Abstract
Synovial fluid-derived mesenchymal stem cells (SF-MSCs) represent a superior source of stem cells and have great potential for autologous transplantation for cartilage regeneration. Transforming growth factor-β3 (TGF-β3) has been demonstrated to stimulate the chondrogenic differentiation of MSCs. Recently, the small molecule kartogenin (KGN) was reported to enhance chondrogenic differentiation and cartilage regeneration. The effects of KGN and TGF-β3 on the in vitro chondrogenic differentiation of rabbit SF-MSCs were studied. The monolayer and pellet cultures of rabbit SF-MSCs were stimulated in vitro using either KGN or TGF-β3 alone or in combination for 21 days. The in vivo therapeutic effects of KGN combined with TGF-β3 were studied using an intra-articular delivery of autologous rabbit SF-MSCs to cartilage defects in a rabbit model. Compared to a single treatment, the in vitro results demonstrated that the combination of KGN and TGF-β3 resulted in significantly increased protein expression levels of type II collagen (COL II) and SRY-box 9 (SOX9) and decreased the expression level of type X collagen (COL X). Compared with the regenerated cartilage in the single treatment groups, the intra-articular injection of rabbit SF-MSCs mixed with TGF-β3 and KGN exhibited substantial amounts of regenerated cartilage in the defective areas in the medial femoral condyles. We noted that the thicker, hyaline-like cartilaginous tissue contained abundant levels of extracellular matrix, which is characteristic of cartilage. This study demonstrated that TGF-β3 and KGN exhibit synergistic effects for the promotion of the chondrogenesis of rabbit SF-MSCs and can effectively repair cartilage defects through the regeneration of hyaline cartilage.
Keywords: Mesenchymal stem cells, rabbit synovial fluid, chondrogenic differentiation, kartogenin, transforming growth factor-β3, cartilage regeneration, cell therapy
Introduction
Articular cartilage plays a critical role in the body’s skeletal system because hyaline cartilage prevents bone abrasion and absorbs concussion [1]. The innate structural characteristics of articular cartilage lack the ability to regenerate after trauma or in cases of pathologic conditions. This is a result of poor vascular supply, lymphatic drainage, and poor progenitor cell access to the lesion location [2]. Upon damage, it is difficult for the cartilage to self-repair in the absence of intervention. Current treatments for damaged articular cartilage include microfracture (MF), mosaicplasty (MO), autologous chondrocyte implantation (ACI) and matrix-induced autologous chondrocyte implantation (MACI) [3]. However, these treatment methods have been demonstrated to have limited restorative effects on the native articular cartilage structure and function in clinical applications [4]. To overcome these limitations, regenerative medicine strategies, including cartilage tissue engineering (CTE) using mesenchymal stem cells (MSCs), have gained increasing attention in regard to defects in cartilage repair [5].
MSCs are easily isolated, expand in sufficient numbers without malignant changes and have been demonstrated to be able to differentiate into chondrocytes. Therefore, these offer great potential for cartilage regeneration [6,7]. MSCs can be isolated from numerous different tissues, including the bone marrow, adipose tissue, periosteum, and synovium [8-10]. The presence of MSCs in synovial fluid (SF-MSCs) has also been recently demonstrated [11]. Further studies have demonstrated that MSCs are substantially increased in the synovial fluid of patients with joint disease and injury [12,13]. We have recently demonstrated that SF-MSCs isolated from human synovial fluid are capable of differentiating into chondrocytes, osteoblasts, and adipocytes [14]. In this study, we aimed to use autologous SF-MSCs as a promising candidate cell source for cartilage repair.
The terminal induction of MSCs toward hyaline-like chondrocytes is a critical step for the use of MSCs in cartilage repair. It has been well established that the use of growth factors, specifically TGF-β1 and TGF-β3, can function to promote chondrogenic differentiation and to induce the expression of cartilage matrix formation [15-17]. In comparison to TGF-β1, TGF-β3 has been shown to have better effects on MSC chondrogenesis [18]. TGF-β3 has been demonstrated to have the ability to promote aggrecan and type II collagen expression. However, it has also been shown to promote type X collagen gene expression in the late stages of induction. This has been shown to lead to hypertrophy, and this ultimately results in the formation of nonfunctional fibrocartilage, instead of hyaline cartilage [18,19]. In addition, numerous studies have suggested that a concentration of 10 ng/mL of TGF-β3 is optimal for the promotion of MSC chondrocyte differentiation in vitro [20-23].
Recently, kartogenin (KGN) was identified as a novel, small molecule for the promotion of bone marrow-derived mesenchymal stem cell (BM-MSC) chondrogenic differentiation, and KGN was shown to stimulate the runt-related transcription factor (RUNX) pathway [24]. KGN that was encapsulated within scaffolds, such as thermogel or nanoparticles, was shown to effectively aid cartilage regeneration in vivo, thereby making this small molecule a critical additive for cartilage tissue engineering [25,26]. In addition, studies have demonstrated that KGN may function to suppress hypertrophic differentiation through the inhibition of terminal chondrocyte differentiation [24]. Based on these data, we hypothesized that the combination of KGN and TGF-β3 may function to synergistically promote the formation of hyaline chondrocytes.
In this study, we studied the chondrogenic differentiation effects of TGF-β3 and KGN using both monolayer culture and pellet culture. In addition, we injected autologous rabbit SF-MSCs mixed with TGF-β3 and KGN into the defective cartilage to gain a better understanding of the synergistic effects on articular cartilage regeneration in a rabbit model. Studies have demonstrated that different KGN concentrations were capable of differentiating BM-MSCs into chondrocytes, and a concentration of 10 μM KGN exhibited the most significant effect [24]. Therefore, 10 ng/mL TGF-β3 and 10 μM KGN were adopted for the induction of rabbit SF-MSCs chondrocyte differentiation in this study. We demonstrated that the combination of TGF-β3 and KGN could function to increase the gene expression of hyaline-like cartilage marker in vitro and demonstrated that the combination led to an improved repair effect in vivo. In summary, the costimulation induced by TGF-β3 and KGN is a viable strategy for SF-MSC-based articular cartilage regeneration.
