Abstract
Osteogenesis is a complex process which relies on the coordination of signals and transcription factors. Recent evidence indicates that microRNAs (miRNAs) act as important post-transcriptional regulators in a large number of biological processes including osteoblast differentiation. In this study, we investigated the expression and biological effect of miR-125a-3p during osteogenic differentiation of human adipose derived mesenchymal stem cells (hADSCs). We observed an obvious decrease in miR-125a-3p level during osteogenic differentiation. By using gain- and loss-of function experiments, we noticed that miR-125a-3p could suppress the osteogenic differentiation of hADSCs. Moreover, miR-125a-3p over-expression in hADSCs by transfection with miR-125a-3p mimics significantly inhibited cell proliferation by MTT. Flow cytometry analysis further demonstrated that forced expression of miR-125a-3p induced cell cycle G1/S phase arrest and apoptosis. In addition, we performed bioinformatic analysis, luciferase reporter assay and western blot to confirm that miR-125a-3p could regulate Smad4 and Jak1 expression negatively. Meanwhile, Smad4 and Jak1 were up-regulated after osteogenic differentiation and the down-regulation of endogenous Smad4 and Jak1 suppressed the osteogenic differentiation of hADSCs. Taken together, these data indicated that miR-125a-3p is Smad4 and Jak1 regulator, and it has a crucially physiological function in osteogenic differentiation of hADSCs.
Keywords: Human adipose derived mesenchymal stem cells, osteogenic differentiation, miR-125a-3p
Introduction
Stem cells have provided promising therapeutic applications with regard to biological and functional restoration of tissue defects. Mesenchymal stem cells (MSCs) isolated from bone marrow have been used in clinical trials for bone damage treatment [1,2]. It has been recently demonstrated that MSCs isolated from adipose tissue (hADSC) displayed a significant higher proliferative rate and more potent for lineage-specific differentiation along the three lineages: adipogenic, osteogenic and chondrogenic, compared to bmMSCs [3,4], thus confirming adipose tissue as the election source to isolate MSCs suitable for cell replacement therapies. Undeniably, hADSCs are either readily available in large quantities and exhibit a very high capability for proliferation and differentiation. However, the efficiency of hADSCs osteogenic differentiation is low. Therefore, the elucidation of the molecular mechanisms underlying the osteogenic differentiation of ADSCs is critical prior to their use in osteogenic tissue engineering applications.
Numerous regulatory pathways and stimulus of specific factors are implicated in the progression of osteogenesis [5]. Accumulating evidence suggests that osteoblastic induction and differentiation are also regulated by post-transcriptional mechanisms, partly through temporarily-expressed microRNAs (miRNAs) [6]. miRNAs have been shown to be involved in diverse biological processes including cellular differentiation [7], proliferation [8] and apoptosis [9]. miRNAs also have important roles in the self-renewal and pluripotency of stem cells [10]. The role of miR-125a-3p, have been investigated in several cell lines, such as differentiation and apoptosis of macrophages [11], tumorigenesis in non-small cell lung cancer [12], migration, invasion and pathological angiogenesis in colorectal cancer cell [13], osteoblastic proliferation and differentiation in BMSCs [14]. To the best of our knowledge, the putative role of miR-125a-3p in hADSCs osteogenic differentiation has not been reported yet.
The aim of the present study was to investigate the differential expression of miR-125a-3p during osteogenic differentiation of hADSCs and explore its biological actions. Results demonstrated that miR-125a-3p was Smad4 and Jak1 regulator, and it had a crucially physiological function in osteogenic differentiation of hADSCs, which suggested that miR-125a-3p might be a potential therapeutic agent for the treatment of patients with osteogenic disorders.
Materials and methods
Isolation and culture of cells
hADSCs were derived from adipose tissue biopsies/lipoaspirates supplied by mediterranean institute of oncology (IOM) (Viagrande, Italy) under an approved Institutional Review Board protocol (project ID code: 829-1 of 8 February 2013, IOM Institutional Review Board). Written informed consent has been obtained from all donor patients who agreed to provide samples for the present study. Isolation of hADSCs from adipose tissue was performed as previously reported [15]. After isolation, cells were characterized by immunocytochemistry and flow cytometry analysis using several positive (CD105, CD90, CD73) and negative (CD45, CD34 and CD31) mesenchymal stem cells surface markers as previously reported [16].
hADSCs osteogenic differentiation
For the induction of osteogenic differentiation, 2×106 hADSCs at passage 3 were slowly drip seeded onto collagen I (Serva, Heidelberg, Germany) coated culture slides in 24-well culture plates in ADSC-GM medium (2 ml) (Lonza, Basel, Switzerland). After 24 hours, the medium was completely replaced with MSC-GM medium supplemented with osteogenic differentiation promoting factors (hMSC osteogenic differentiation BulletKit, Lonza, Basel, Switzerland). The osteogenic medium was completely replaced twice a week.
