integrity [24]. In the present study, increased presences of Cx43 protein, both phosphorylated form and total protein, indicates that Cx43 plays a key role in the process of POCD. Of note, the increased level of phosphorylated Cx43 at s368 exceeded the upregulation of total Cx43 protein, indicating that surgery facilitates HCs opening, but not GJ, since Cx43s368 phosphorylation is reported to inhibit information exchanging between two adjacent cells [15,17].
Inflammation is an important mechanism of HCs opening [7,25]. Proinflammatory cytokines, such as IL-1β and TNF-α, are essential to increase Cx43 HCs activity [6,8,21]. In a cerebral ischemic model, Cx43 HCs are open when sheep are subjected to cerebral ischemia [10]. In the present study, the surgical fixation of tibial fractures increased productions of proinflammatory cytokines and enhanced protein presences of HCs, confirming that proinflammatory cytokines and Cx43 HCs are team players in the process of POCD.
Gap26 is an HC peptide mimetic, which specifically blocks Cx43 HCs, but not GJs [9,12,26]. It is reported that Gap26 treatment reduces the area of cerebral infarction and improves cognitive function in the rat, shown as better performances in water maze tests [12]. In an ischemic sheep model, blockade of Cx43 HCs promotes electroencephalographic power recovery and improves the prognosis of stroke [10]. In the present study, Gap26 treatment reduced mRNA expressions of IL-1β and TNF-α in the hippocampus, suggesting that the activation of astrocyte is essential for the upregulation of proinflammatory cytokines. Gap26 treatment improved postoperative cognitive function, confirming the critical role of Cx43 HCs in the occurrence of POCD.
In summary, aged mice undergoing tibial fractures fixation exhibit a cognitive defect. Blockade of Cx43 HCs reduces inflammatory responses in the hippocampus and improves cognitive function in aged mice. Thus, the opening of Cx43 HCs plays a prominent role in the neuroinflammation as well as in the occurrence of POCD. Blockade of Cx43 hemichannels shed a light of a promising future strategy for the treatment of POCD.
Acknowledgements
This work was sponsored by the Natural Science Foundation of Shanghai (17ZR1404300), the Natural Science Project of Minhang district of Shanghai (2018MHZ107).
Disclosure of conflict of interest
None.
References
- 1.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y, Lee SR, Choi SS, Yeo HG, Chang KT, Lee HJ. Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int. 2014;2014:297241. doi: 10.1155/2014/297241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pannasch U, Vargová L, Reingruber J, Ezan P, Holcman D, Giaume C, Syková E, Rouach N. Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A. 2011;108:8467–8472. doi: 10.1073/pnas.1016650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheckenbach KE, Crespin S, Kwak BR, Chanson M. Connexin channel-dependent signaling pathways in inflammation. J Vasc Res. 2011;48:91–103. doi: 10.1159/000316942. [DOI] [PubMed] [Google Scholar]
- 5.Belousov AB, Fontes JD, Freitas-Andrade M, Naus CC. Gap junctions and hemichannels: communicating cell death in neurodevelopment and disease. BMC Cell Biol. 2017;18:4. doi: 10.1186/s12860-016-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Sáez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abudara V, Roux L, Dallérac G, Matias I, Dulong J, Mothet JP, Rouach N, Giaume C. Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia. 2015;63:795–811. doi: 10.1002/glia.22785. [DOI] [PubMed] [Google Scholar]