Materials and methods
Ethics statement
All of the animal experiments were conducted in accordance with the institution’s or the National Research Council’s Guide for the care and use of laboratory animals. This study was approved by the Institutional Animal Care and Use Committee of Shenzhen People’s Hospital at Jinan University.
Collection of the rabbit synovial fluid
The New Zealand white rabbits (2.5-3.0 kg, 6 months old) were placed in the supine position after general anesthetization. The knee cavity arthrocentesis procedure was performed for rabbit synovial fluid collection.
Two milliliters of isotonic saline solution was injected into the rabbit knee joint cavity from the lateral articular space. Then, the knee was fully extended and flexed several times. Subsequently, the synovial fluid and the saline solution were collected using a sterile injection syringe.
Cell isolation and culture
Adhesive cultivation with specific MSC culture medium was used to isolate and culture the primary rabbit SF-MSCs. Within 4 hours of aspiration, the synovial fluid was diluted with phosphate-buffered saline, filtered through a 40-mm nylon filter (Corning, USA) to remove debris, plated in 100-mm dishes, and cultured for 1 week as passage 0 in complete culture medium with basic DMEM (Gibco, USA) that was supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin-streptomycin (HyClone, USA). The dishes were incubated at 37°C in an atmosphere of 5% humidified CO2. After 48 hours, the medium was discarded to remove non-adherent cells and was replaced with fresh medium.
Identification of surface markers of rabbit SF-MSCs
The passage 3 rabbit SF-MSCs were suspended in PBS containing 5% bovine serum albumin (Sigma Aldrich, USA) at a concentration of 3×105 cells/0.05 mL and were incubated with mouse anti-rabbit CD34, CD45, CD73, CD90 and CD105, and monoclonal antibodies (mAb) (1:100 dilution, BD Biosciences, USA) at 4°C for 1 hour. The cells were washed three times with PBS and were then incubated with an FITC-labeled secondary anti-mouse antibody (1:200 dilution, Invitrogen) at 4°C for 30 min. The appropriate rabbit isotype antibodies were used as controls. The samples were processed using a FACS Canto flow cytometer (BD Biosciences, USA) and were analyzed with FlowJo software.
Multidifferentiation of the rabbit SF-MSCs
Osteogenic differentiation
Rabbit SF-MSCs from passage 3 were seeded into a 6-well culture plate at a density of 103 cells/cm2 and were cultured in osteogenic induction medium (BioWit Technologies, China). The medium was changed every 3 days for 3 weeks. The cells were fixed with 4% formaldehyde for 30 min at room temperature and were stained with 1% Alizarin Red for 15 min [27].
Adipogenic differentiation
Rabbit SF-MSCs from passage 3 were seeded into a 6-well culture plate at a density of 103 cells/cm2 and were induced by adipogenic induction medium (BioWit Technologies, China). After 3 weeks, the neutral lipid vacuoles were stained with Oil Red O to confirm adipogenic differentiation. The cells were fixed with 4% formaldehyde solution for 30 min at room temperature in advance [28].
Chondrogenic differentiation
Chondrogenic induction was performed in pellet culture. A total of 5×105 of rabbit SF-MSCs from passage 3 were centrifuged (1,500 rpm, 10 min) in a 15 mL polypropylene tube to form a pellet. The cell pellet was cultured for 3 weeks with chondrogenic induction medium (BioWit Technologies, China). The medium was changed every 3 days for 3 weeks. After 3 weeks, the cell pellet was embedded in paraffin, cut into 4 μm slices, and stained with 0.1% toluidine blue for 30 min at room temperature [29].
In vitro chondrogenic induction of rabbit SF-MSCs
Two-dimensional monolayer culture
A total of 1×105 rabbit SF-MSCs were seeded into glass-bottom cell culture dishes (NEST Biotechnology, China). After 24 hours, the media was changed to serum-free chondrogenic medium consisting of DMEM, 1% insulin-transferrin-selenous acid premix (BD Biosciences, USA), 100 nM dexamethasone, 50 mM L-ascorbic acid-2-phosphate, 1 mM sodium pyruvate, and 0.35 mM L-proline (Sigma-Aldrich, USA). Then, the cells were treated with 10 ng/mL TGF-β3 (RD Systems, USA) or 10 μM KGN (RD Systems, USA) or both for 21 days. The experimental groups were treated as follows: control group (serum-free chondrogenic medium without TGF-β3 and KGN), TGF-β3 group, KGN group, KGN + TGF-β3 group.
High-density pellet culture
For the chondrogenic differentiation experiments from high-density pellet cultures, 5×105 rabbit SF-MSCs from passage 3 were placed into a 15 mL polypropylene tube (BD Biosciences, USA) and were centrifuged (1,500 rpm, 10 min) to form a pellet. The pellets were cultured in serum-free chondrogenic medium, which consisted of DMEM, 1% insulin-transferrin-selenous acid premix (BD Biosciences, USA), 100 nM dexamethasone, 50 mM L-ascorbic acid-2-phosphate, 1 mM sodium pyruvate, and 0.35 mM L-proline (Sigma-Aldrich, USA). Then, 10 ng/mL TGF-β3, 10 μM KGN or 10 μM KGN + 10 ng/mL TGF-β3 was added to the medium, according to the above groups. The medium was changed twice a week for 3 weeks. The cartilage nodules formed by these cell pellets were embedded with O.C.T (optimal cutting temperature compound) (Tissue-Tek, Sakura, USA), frozen, sectioned into 4 μm slices and then stained with H&E (Sigma-Aldrich, USA) and toluidine blue (Sigma-Aldrich, USA).
Immunofluorescence staining of collagen II and aggrecan
The rabbit SF-MSCs induced for 3 weeks were fixed using 4% phosphate paraformaldehyde (Sigma-Aldrich, USA) for 20 min at room temperature followed by incubation with 0.2% Triton X-100 solution (Sigma-Aldrich, USA) for 30 min at room temperature to permeabilize the cells. The cells were blocked with 5% bovine serum albumin for one hour at room temperature. Then, the cells were incubated with fluorophore-conjugated goat anti-rabbit antibodies against COL II and AGG (1:100, RD Systems, USA) for one hour at room temperature. Subsequently, the samples were washed with PBS three times, and the nuclei were stained blue with 4, 6-diamidino-2-phenylindole DAPI (1:1000, Invitrogen, USA) for 5 min and rinsed with PBS. Then, the cells were rinsed with PBS, and the cells were visualized using laser scanning confocal microscopy (ZEISS, Germany).