Evaluation of cell proliferation
hADSCs were transfected with miRNA or small interfering RNA (siRNA). After a 48 h incubation, cells were detached and the plated in 6-well plates at a density of 1×104 cells per well. The number of cells was counted at the indicated days after plating with a Countess Automated Cell Counter (Invitrogen, Carlsbad, CA, USA).
Apoptosis detection by TUNEL
Tunel assay was used to measure DNA fragmentation [4]. Briefly, cells were plated on autoclaved glass cover-slips in six-well culture plates and treated with TNF-α, or recombinant JAG2, with or without Notch2/Hes1/Hey2 siRNA. Cellular DNA was stained with apoptosis detection kits (Millipore), and the assay was performed according to recommendations from the manufacturer.
Flow cytometric (FCM) analysis for cell apoptosis
NP cells were trypsinized and collected for detection of apoptosis by using annexin V-FITC apoptosis detection Kit (BD), according to instructions of the manufacturer. Briefly, NP cells were detached from culture plates using 0.25% trypsin/EDTA (Invitrogen). Apoptotic NP cells were identified by staining with FITC-annexin V/PI (BD Biosciences, San Diego, CA, USA) and then analysed by FCM. After washing twice with PBS, 1×106 cells were resuspended in binding buffer (10 mm HEPES, pH 7.4; 140 mm NaCl; 2.5 mm CaCl2). PI and FITC-annexin V were added, and the cells were incubated at room temperature for 10 min before analysis. The index for apoptosis was calculated as the number of cells undergoing apoptosis to the number of total cells.
Gene expression analysis
To confirm the osteogenic induction, total RNA from differentiated hADSCs was isolated through RNeasy minikit (Qiagen, Germantown, MD, USA) according to manufacturer’s instructions. Three independently isolated and cultured samples were used for each of the time points in osteogenic differentiation. cDNA were generated from mRNA using random primer and ReverTraAca® qPCR RT kit (Toyobo, Osaka, Japan). cDNA were generated from miRNA using stem-loop primer and Reverse Transcriptase M-MLV (Rnase H-) (Takara, Dalian, China) according to the manufacturer’s instructions. Quantitative real-time PCR was performed by triplicate using Bio-Rad SYBR green super mix (Bio-Rad, Hercules, USA). β-Actin and U6 were reference gene of mRNA and miRNA, respectively. MiRNA stem-loop primer sequences were as follows: miR-125a-3p, 5’-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACG GCT CC-3’; synthesized by GenePharma (Shanghai, China). The following primers for real-time PCR were showed in Table 1. Results were normalized to the levels of β-actin.
Table 1.
Primer sequences for qRT-PCR
| Gene | Forward (5’-3’) | Reverse (3’-5’) |
|---|---|---|
| miR-125a-3p | GGC GAC AGG TGA GGT TCT T | GCA GGG TCC GAG GTA TTC |
| U6 | CTC GCT TCG GCA GCA CA | AAC GCT TCA CGA ATT TGC GT |
| miR-125a-3p mimics | ACA GGU GAG GUU CUU GGG AGC C | CUC CCA AGA ACC UCA CCU GUU U |
| miR-NC | UUC UCC GAA CGU GUC ACG UTT | ACG UGA CAC GUU CGG AGA ATT |
| ALP | CGA GAT ACA AGC ACT CCC ACT TC | CTG TTC AGC TCG TAC TGC ATG TC |
| Runx2 | CAA GGA CAG AGT CAG ATT AC | GTG GTA GAG TGG ATG GAC |
| OCN | GGT GCA GCC TTT GTG TCC AAG C | GTC AGC CAA CTC GTC ACA GTC C |
| Osterix | TGC TTG AGG AGG AAG TTC | CTT TGC CCA GAG TTG TTG |
| GAPDH | CCA TCT TCC AGG AGC GAG ATC | GCC TTC TCC ATG GTG GTG AA |
Protein extraction and western blotting
Cells were placed on ice immediately following treatment. They were lifted, washed with ice-cold PBS, and harvested in mammalian protein extraction reagent buffer (Pierce). Total cell proteins were resolved on 8-12% SDS-polyacrylamide gels and transferred by electroblotting to PVDF membranes (Bio-Rad). The membranes were blocked with 5% nonfat dry milk in TBST and incubated overnight at 4°C in 3% nonfat dry milk in TBST with the antibodies against anti-Smad4 (CST, #38454; 1:2000 dilution 70 kDa), anti-Jak1 (Abcam, ab138005; 1:2000 dilution 133 kDa), anti-Sp7/Osterix (Abcam, ab22552; 1:2000 dilution, 46 kDa), anti-ALP (Abcam, ab95462; 1:2000 dilution, 57 kDa), anti-OCN (Abcam, ab93876; 1:500 dilution, 11 kDa), anti-OPN (Abcam, ab69498; 1:1000 dilution, 33 kDa), anti-Runx2 (Abcam, ab76956; 1:1000 dilution, 57 kDa), anti-Gapdh (CST, #2118; 1:2000 dilution 37 kDa).