- 8.Froger N, Orellana JA, Calvo CF, Amigou E, Kozoriz MG, Naus CC, Sáez JC, Giaume C. Inhibition of cytokine-induced connexin43 hemichannel activity in astrocytes is neuroprotective. Mol Cell Neurosci. 2010;45:37–46. doi: 10.1016/j.mcn.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Rovegno M, Soto PA, Sáez PJ, Naus CC, Sáez JC, von Bernhardi R. Connexin43 hemichannels mediate secondary cellular damage spread from the trauma zone to distal zones in astrocyte monolayers. Glia. 2015;63:1185–1199. doi: 10.1002/glia.22808. [DOI] [PubMed] [Google Scholar]
- 10.Davidson JO, Green CR, Nicholson LF, O’Carroll SJ, Fraser M, Bennet L, Gunn AJ. Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol. 2012;71:121–132. doi: 10.1002/ana.22654. [DOI] [PubMed] [Google Scholar]
- 11.Vicario N, Calabrese G, Zappalà A, Parenti C, Forte S, Graziano ACE, Vanella L, Pellitteri R, Cardile V, Parenti R. Inhibition of Cx43 mediates protective effects on hypoxic/reoxygenated human neuroblastoma cells. J Cell Mol Med. 2017;21:2563–2572. doi: 10.1111/jcmm.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Zhao H, Tan X, Kostrzewa RM, Du G, Chen Y, Zhu J, Miao Z, Yu H, Kong J, Xu X. Inhibition of connexin43 improves functional recovery after ischemic brain injury in neonatal rats. Glia. 2015;63:1553–1567. doi: 10.1002/glia.22826. [DOI] [PubMed] [Google Scholar]
- 13.Zhu YJ, Peng K, Meng XW, Ji FH. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 2016;1644:1–8. doi: 10.1016/j.brainres.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 14.Alstrom JS, Stroemlund LW, Nielsen MS, MacAulay N. Protein kinase C-dependent regulation of connexin43 gap junctions and hemichannels. Biochem Soc Trans. 2015;43:519–523. doi: 10.1042/BST20150040. [DOI] [PubMed] [Google Scholar]
- 15.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Márquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD. Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta. 2012;1818:1985–1992. doi: 10.1016/j.bbamem.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimlamool W, Andrews RM, Falk MM. Connexin43 phosphorylation by PKC and MAPK signals VEGF-mediated gap junction internalization. Mol Biol Cell. 2015;26:2755–2768. doi: 10.1091/mbc.E14-06-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orellana JA, von Bernhardi R, Giaume C, Sáez JC. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev Neurosci. 2012;23:163–177. doi: 10.1515/revneuro-2011-0065. [DOI] [PubMed] [Google Scholar]
- 19.Orellana JA, Retamal MA, Moraga-Amaro R, Stehberg J. Role of astroglial hemichannels and pannexons in memory and neurodegenerative diseases. Front Integr Neurosci. 2016;10:26. doi: 10.3389/fnint.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Même W, Calvo CF, Froger N, Ezan P, Amigou E, Koulakoff A, Giaume C. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB J. 2006;20:494–496. doi: 10.1096/fj.05-4297fje. [DOI] [PubMed] [Google Scholar]
- 21.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovens IB, Schoemaker RG, van der Zee EA, Absalom AR, Heineman E, van Leeuwen BL. Postoperative cognitive dysfunction: Involvement of neuroinflammation and neuronal functioning. Brain Behav Immun. 2014;38:202–210. doi: 10.1016/j.bbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Contreras JE, Sánchez HA, Véliz LP, Bukauskas FF, Bennett MV, Sáez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013;61:1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagos-Cabré R, Alvarez A, Kong M, Burgos-Bravo F, Cárdenas A, Rojas-Mancilla E, Pérez-Nuñez R, Herrera-Molina R, Rojas F, Schneider P, Herrera-Marschitz M, Quest AFG, van Zundert B, Leyton L. alphaVbeta3 Integrin regulates astrocyte reactivity. J Neuroinflammation. 2017;14:194. doi: 10.1186/s12974-017-0968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawat G, Hélie P, Baroudi G. Single intravenous low-dose injections of connexin 43 mimetic peptides protect ischemic heart in vivo against myocardial infarction. J Mol Cell Cardiol. 2012;53:559–566. doi: 10.1016/j.yjmcc.2012.07.008. [DOI] [PubMed] [Google Scholar]