RNA purification and quantitative polymerase chain reaction (qPCR)
After 3 weeks of in vitro chondrogenic induction, the cells were lysed with TRIzol Reagent (Invitrogen, USA), and the total RNA was extracted following the manufacturer’s instructions. The total RNA from each sample was reverse transcribed to cDNA using RT-PCR for first-strand cDNA synthesis (US Everbright Inc., USA) following standard protocols and primers (Table 1). The mRNA expression levels of COL2A1, SOX9, ACAN and COL10A1 were determined by real-time PCR using SYBR Premix EX Taq (Takara, China) following the recommended protocols. GAPDH was used as an internal control. Each sample was analyzed in triplicate. The data were analyzed using the comparative CT method (2-ΔΔCT method). The relative fold transcript levels were expressed as the mean ± SD and were plotted as bar graphs. The statistical analysis was performed at the level of ∆CT to exclude potential bias due to averaging data that had been transformed through the 2-ΔΔCT equation [30].
Table 1.
Primer sequences used for real-time PCR
| Genes | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| GAPDH | GGAGAAAGCTGCTAA | ACGACCTGGTCCTCGGTGTA |
| COL2A1 | CAGGCAGAGGCAGGAAACTAAC | CAGAGGTGTTTGACACGGAGTAG |
| SOX9 | GTACCCGCACCTGCACAAC | TCCGCCTCCTCCACGAAG |
| ACAN | GCTACGGAGACAAGGATGAGTTC | CGTAAAAGACCTCACCCTCCAT |
| COL10A1 | CACCTTCTGCACTGCTCATC | GGCAGCATATTCTCAGATGGA |
Cartilage defects in a rabbit model and cartilage repair by rabbit SF-MSCs
Defective cartilage establishment
Each rabbit was anesthetized with a marginal ear vein injection of 3% pentobarbital sodium. The rabbit was placed in a dorsal-recumbent position, and the rabbit’s right knee joint was operated on with a medial para-patellar approach. Then, a cylindrical full-thickness cartilage defect (4.0 mm in diameter and 2.5 mm in depth) was created on the trochlear groove using a special drill. The joint capsule was sutured, and the incision was closed layer by layer.
Postoperative treatment
After surgery, the rabbits were allowed free movement in their cages, and the wounds were disinfected with 0.1% povidone iodine twice a day for 3 days. Wound healing was observed for 3 weeks to ensure that there were no infections in the wounds.
Cartilage repair by intra-articular injection of autologous rabbit SF-MSCs with KGN and TGF-β3
In this study, the rabbits were randomized into the following four experimental groups (6 in each group): control group (1×107/mL rabbit SF-MSCs in 1 mL isotonic saline application), TGF-β3 group (1×107/mL rabbit SF-MSCs + 10 ng/mL TGF-β3), KGN group (1×107/mL rabbit SF-MSCs + 10 μM KGN), KGN + TGF-β3 group (1×107/mL rabbit SF-MSCs + 10 μM KGN + 10 ng/mL TGF-β3). Each rabbit was treated with autologous rabbit SF-MSCs, which were cultured in vitro. Three weeks after the operation, all the wounds healed well, and the stitches were removed. All animals were injected with different 1 mL isotonic saline solutions according to the above treatment groups; the injection was done using an 18G sterile hypodermic syringe into the knee joint cavity from the lateral articular space. The rabbits were administered an intra-articular injection once a week for 3 weeks.
Macroscopic score evaluation of repaired cartilage
The rabbits in each group were sacrificed, and the operated femur condyles were dissected and harvested for macroscopic score evaluation 12 weeks after the surgery. The gross appearance of the defect sites was photographed and blindly scored by 3 independent observers using the International Cartilage Repair Society (ICRS) macroscopic scoring system, which contains the four following categories: degree of repair, integration to border zone, macroscopic appearance and overall repair assessment [31] (Table S1).
Histological examination of the repaired cartilage
The specimens for histological analysis were fixed in 10% neutral formalin (Sigma-Aldrich, USA) after macroscopic evaluation for 24 hours at 4°C and were then decalcified with a 10% ethylenediaminetetraacetate (EDTA)-buffered saline solution (Sigma-Aldrich, USA) for 28 days. The specimens were embedded in paraffin (Sigma-Aldrich, USA) and were cut into 5 μm thick sections. The coronal sections were stained with hematoxylin-eosin (Sigma-Aldrich, USA) and toluidine blue (Sigma-Aldrich, USA). They were then immunostained for COL II following the manufacturer’s protocol (Abcam, UK). The sections were blindly scored by 3 different experienced pathologists using an ICRS Visual Histological Assessment Scale. This scale had a maximum score of 25 and a minimum score of 0 [32,33] (Table S2).
Statistical analysis
All data are presented as the mean ± standard deviation (SD). All experiments were repeated three times. The statistical significance was determined using the SPSS 18.0 statistical analytical software (SPSS Inc., USA). In all cases, P-values less than 0.05 were considered to indicate statistically significant differences, and P-values less than 0.01 and 0.001 were considered to be highly significant. Graph-Pad Prism version 6.0 was used for the statistical analysis.
Results
Characterization of the rabbit SF-MSCs
After one week of pellet culturing the rabbit synovial fluid, we observed the formation of cell colonies. These cells were observed to be spindle-shaped and were attached to the plastic plates. The cells were observed to have diverse cell types and sizes. Upon subculturing the cells, the spindle-shaped cells were observed to predominate and proliferate over the course of numerous passages (Figure 1).
Figure 1.