Cell transfection
hADSCs (~2.5×105 cells/well) were plated into 6-well plates to ensure that a confluence of ~70-80% was reached. On the following day, hADSCs were transfected with 100 nM miR-125a-3p mimics or 100 nM miR-125a-3p inhibitor (all from GenePharma, Shanghai, China) using Lipofectamine™ 2000 transfection reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer’s instructions; miR-NC and miR-NC inhibitor were used as negative control, respectively. miR-125a-3p mimics sense: 5’-ACA GGU GAG GUU CUU GGG AGC C-3’, miR-125a-3p mimics antisense: 5’-CUC CCA AGA ACC UCA CCU GUU U-3’; miR-NC sense: 5’-UUC UCC GAA CGU GUC ACG UTT-3’, miR-NC antisense: 5’-ACG UGA CAC GUU CGG AGA ATT-3’; The medium was replaced after 6 h, and the efficiency of transfection was determined by qPCR 3 days later. Osteogenicity was also accessed via the aforementioned differentiation assays following transfection.
Alkaline phosphatase staining
The detailed procedure of cell culture was explained above. Alkaline phosphatase (ALP) staining was performed with BCIP/NBT alkaline phosphatase color development kit (Beyotime, China) according to manufacturer’s instructions. In brief, cells were carefully rinsed with PBS and fixed with 10% neutral buffered formalin to cover the cellular monolayer at room temperature for 15 min. After fixation, cells were rinsed in washing buffer and then incubated in BCIP/NBT liquid substrate for 1-24 hr. Sample preparation and incubation were performed at room temperature while protected from exposure to light. Color change was monitored (osteoblast cells will stain blue/purple) to avoid non-specific staining and cells were observed under a CCD microscope, and the stained cell cultures were imaged. All the staining data were confirmed by three repeated tests.
Alizarin red staining
hADSCs were seeded into 24-well plates at a density of ~1×105 cells/well. When the cells had reached ~80% confluence, the culture medium was replaced with standard osteogenic differentiation induction medium as aforementioned and cultured for a further 3 weeks. The induction medium was replaced every 3 days. Following 3 weeks of culture, cells were stained with Alizarin red (pH 4.1) and analyzed under an inverted microscope.
miRNA target prediction
To identify potential targets of miR-125a-3p, two public available algorithms, TargetScan (www.targetscan.org/) and mirbase (www.mirbase.org) were used. Another database, miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2) was used for the prediction and validation of miRNAs targets. Computational target predictions were ranked according to the total context score of the conserved sites.
Reporter vectors and DNA consturcts
Putative miR-125a-3p-recognition elements (as single copy) from the Smad4 and Jak1 were cloned in the 3’-UTR of the firefly luciferase reporter vector (pMIR-Report, Ambion) according to the manufacturer’s specified guidelines. The oligonucleotide sequences were designed to carry the HindIII and SpeI sites at their extremities facilitating ligation into the HindIII and SpeI sites of pMIR-Report (Ambion).
Reporter gene assay
All transient transfections were conducted by using Dharmafect Duo (Dharmacon, Thermo Scientific). The pMIR-Smad4 and pMIR-Jak1 plasmids were used as reporter constructs. The cells were harvested 72 h after transfection, lysed in reporter lysis buffer, and subsequently assayed for their luciferase activity (Luciferase Assay System, Promega). The transfections were performed in duplicate per each experiment. The luciferase assays were normalized according to their β-galactosidase activity.
Statistical analysis
Data are expressed as mean ± standard deviation. Comparisons between groups were performed using an independent samples t-test. Comparisons among ≥3 groups were performed using one-way analysis of variance and a Bonferroni post hoc test. All statistical analyses were performed with SPSS 13.0 software and a p-value < 0.05 was considered to indicate statistical significance. All assays were repeated three times.
Results
miR-125a-3p inhibits the osteogenic differentiation of hADSCs
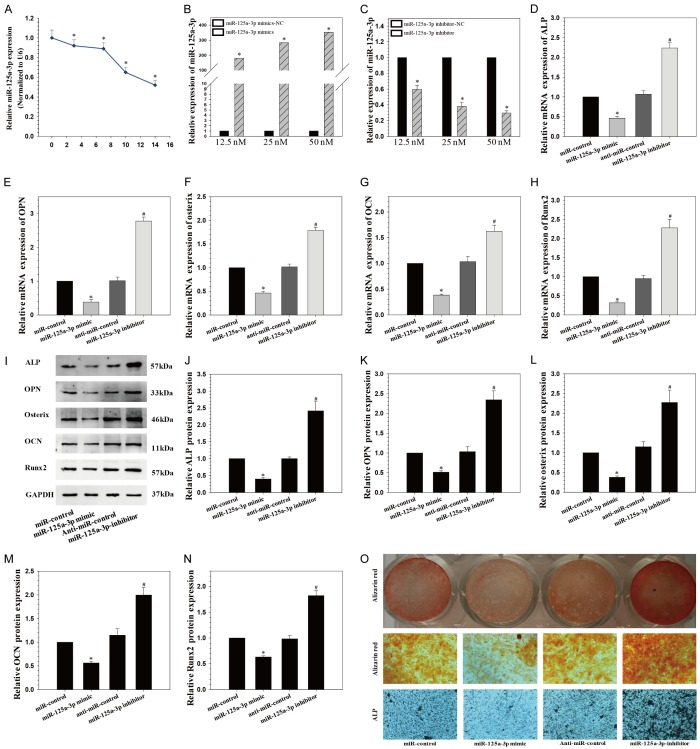
We first determined the temporospatial expression pattern of miR-125a-3p in hADSCs cultured in osteogenic medium by qPCR to investigate the potential role of miR-125a-3p in the osteogenic differentiation of hADSCs. Expression of miR-125a-3p decreased at day 3 compared with that at day 0 and continuously decreased to day 14 (Figure 1A). This suggested that miR-125a-3p might negatively regulate osteogenic differentiation of hADSCs.