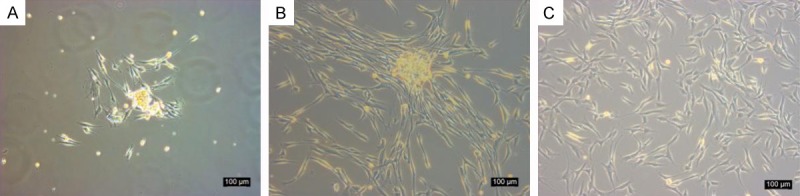
Morphological features of rabbit SF-MSCs. A. Passage 0; B. Passage 1; C. Passage 3. Scale bar = 100 μm.
To further characterize the rabbit SF-MSCs, we utilized flow cytometry to determine the presence of surface markers. We analyzed the mesenchymal markers (CD34, CD45, CD73, CD90 and CD105) of passage 3 cells as described by the MSC identification criteria recommended by the International Society for Cellular Therapy [34]. These results demonstrated that the cultured rabbit SF-MSCs were negative for CD34 (0.49%) and CD45 (0.32%), and were positive for CD90 (94.6%), CD73 (97.3%) and CD105 (98.9%) (Figure 2). Thus, these cultured rabbit SF-MSCs met the identification criteria of MSCs.
Figure 2.
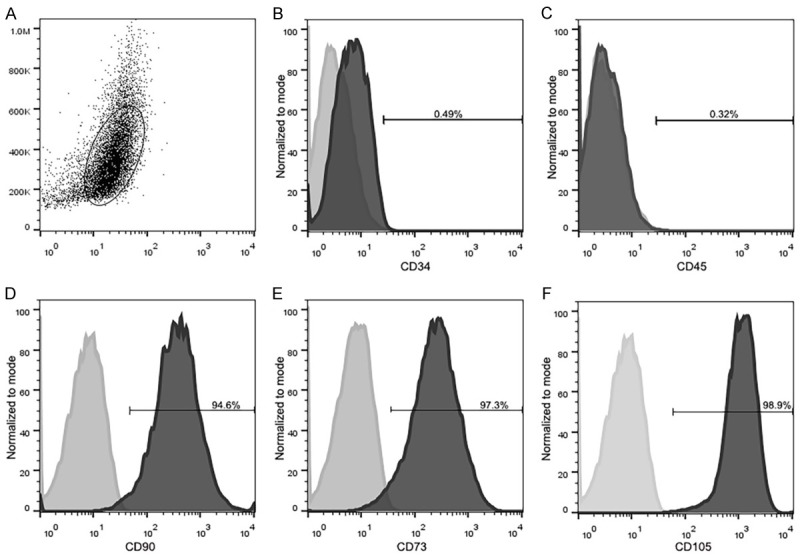
Flow cytometry analysis of the expression of rabbit SF-MSC surface markers. A. Representative dot plots and histograms (overlaid) of marked cell populations (oval). B, C. The cells were negative for the endothelial cell marker CD34 (0.49%) and hematopoietic cell marker CD45 (0.32%). D-F. The cells were positive for CD90 (94.6%), CD73 (97.3%), and CD105 (98.9%).
In addition to these cell surface markers, the tri-lineage differential potential is another indicator of MSCs; thus, we further induced osteogenesis, adipogenesis, and chondrogenesis in rabbit SF-MSCs (Figure 3). Alizarin red staining of calcium compounds revealed the formation of mineralized nodules following 3 weeks of osteogenic induction (Figure 3D). We observed the accumulation of lipid-rich vacuoles in rabbit SF-MSCs using intracellular Oil Red O staining, and this is indicative of the success of adipogenic differentiation (Figure 3E). Under chondrogenic conditions, the rabbit SF-MSC pellet was positively stained with toluidine blue, and this indicated the emergence of chondrocyte-like cells (Figure 3F).
Figure 3.
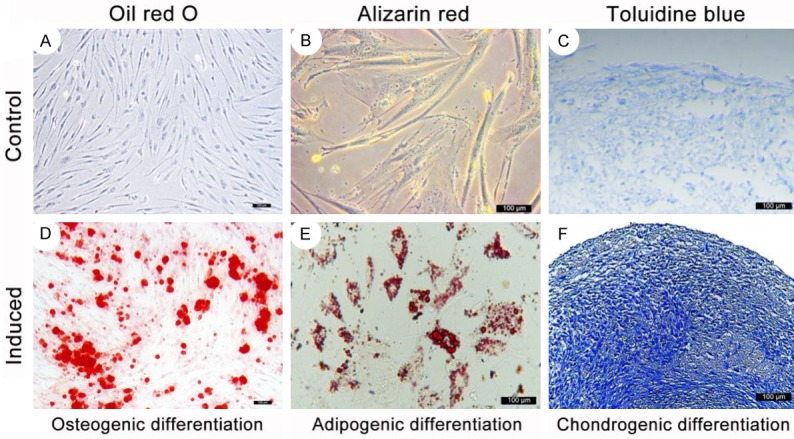
Osteogenic, adipogenic and chondrogenic differentiation of rabbit SF-MSCs 21 days after induction. A-C. Differentiation medium in the absence of an induced cytokine served as the negative control. D. The positive staining of Alizarin red demonstrated the formation of mineralized nodules in the rabbit SF-MSCs 21 days after osteogenic induction. E. 21 days after adipogenic induction, the presence of intracellular Oil Red O staining indicated the formation of small lipid-rich vesicles in the cells. F. Toluidine blue staining of the rabbit SF-MSC pellet 21 days after chondrogenic induction. The scale bar is 100 μm.
The chondrogenic induction of KGN and TGF-β3 in vitro
To compare the effects of KGN and TGF-β3 on the chondrogenic differentiation of rabbit SF-MSCs, we first carried out in vitro experiments. After incubating the cells under the indicated conditions for 21 days, we analyzed the expression levels of numerous genes using qPCR (Figure 4). Relative to the expression levels in the control cells, KGN or TGF-β3 treatment alone or a combined TGF-β3 and KGN treatment significantly increased the expression levels of COL2A1, SOX9, ACAN, and COL10A1. The combined treatment of TGF-β3 and KGN was found to result in significantly higher expression levels of the chondrogenic genes COL2A1 and ACAN relative to the expression levels from either KGN or TGF-β3 treatment alone. As reported previously, TGF-β3 is able to induce the formation of hypertrophic chondrocytes in the later stages. This phenomenon was confirmed by the observation of high COL10A1 expression levels, which led to the formation of fibrocartilage in vivo. Fortunately, with KGN addition, the COL10A1 expression levels were found to decrease significantly. In summary, the combination of TGF-β3 and KGN demonstrates a promising strategy for cartilage repair because it was found to favor the formation of hyaline cartilage.
Figure 4.
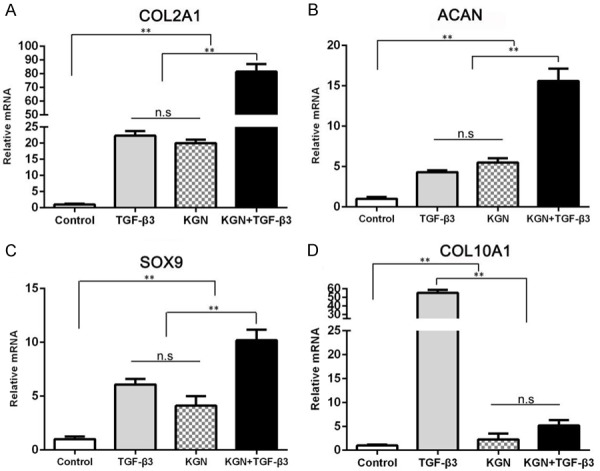
Analysis of the expression levels of COL2A1 (A), ACAN (B), SOX9 (C) and COL10A1 (D) using real-time qPCR over the 3 weeks of chondrogenic differentiation in the presence of TGF-β3 or KGN alone or together. The housekeeping gene (GAPDH) was used as an internal control. The data are presented as the mean ± SD. The statistical significance indicated by ** (P<0.01). No significant differences are indicated by n.s.
To further confirm the chondrogenic differentiation of rabbit SF-MSCs, we immuno-stained cells for COL II and AGG protein expression following induction (Figure 5). Confocal microscopy images demonstrated the expression of AGG and COL II protein expression in rabbit SF-MSCs that was induced by either KGN or TGF-β3 treatment alone or together. The control group exhibited the weakest fluorescence signal among all the experimental groups. This suggests that the rabbit SF-MSCs do not undergo chondrogenesis in the absence of external cytokines. The KGN-induced group exhibited a similar staining pattern as the TGF-β3-induced group. The KGN and TGF-β3 dual-induced group exhibited increased staining compared to the staining in both the single-induced groups. These findings correlated well with the qPCR results and further confirmed the pronounced effects of KGN and TGF-β3 on chondrogenic induction.
Figure 5.
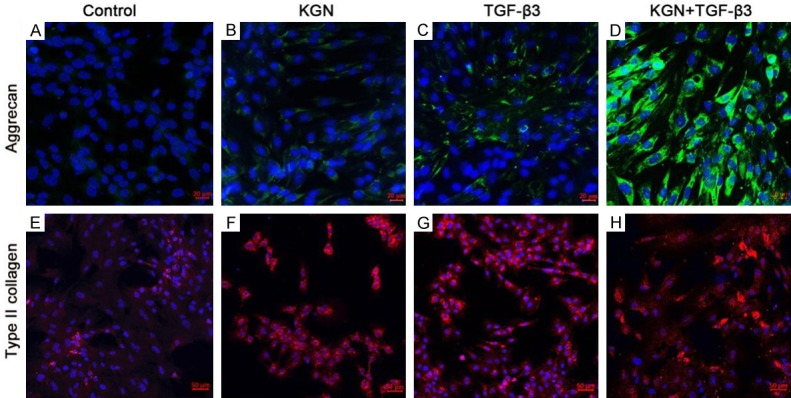
Immuno-staining of chondrocyte marker proteins in the induced cells. Confocal images of immunofluorescence staining with AGG antibodies (A-D, green) and COL II antibodies (E-H, red) are shown, and the cell nuclei were labeled with DAPI (blue). Original magnification: 20× for (A-D) (scale bar is 20 μm), 10× for (E-H) (scale bar is 50 μm).
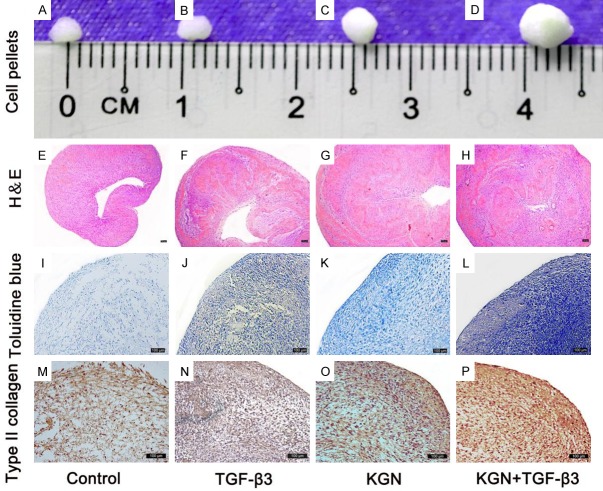
Because chondrogenic differentiation has been demonstrated to work better with high-density cell cultures, we carried out an in vitro chondrogenesis experiment in a pellet culture system. Under chondrogenic conditions, the cell pellet will increase in size due to the production of extracellular matrix [35]. We observed that the KGN + TGF-β3 group exhibited the largest pellet among all of the groups, which could be indicative that this group has the highest level of extracellular matrix production (Figure 6A-D). We then stained the cells using H&E, toluidine blue and anti-COL II. These results demonstrated that the KGN + TGF-β3 group exhibited greater cartilage matrix synthesis following induction, which was found to be especially true in the case of the hyaline cartilage marker protein COL II (Figure 6E-P).
Figure 6.
Chondrogenic differentiation of rabbit SF-MSCs by pellet culture for 21 days. The cartilage nodule formed by rabbit SF-MSCs following the induction period is shown (A-D). H&E staining (E-H), toluidine blue (I-L) and type II collagen immunohistochemical staining (M-P) of cartilage nodules formed by rabbit SF-MSCs in different experimental groups (10 ng/mL TGF-β3 and 10 μM KGN as group (K + T), KGN group and TGF-β3 group, and control pellets) are shown. The scale bar is 100 μm in (E-P).