Figure 1.
miR-125a-3p inhibits the osteogenic differentiation of hADSCs. A. Endogenous miR-125a-3p expression levels were measured via qRT-PCR at different time points during osteogenic differentiation of hADSCs; *P < 0.05 compared with day 0; B. miR-125a-3p expression was assessed via qRT-PCR in ADSCs transfected with miRNA mimics; *P < 0.05 compared with nonspecific microRNA (miR-NC) group; C. miR-125a-3p expression in hADSCs transfected with miRNA inhibitors; *P < 0.05 compared with anti-miR-NC group; D-H. Relative mRNA expression of osteogenic marker after the treatment of miR-125-3p mimics or miR-125-3p inhibitor at day 14; F, G. alkaline phosphatase (ALP); I-N. Western blot analysis of osteogenic marker protein expression. O. Alkaline phosphatase (ALP) staining and Alizarin Red staining at day 14 showed ALP activity and calcification after miR-125a-3p over-expression and suppression. *P < 0.05 compared with miR-control group, #P < 0.05 compared with anti-miR-control group.
To further elucidate the role of miR-125a-3p in the regulation of osteogenic differentiation of hADSCs, synthetic mimics of miR-125a-3p or inhibitors were transfected into hADSCs, and the osteogenic capacity was examined. Intracellular miR-125a-3p level was markedly up-regulated by miR-125a-3p mimics (Figure 1B) and substantially down-regulated by miR-125a-3p inhibitors (Figure 1C). Furthermore, osteogenic differentiation was significantly inhibited after over-expression of miR-125a-3p and promoted after reduction of miR-125a-3p (Figure 1D-N), as indicated by the expression change of the osteogenic transcription factors, RUNX2 and Osterix, and osteoblastic markers, alkaline phosphatase (ALP), osteopontin (OPN) and osteocalcin (OCN), as well as ALP and Alizarin Red staining (Figure 1O).
miR-125a-3p inhibits the proliferation of hADSCs
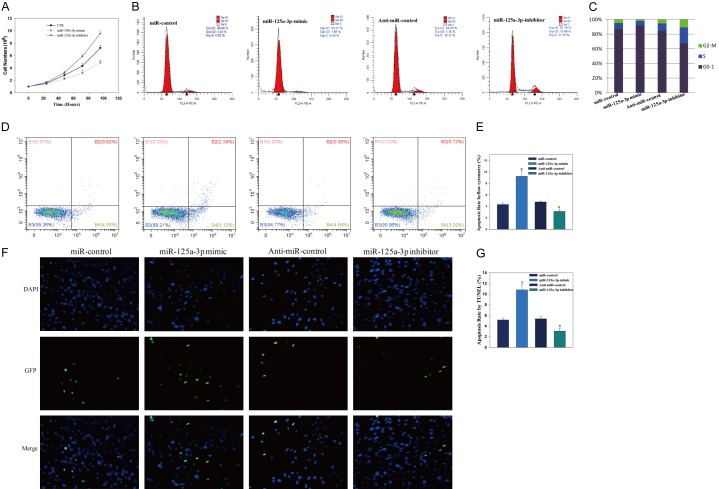
To test the effect of miR-125a-3p on hADSCs proliferation, synthetic mimics of miR-125a-3p or inhibitors were transfected into hADSCs, which were plated (1×104 cells per well) on culture plates and the number of cells was counted on specific days. Results showed that miR-125a-3p mimics inhibited the proliferation of hADSCs, while hADSCs treated with miR-125a-3p inhibitors had faster growth than control cells (Figure 2A). As cell proliferation is closely related to cell cycle progression, we analyzed the effects of miR-125a-3p on cell cycle distribution. Cell cycle progression can be promoted by miR-125-3p inhibitor and suppressed by miR-125-3p mimics, as characterized by an accumulation of cells in G0/G1 hADSCs (Figure 2B, 2C).