In vivo cartilage regeneration assisted by TGF-β3 and KGN
A cartilage defect with a 4.0 mm diameter and a 2.5 mm depth was successfully created on the trochlear groove of the knee. The intra-articular injection procedure of rabbit SF-MSCs combined with TGF-β3 and KGN treatment was completed successfully three weeks following the model operation (Figure S1A, S1B). All rabbits survived the surgical procedure and the postoperative treatment. All rabbits were observed to have returned to normal activity. No surgical or intra-articular injection site infections were detected. The rabbits were sacrificed 12 weeks following intra-articular injection and the cartilage specimens were obtained for both macroscopic gross evaluation and histological evaluation.
Macroscopic evaluation of cartilage repair
Following 12 weeks, the cartilage defects remained unrepaired in the control group. The defect border was found to be destroyed and resulted in an even worse defect (Figure 7A1). Fibrocartilage-like tissue, which indicates overgrowth on the cartilage surface, was found to have filled in the cartilage defects in the TGF-β3 group (Figure 7B1). The cartilage defects in the KGN group were approximately 60% covered with cartilage-like tissue (Figure 7C1). At 12 weeks, the cartilage defects in the KGN + TGF-β3 group were almost 100% covered with cartilage-like tissue, which was found to integrate well with the surrounding cartilage (Figure 7D1). The defect sites of the femoral condyles were imaged and blindly evaluated by 3 different investigators based on the International Cartilage Repair Society (ICRS) scoring system (Table S1). All experimental groups exhibited significantly higher ICRS scores compared to those in the control group. The KGN group exhibited a similar, but slightly higher score compared to the TGF-β3 group. The KGN + TGF-β3 group possessed the most significant, and the highest scores, which were 4.6-fold greater than the control group at 12 weeks, and almost reached the identical level of normal cartilage. Therefore, the combination of TGF-β3 and KGN was found to greatly assist the cartilage regeneration ability of rabbit SF-MSCs.
Figure 7.
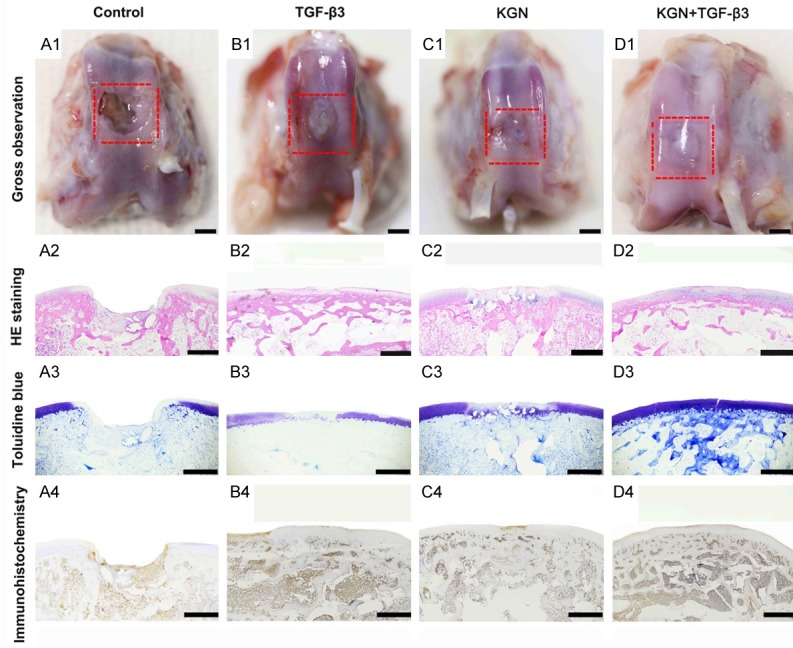
Cartilage repair following 12 weeks of rabbit SF-MSCs administration combined with TGF-β3 or KGN or both. (A1-D1) The macroscopic appearance of the femoral condylar specimens harvested at 12 weeks is shown with (A2-D2) H&E staining, (A3-D3) toluidine blue staining, (A4-D4) and immunohistochemistry staining of type II collagen. All results indicate that the KGN + TGF-β3 group exhibited the best repair effects for hyaline-like cartilage regeneration. Scale bar: 2 mm in (A1-D1), 1.5 mm in (A2-D4).
Histological evaluation of cartilage repair
Following macroscopic evaluation, specimens were cut into 4 μm sections and were stained with hematoxylin and eosin (H&E), toluidine blue, and anti-COL II following the standard protocols. From the histological images, we observed obvious cartilage defects in the control group, and the defect area was found to be covered with inflammatory cells and necrotic tissue (Figure 7A2-A4). In the TGF-β3 treatment group, the defect was replaced by non-functional fibrous scar tissue, which was observed to protrude out of the normal cartilage tissue. The control sections of toluidine blue and COL II staining indicated that the regenerated tissue was not hyaline-like cartilage (Figure 7B2-B4). In the case of the KGN group, the defect was observed to be partially covered with hyaline-like cartilage (Figure 7C2-C4). Unsurprisingly, the KGN + TGF-β3 group exhibited the best repair effect based on the histological staining results, as the defect was entirely filled with hyaline-like cartilage and had a smooth and shiny surface. In addition, the thickness of the regenerated hyaline-like cartilage was observed to be similar to the native cartilage, and the regenerated tissue was found to be well integrated with the surrounding normal cartilage (Figure 7D2-D4). Altogether, this result indicated that we were able to successfully regenerate tissue that was similar to normal cartilage characteristics with the assistance of TGF-β3 and KGN treatment.
We also studied regenerated cartilage using the ICRS visual histological scoring evaluation system (Figure 8). All of the experimental groups were found to have significantly higher scores compared to the scores from the control group. The KGN group (14.7±0.5) was found to have a comparable but lower score compared to the score from the TGF-β3 group (17.6±0.5). The KGN + TGF-β3 group exhibited the highest scores (23.7±0.2), which correlated well with the results described above.
Figure 8.