Figure 2.
miR-125a-3p inhibits the proliferation of hADSCs and induces the apoptosis of hADSCs. A. hADSCs proliferation was determined by direct cell counting after the treatment of miR-125-3p mimics or miR-125-3p inhibitors at different time points. B, C. miR-125-3p mimics led to an arrest in cell cycle progression, as characterized by an accumulation of cells in G0/G1 in NP cells. D, E. miR-125a-3p mimics treatment increased the apoptotic rate of hADSCs, which was reversed in the presence of miR-125-3p inhibitors. F, G. TUNEL assay showed similar results.
miR-125a-3p induces the apoptosis of hADSCs
To further evaluate the role of miR-125a-3p on the apoptosis of hADSCs, synthetic mimics of miR-125a-3p or inhibitors were transfected into hADSCs. FITC-annexin V/PI FCM assay was performed to assess the rate of hADSCs. Results showed miR-125a-3p mimics treatment increased the apoptotic rate of hADSCs, which was reversed in the presence of miR-125-3p inhibitors with decreased apoptotic rate (Figure 2D, 2E).
TUNEL staining demonstrated that miR-125a-3p mimics could significantly induced hADSCs cells apoptosis compared with control. While miR-125-3p inhibitors decreased hADSCs apoptosis (Figure 2F, 2G). This is similar with flow cytometry, indicating that miR-125a-3p plays an important role in the regulation of hADSCs apoptosis.
miR-125a-3p directly targets Smad4 and Jak1
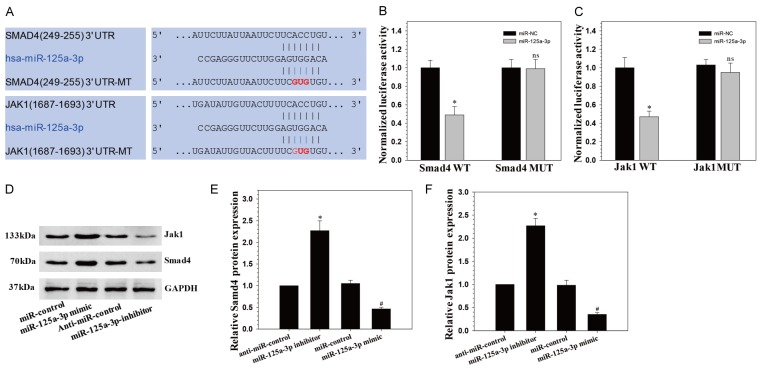
To reveal the molecular mechanism by which miR-125a-3p regulates the osteogenic differentiation of hADSCs, TargetScan (www.targetscan.org/) and mirbase (www.mirbase.org) were used to forecast potential miR-125a-3p targets. Among the candidates, we found that two osteogenesis-related genes, Smad4 and Jak1, contain miR-125a-3p binding sites in their 3’-UTRs. Next, we constructed luciferase reporters for each gene that contained either a wild-type (WT) 3’-UTR or a mutant (mut) 3’-UTR with mutant sequences of the miR-125a-3p binding site (Figure 3A). The results showed that miR-125a-3p repressed the luciferase activity of the 3’-UTR of Smad4 and Jak1 when compared to the nonspecific microRNA (miR-NC) control group, respectively. Additionally, no statistically significant alteration in luciferase activity was observed in the presence of the mutated 3’-UTR site (Figure 3B, 3C).
Figure 3.
miR-125a-3p directly targets Smad4 and JAK1. A. The wild-type and mutated type miR-125a-3p binding sites in the Smad4 and JAK1 3’-UTR. The sequences in red font showed the blinding sites; B, C. The wild-type (WT) 3’-UTR or mutant (MUT) 3’-UTR reporter plasmids of the three genes were co-transfected into HEK293T cells with either miR-125a-3p or miR-NC and fluorescence was quantified; *P < 0.05 compared with miR-NC group; D-F. Smad4 and JAK1 protein expression levels were examined via Western blot following miR-125a-3p mimics and inhibitors transfection in hADSCs.
Next, we detected the protein expression of Smad4 and Jak1 after transfecting hADSCs with the mimics of miR-125a-3p and the inhibitor. We confirm that miR-125a-3p over-expression resulted in down-regulation of Smad4 and Jak1 in hADSCs based on Western blot analysis, miR-125a-3p reduction resulted in the opposite effects (Figure 3D-F). These suggested that miR-125a-3p regulated osteogenic differentiation of hADSCs probably by targeting Smad4 and Jak1.
Smad4 and Jak1 knockdown inhibits osteogenic differentiation and proliferation in hADSCs
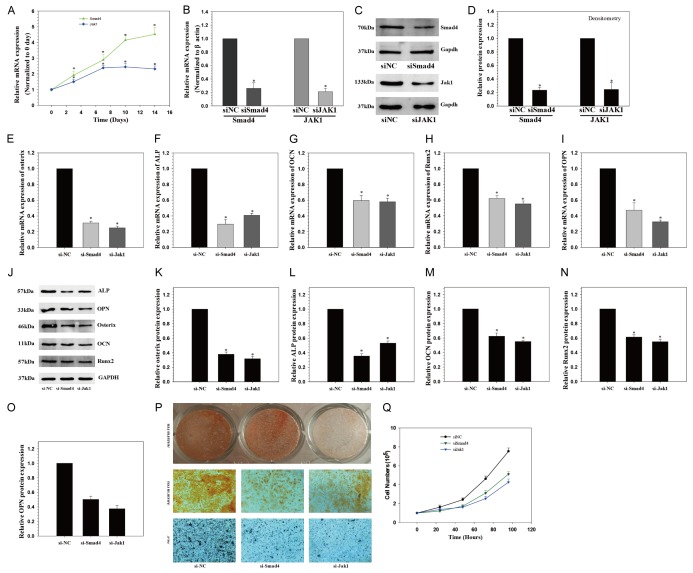
The mRNA levels of Smad4 and Jak1 were determined by qRT-PCR, which were up-regulated after osteogenic differentiation compared with day 0 (Figure 4A).
Figure 4.
Smad4 and JAK1 knockdown inhibits osteogenic differentiation of hADSCs. A. Smad4 and JAK1 mRNA expression levels examined via qRT-PCR at different time points during osteogenic differentiation of hADSCs; B-D. Smad4 and JAK1 mRNA and protein expression level examined via qRT-PCR and Western blot following siRNAs transfection in hADSCs; E-O. Osteogenic marker protein expression examined via Western blot at day 14 after Smad4 and JAK1 knockdown; P. ALP staining and Alizarin Red staining at day 14 showed inhibited ALP activity and calcification following Smad4 and JAK1 knockdown when compared with siNC group (×10). Q. hADSCs proliferation was determined by direct cell counting after the treatment of Smad4 and JAK1 knockdown at different time points. *P < 0.05 compared with non-targeting siRNA (si-NC) group.
To examine the functional effects of Smad4 and Jak1 on the osteogenic differentiation of hADSCs, siRNA-induced mRNA knockdown for each gene was employed, which significantly reduced both mRNA and protein level of Smad4 and Jak1 (Figure 4B-D). Furthermore, Smad4 and Jak1 knockdown inhibited the osteogenic differentiation of hADSCs, as indicated by altered expression of ALP, OPN, OCN, Runx2 and Osterix (Figure 4E-O), and ALP and Alizarin Red staining (Figure 4P).
The effect of Smad4 and Jak1 on hADSCs proliferation was also determined. Direct cell counting showed that Smad4 and Jak1 siRNA-transfected hADSCs proliferated less than control cells (Figure 4Q).
Discussion
ADSCs derived from the adipose tissue is an alternative source of multipotent stem cells, with similar properties to those of BMSCs [17]. ADSCs can be easily harvested, obtaining large amount with minimal risk, and moreover they show the ability to be readily expanded, and also the capacity to undergo adipogenic, osteogenic, chondrogenic, neurogenic, and myogenic differentiation in vitro [18,19]. Besides, appear to be more genetically stable in long-term cultures compared to BMSCs [20]. In view of all these properties, ADSCs is suitable for bone regeneration, but more knowledge of the features of this cell population is needed in order to use in osteogenic tissue engineering applications.
Numerous regulatory pathways and stimulus of specific factors are implicated in the progression of osteogenesis [5]. miRNAs had been detected dysregulated expression and verified to act as an important role during osteogenic differentiation of various types of human cells [21]. The role of miR-125a-3p, have been investigated in several cell lines, such as differentiation and apoptosis of macrophages [11], tumorigenesis in non-small cell lung cancer [12], migration, invasion and pathological angiogenesis in colorectal cancer cell [13], osteoblastic proliferation and differentiation in BMSCs [14]. In the current study, we investigated whether miR-125a-3p was involved in proliferation and osteogenic differentiation of hADSCs. The data in this study demonstrated that miR-125a-3p over-expression inhibited proliferation and osteogenic differentiation of hADSCs, and that miR-125a-3p inhibition by transfection of inhibitor oligonucleotides increased osteogenic differentiation and proliferation. These results indicated that miR-125a-3p was a negative regulator of osteogenic differentiation and proliferation in hADSCs.
TGF-β signaling is important for osteogenesis. TGF-β signals are known to be transduced to the nuclei by the intracellular mediators, Smads [22]. Smad4 is a common mediator; previous studies demonstrated that Smad4 was enrolled in the regulation of osteogenic differentiation. Such as LncRNA Malat1 sponges miR-204 to promote osteoblast differentiation of human aortic VICs through up-regulating Smad4 [23], Smad4 in regulating osteogenic differentiation of human aortic valve interstitial cells [23], miR-449c-5p inhibits osteogenic differentiation of human VICs through Smad4-mediated pathway [24]. In the present study, we proved that Smad4 was a direct target gene of miR-125a-3p using dual-luciferase reporter assay and WB test. At the same time, we also found that Smad4 plays a crucial part in regulating hADSCs osteogenic differentiation.