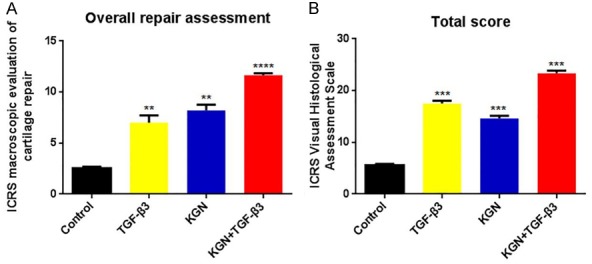
ICRS macroscopic scores and the visual histological assessment scales of the repaired cartilage at 12 weeks. The KGN + TGF-β3 group had the highest score compared to the scores of any other group. The data are presented as the mean ± SD (n=6, **P<0.01, ***P<0.001, ****P<0.0001).
In summary, we obtained excellent cartilage repair using the coinjection of TGF-β3 and KGN with rabbit SF-MSCs.
Discussion
Cartilage defects are a leading cause of disability among adults [1,2]. Thus, novel strategies that can induce hyaline-like cartilage regeneration are needed. In this study, we investigated the effect of two additives, KGN and TGF-β3, on chondrogenic differentiation in vitro and on hyaline-like cartilage formation in vivo using rabbit SF-MSCs. The results from our study demonstrated that both KGN and TGF-β3 were able to increase the expression levels of chondrocyte marker genes in vitro and benefited cartilage repair in vivo. In addition, the combination of these two additives was found to exhibit the best effect both in vitro and in vivo. In the case of chondrogenic differentiation, KGN was shown to inhibit the hypertrophy induced by TGF-β3, while TGF-β3 was shown to increase the differentiation capability of KGN. For in vivo cartilage regeneration, the addition of KGN was able to counteract the formation of the fibrocartilage that was induced by TGF-β3; TGF-β3 was able to enhance the regenerative capability of KGN. In summary, we observed that TGF-β3 and KGN work synergistically to promote hyaline-like cartilage regeneration.
Due to the limited source of autologous chondrocytes, the differentiation of MSCs into cartilage chondrocytes has emerged as the most promising potential strategy for cartilage regeneration [5,36]. MSCs can be isolated from numerous tissues [8-10]. Among all MSCs, SF-MSCs are superior because they can be easily and noninvasively obtained from patients with joint injuries or disease during the treatment process. In addition, the amount of SF-MSCs in the synovial fluid has been shown to dramatically increase in joint disease cases [11-14], and this allows autologous MSC implantation to be available.
MSCs can differentiate into chondrocytes with the addition of special growth factors that accelerate cartilage repair [21,37]. While TGF-β3 is well known for its chondrogenic induction of MSCs, the cells are always differentiated into hypertrophic chondrocytes, and this results in the formation of nonfunctional cartilage in vivo [18,38]. In this study, we observed that the small molecule, KGN, favored the formation of hyaline cartilage. This was likely through the activation of the CBFβ-RUNX1 pathway to induce early chondrocyte differentiation without inducing late hypertrophic differentiation. Thus, the COL X expression level was observed to be decreased upon the addition of KGN to TGF-β3-induced cells. In addition, numerous studies have reported a crosstalk mechanism between the CBFβ/RUNX and Smad pathways for the modulation of chondrogenesis [39,40]. It has been well established that TGF-β functions to activate the Smad2/3 pathway during chondrogenic differentiation [41]. However, the TGF-β family also functions to promote Runx2 through the smad1/5/8 pathway. This leads to the formation of hypertrophic chondrocytes. KGN demonstrated a chondroprotective effect by increasing the Smad2/3 expression levels while suppressing the Smad1/5/8 expression levels [24]. Therefore, the combination of TGF-β3 and KGN was shown to synergistically promote chondrogenic differentiation via the Smad2/3 pathway, and KGN was shown to simultaneously inhibit the hypertrophy induced by TGF-β3 via the Smad1/5/8 pathway. Overall, we found that TGF-β3 and KGN function to assist the generation of hyaline-like cartilage both in vitro and in vivo.
Intra-articular MSC injections are widely used in clinical trials to study the effects on cartilage repair [42,43]. However, no relevant research has been applied to SF-MSCs for cartilage repair in an animal model with cartilage defects. For the first time, we have obtained successful results from in vivo experiments in the present study.
Currently, over 5 cases of autologous SF-MSC-based cell therapy for cartilage injury repair have been carried out in our hospital. However, these therapies are processed though the transplantation of differentiated SF-MSCs, which possesses limitations and require improvement. The animal experimental results presented here can lead to new clinical trials with the direct, intra-articular injection of SF-MSCs/TGF-β3/KGN. This can simplify the process and potentially result in an improved outcome.
We note that this study possesses several limitations. The first limitation is that the optimal cell number of rabbit SF-MSCs for intra-articular injection is currently uncertain. Second, it remains unknown if the newly regenerative tissue was completely induced by the injected rabbit SF-MSCs. Third, in animal studies; we observed and evaluated the 12 week results. This follow-up time may be too short to exhibit more significant differences in repair quality.
Conclusions
This study demonstrates that KGN could function to inhibit the hypertrophic differentiation induced by TGF-β3. Simultaneously, KGN works synergistically with TGF-β3 to promote the formation of hyaline-like cartilage both in vitro and in vivo. Thus, the use of SF-MSCs induced by the combination of TGF-β3 and KGN represents a promising strategy for cartilage repair in the future.
Acknowledgements
All authors have approved the final version of the manuscript and read the journal’s authorship agreement. This study was supported financially by the following grants: Natural Science Foundation of China (No. 21602137); Shenzhen Science and Technology Projects (No. JCYJ2016042510); Health and Family Planning Commission of ShenZhen (No. SZXJ2018035).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24:1–12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Li XZ, Ding JX, Wang JC, Zhuang XL, Chen XS. Biomimetic biphasic scaffolds for osteochondral defect repair. Regener Biomater. 2015;2:221–228. doi: 10.1093/rb/rbv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollander AP, Dickinson SC, Kafienah W. Stem cells and cartilage development: complexities of a simple tissue. Stem Cells. 2010;28:1992–1996. doi: 10.1002/stem.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampat SR, O’Connell GD, Fong JV, Alegre-Aguaron E, Ateshian GA, Hung CT. Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17:2259–2265. doi: 10.1089/ten.tea.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mardones R, Jofre CM, Minguell JJ. Cell therapy and tissue engineering approaches for cartilage repair and/or regeneration. Int J Stem Cells. 2015;8:48–53. doi: 10.15283/ijsc.2015.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 10.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 12.Morito T, Muneta T, Hara K, Ju YJ, Mochizuki T, Makino H, Umezawa A, Sekiya I. Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology. 2008;47:1137–1143. doi: 10.1093/rheumatology/ken114. [DOI] [PubMed] [Google Scholar]
- 13.Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, Tsuji K, Miyaguchi K, Ogishima S, Tanaka H, Muneta T. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2012;30:943–949. doi: 10.1002/jor.22029. [DOI] [PubMed] [Google Scholar]
- 14.Jia Z, Liang Y, Xu X, Li X, Liu Q, Ou Y, Duan L, Zhu W, Lu W, Xiong J, Wang D. Isolation and characterisation of human mesenchymal stem cells derived from synovial fluid by magnetic activated cell sorting (MACS) Cell Biol Int. 2018;42:262–271. doi: 10.1002/cbin.10903. [DOI] [PubMed] [Google Scholar]
- 15.Boeuf S, Richter W. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Res Ther. 2010;1:31. doi: 10.1186/scrt31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roelen BA, Dijke P. Controlling mesenchymal stem cell differentiation by TGF-Beta family members. J Orthop Sci. 2003;8:740–748. doi: 10.1007/s00776-003-0702-2. [DOI] [PubMed] [Google Scholar]
- 17.Jones AR, Flannery CR. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 2007;13:40–45. doi: 10.22203/ecm.v013a04. [DOI] [PubMed] [Google Scholar]
- 18.Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, Kujat R, Nerlich M, Tuan RS, Angele P. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-β isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010;192:158–166. doi: 10.1159/000313399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 20.Bosnakovski D, Mizuno M, Kim G, Ishiguro T, Okumura M, Iwanaga T, Kadosawa T, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol. 2004;32:502–509. doi: 10.1016/j.exphem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Feng Q, Lin S, Zhang K, Dong C, Wu T, Huang H, Yan X, Zhang L, Li G, Bian L. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017;53:329–342. doi: 10.1016/j.actbio.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Richter W. Mesenchymal stem cells and cartilage in situ regeneration. J Intern Med. 2009;266:390–405. doi: 10.1111/j.1365-2796.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- 23.Mara CS, Sartori AR, Duarte AS, Andrade AL, Pedro MA, Coimbra IB. Periosteum as a source of mesenchymal stem cells: the effects of TGF-β3 on chondrogenesis. Clinics. 2011;66:487–492. doi: 10.1590/S1807-59322011000300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X, Schultz PG. A stem cell-based approach to cartilage repair. Science. 2012;336:717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Ding J, Zhang Z, Yang M, Yu J, Wang J, Chang F, Chen X. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl Mater Interfaces. 2016;8:5148–5159. doi: 10.1021/acsami.5b12212. [DOI] [PubMed] [Google Scholar]
- 26.Shi D, Xu X, Ye Y, Song K, Cheng Y, Di J, Hu Q, Li J, Ju H, Jiang Q, Gu Z. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano. 2016;10:1292–1299. doi: 10.1021/acsnano.5b06663. [DOI] [PubMed] [Google Scholar]
- 27.Koyama N, Okubo Y, Nakao K, Osawa K, Fujimura K, Bessho K. Pluripotency of mesenchymal cells derived from synovial fluid in patients with temporomandibular joint disorder. Life Sci. 2011;89:741–747. doi: 10.1016/j.lfs.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Kim YS, Lee HJ, Yeo JE, Kim YI, Choi YJ, Koh YG. Isolation and characterization of human mesenchymal stem cells derived from synovial fluid in patients with osteochondral lesion of the talus. Am J Sports Med. 2015;43:399–406. doi: 10.1177/0363546514559822. [DOI] [PubMed] [Google Scholar]
- 29.Vereb Z, Vancsa A, Pilling M, Rajnavolgyi E, Petrovski G, Szekanecz Z. Immunological properties of synovial fluid-derived mesenchymal stem cell-like cells in rheumatoid arthritis. Ann Rheum Dis. 2015;74:A64–A65. [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Van den Borne M, Raijmakers N, Vanlauwe J, Victor J, de Jong S, Bellemans J, Saris DB International Cartilage Repair Society. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15:1397–1402. doi: 10.1016/j.joca.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E International Cartilage Repair Society. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85:45–57. [PubMed] [Google Scholar]
- 33.Hoemann C, Kandel R, Roberts S, Saris DB, Creemers L, Mainil-Varlet P, Méthot S, Hollander AP, Buschmann MD. International Cartilage Repair Society (ICRS) Recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage. 2011;2:153–172. doi: 10.1177/1947603510397535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 35.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R, Xu J, Wong DSH, Li J, Zhao P, Bian L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials. 2017;145:33–43. doi: 10.1016/j.biomaterials.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 37.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kundu M, Javed A, Jeon JP, Horner A, Shum L, Eckhaus M, Muenke M, Lian JB, Yang Y, Nuckolls GH, Stein GS, Liu PP. Cbfβ interacts with Runx2 and has a critical role in bone development. Nat Genet. 2002;32:639–644. doi: 10.1038/ng1050. [DOI] [PubMed] [Google Scholar]
- 40.Zhang T, Feng W, Wu Y, Goh GS, Ge Z, Tan LP, Hui JH, Yang Z. Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials. 2015;38:72–85. doi: 10.1016/j.biomaterials.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O’Keefe RJ. Smad2 and 3 mediate transforming growth factor-β1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141:4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 42.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 43.Jia Z, Liu Q, Liang Y, Li X, Xu X, Ouyang K, Xiong J, Wang D, Duan L. Repair of articular cartilage defects with intra-articular injection of autologous rabbit synovial fluid-derived mesenchymal stem cells. J Transl Med. 2018;16:123. doi: 10.1186/s12967-018-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.