The Janus kinases (Jaks) are involved in various cellular events depending on the type of cells [25]. It has been demonstrated that Jak1 involved in the process of osteoblasts, inhibition of Jak1 and/or Jak2 in osteoblast-lineage cells led to impaired osteoclastogenesis [26]. Besides, synergistic osteogenic activity between BMP9 and GH can be significantly blunted by JAK inhibitors, leading to a decrease in GH-regulated IGF1 expression in murine multilineage cells [27]. IL-6 in collaboration with sIL-6R may modulate differentiation and proliferation of osteoblastic cells by activating JAK-STAT signaling pathway [28]. In the present study, we proved that Jak1 was a direct target gene of miR-125a-3p using dual-luciferase reporter assay and WB test. At the same time, we also found that Jak1 plays a crucial part in regulating hADSCs osteogenic differentiation. Jak1 knockdown suppressed osteogenic differentiation of hADSCs, indicated by decreased expression of ALP, OCN, Runx2 and Osterix. Jak1 silence also suppressed the proliferation of hADSCs.
In conclusion, we demonstrated that miR-125a-3p negatively regulated hADSCs osteogenic differentiation by directly targeting Smad4 and Jak1. This is the first report to study the regulatory role of miR-125a-3p for hADSCs osteogenic differentiation. Furthermore, our study suggested miR-125a-3p might be identified as potential contributors to the osteogenic differentiation of hADSCs. However, various bioactive factors are present in hADSCs, and the functions of hADSCs are likely a result of the integrated effects of all these factors combined. Therefore, other potential mechanisms require investigation to elucidate the paracrine effects of hADSCs in bone regeneration.
Acknowledgements
This work was supported by grants from Funding for this research was received from the National Natural Science Foundation of China (Grant No. 81371984, 81401840), Sun Yat-sen University Starting Funds for Young Teachers (Grant No. 16ykpy31), Shanghai Municipal Health and Family Planning Commission Fund Project (Grant No. 201640057).
Disclosure of conflict of interest
None.
References
- 1.He M, Gan AW, Lim AY, Goh JC, Hui JH, Chong AK. Bone marrow derived mesenchymal stem cell augmentation of rabbit flexor tendon healing. Hand Surg. 2015;20:421–9. doi: 10.1142/S0218810415500343. [DOI] [PubMed] [Google Scholar]
- 2.Aykan A, Ozturk S, Sahin I, Gurses S, Ural AU, Oren NC, Isik S. Biomechanical analysis of the effect of mesenchymal stem cells on mandibular distraction osteogenesis. J Craniofac Surg. 2013;24:e169–75. doi: 10.1097/SCS.0b013e31827c8706. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9:168. doi: 10.1186/s13287-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunay O, Can G, Cakir Z, Denek Z, Kozanoglu I, Erbil G, Yilmaz M, Baran Y. Autologous rabbit adipose tissue-derived mesenchymal stromal cells for the treatment of bone injuries with distraction osteogenesis. Cytotherapy. 2013;15:690–702. doi: 10.1016/j.jcyt.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Lamplot JD, Qin J, Nan G, Wang J, Liu X, Yin L, Tomal J, Li R, Shui W, Zhang H, Kim SH, Zhang W, Zhang J, Kong Y, Denduluri S, Rogers MR, Pratt A, Haydon RC, Luu HH, Angeles J, Shi LL, He TC. BMP9 signaling in stem cell differentiation and osteogenesis. Am J Stem Cells. 2013;2:1–21. [PMC free article] [PubMed] [Google Scholar]
- 6.Dong S, Yang B, Guo H, Kang F. MicroRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys Res Commun. 2012;418:587–91. doi: 10.1016/j.bbrc.2012.01.075. [DOI] [PubMed] [Google Scholar]
- 7.Kim DS, Lee SY, Lee JH, Bae YC, Jung JS. MicroRNA-103a-3p controls proliferation and osteogenic differentiation of human adipose tissue-derived stromal cells. Exp Mol Med. 2015;47:e172. doi: 10.1038/emm.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Huang T, Yang X, Liu C, Li P, Wang Z, Zhi S. MicroRNA106a regulates the proliferation and invasion of human osteosarcoma cells by targeting VNN2. Oncol Rep. 2018;40:2251–2259. doi: 10.3892/or.2018.6601. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Li M, Peng Y, Hu X, Xu J, Zhu S, Yu Z, Han S. miR-30c regulates proliferation, apoptosis and differentiation via the Shh signaling pathway in P19 cells. Exp Mol Med. 2016;48:e248. doi: 10.1038/emm.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song JL, Zheng W, Chen W, Qian Y, Ouyang YM, Fan CY. Lentivirus-mediated microRNA-124 gene-modified bone marrow mesenchymal stem cell transplantation promotes the repair of spinal cord injury in rats. Exp Mol Med. 2017;49:e332. doi: 10.1038/emm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun XX, Zhang SS, Dai CY, Peng J, Pan Q, Xu LF, Ma XL. LukS-PV-Regulated MicroRNA-125a-3p promotes THP-1 macrophages differentiation and apoptosis by down-regulating NF1 and Bcl-2. Cell Physiol Biochem. 2017;44:1093–1105. doi: 10.1159/000485415. [DOI] [PubMed] [Google Scholar]
- 12.Hou L, Luo P, Ma Y, Jia C, Yu F, Lv Z, Wu C, Fu D. MicroRNA-125a-3p downregulation correlates with tumorigenesis and poor prognosis in patients with non-small cell lung cancer. Oncol Lett. 2017;14:4441–4448. doi: 10.3892/ol.2017.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang L, Gao C, Li Y, Sun M, Xu J, Li H, Jia L, Zhao Y. miR-125a-3p/FUT5-FUT6 axis mediates colorectal cancer cell proliferation, migration, invasion and pathological angiogenesis via PI3K-Akt pathway. Cell Death Dis. 2017;8:e2968. doi: 10.1038/cddis.2017.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu XM, Gu YL, Ren GQ. miR-125a-3p targetedly regulates GIT1 expression to inhibit osteoblastic proliferation and differentiation. Exp Ther Med. 2016;12:4099–4106. doi: 10.3892/etm.2016.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Curley JL, Floyd ZE, Wu X, Halvorsen YDC, Gimble JM. Isolation of human adipose-derived stem cells from lipoaspirates. Methods Mol Biol. 2018;1773:155–165. doi: 10.1007/978-1-4939-7799-4_13. [DOI] [PubMed] [Google Scholar]
- 16.Vicari L, Calabrese G, Forte S, Giuffrida R, Colarossi C, Parrinello NL, Memeo L. Potential role of activating transcription factor 5 during osteogenesis. Stem cells international. 2016;2016:5282185. doi: 10.1155/2016/5282185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciuffi S, Zonefrati R, Brandi ML. Adipose stem cells for bone tissue repair. Clin Cases Miner Bone Metab. 2017;14:217–226. doi: 10.11138/ccmbm/2017.14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–9. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 19.Guasti L, New SE, Hadjidemetriou I, Palmiero M, Ferretti P. Plasticity of human adipose-derived stem cells-relevance to tissue repair. Int J Dev Biol. 2018;62:431–439. doi: 10.1387/ijdb.180074pf. [DOI] [PubMed] [Google Scholar]
- 20.Meza-Zepeda LA, Noer A, Dahl JA, Micci F, Myklebost O, Collas P. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med. 2008;12:553–63. doi: 10.1111/j.1582-4934.2007.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodges WM, O’Brien F, Fulzele S, Hamrick MW. Function of microRNAs in the osteogenic differentiation and therapeutic application of Adipose-derived stem cells (ASCs) Int J Mol Sci. 2017;18 doi: 10.3390/ijms18122597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–69. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 23.Xiao X, Zhou T, Guo S, Guo C, Zhang Q, Dong N, Wang Y. LncRNA MALAT1 sponges miR-204 to promote osteoblast differentiation of human aortic valve interstitial cells through up-regulating Smad4. Int J Cardiol. 2017;243:404–412. doi: 10.1016/j.ijcard.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Xu R, Zhao M, Yang Y, Huang Z, Shi C, Hou X, Zhao Y, Chen B, Xiao Z, Liu J, Miao Q, Dai J. MicroRNA-449c-5p inhibits osteogenic differentiation of human VICs through Smad4-mediated pathway. Sci Rep. 2017;7:8740. doi: 10.1038/s41598-017-09390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami K, Kobayashi Y, Uehara S, Suzuki T, Koide M, Yamashita T, Nakamura M, Takahashi N, Kato H, Udagawa N, Nakamura Y. A Jak1/2 inhibitor, baricitinib, inhibits osteoclastogenesis by suppressing RANKL expression in osteoblasts in vitro. PLoS One. 2017;12:e0181126. doi: 10.1371/journal.pone.0181126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang E, Zhu G, Jiang W, Yang K, Gao Y, Luo Q, Gao JL, Kim SH, Liu X, Li M, Shi Q, Hu N, Wang L, Liu H, Cui J, Zhang W, Li R, Chen X, Kong YH, Zhang J, Wang J, Shen J, Bi Y, Statz J, He BC, Luo J, Wang H, Xiong F, Luu HH, Haydon RC, Yang L, He TC. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res. 2012;27:1566–75. doi: 10.1002/jbmr.1622. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura R, Moriyama K, Yasukawa K, Mundy GR, Yoneda T. Combination of interleukin-6 and soluble interleukin-6 receptors induces differentiation and activation of JAK-STAT and MAP kinase pathways in MG-63 human osteoblastic cells. J Bone Miner Res. 1998;13:777–85. doi: 10.1359/jbmr.1998.13.5.777. [DOI] [PubMed] [Google Scholar]