Abstract
Background: Ischemic reperfusion injury of kidney is major cause for renal failure, however the involved pathogenesis remains unclear creating an void for its effective treatment. Here we studied involvement of microRNA-424 in renal injury. Methods: For the study, p53 or HIF-1α mice were used, ischemic renal injury was induced using clamping of renal pedicles bilateraly. Proximal kidney tubular cells were used for in vitro studies. Hoechst 33342 analysis was done for apoptosis. Blood urea nitrogen (BUN) and serum creatinine was done for renal function, Hematoxylin-eosin tissue damage and Terminal transferase-dUTP nick-end labeling assay for apoptosis. RT-PCR was done for miRNA and ChIP assay to identify the binding of p53 to miR-424. TargetScan and miRanda data base were scanned to find targets of miR-424. Protein expression was done by western blot analysis. Results: We discovered that, miR-424 was over-expressed in ischemic renal injury mice and in hypoxia exposed renal cells. In cells, miR-424 suppressed the expression levels of death receptor 6 (DR6) and halted the apoptosis mediated by hypoxia. Blocking of miR-424 halted the inhibition of DR6 and caused apoptosis and activation of caspase. In mice, miR-424 mimic inhibited expression of DR6 and attenuated ischemic renal injury. We established that, up-regulation of miR-424 in ischemic reperfusion injury was p53 dependent, also inhibition of p53 caused repression of miR-424 levels in hypoxia induced cells in vitro. The p53 knockout mice showed attenuation in levels of miR-424 confirming role of p53 behind up-regulation of miR-424 in vivo. Conclusion: The study confirmed p53/miR-424/DR6 as a protective cascade during ischemic-reperfusion injury.
Keywords: miR-424, kidney injury, DR6, hypoxia, reperfusion
Introduction
Renal ischemia-reperfusion (I/R) injury has emerged as one of the primary reason for acute kidney injury (AKI). AKI mainly occurs in cases which suffer major cardiac abnormalities like surgery and infraction, vascular obstruction and kidney transplantation [1,2]. Renal injury has resulted in high death rates globally along with end-stage kidney failure. It has also been found that if the patients of AKI survives the initial injury are susceptible to chronic renal disease (CRD) ahead in their life [3]. Number of studies have been done to trace the mechanisms associated with renal injury, different types of pathophysiological changes have been identified including inflammation, oxidative stress, cell injury, renal cells apoptosis and damaged perfusion that lead to AKI [4-6].
MicroRNAs (miRs) are small molecules having length of about 21-25 nucleotides discovered to modulate translation of target genes post-transcriptionally via 3’untranslated region (3’UTR) of mRNAs [7-9]. microRNAs play an important role in altering cellular physiology which in turn may lead to development and progression of certain disorders under various pathologic conditions. Numbers of microRNAs have been discovered to contribute in development of renal function, MiR-10a was reported to be as a renal tubule-specific miRNA released in AKI [10]. MiR-17 has been found to be expressed in animal models of AKI [11]. Elevated levels of miR-21 have been observed in various conditions of kidney injury [12,13]. Levels of miR-24 are up-regulated in kidneys of both rats and patients of AKI [14], tissue levels of miR-26a are decreased in rats induced with AKI [15]. A report recently suggested that miR-29a is over expressed in the kidneys of patients diagnosed for AKI [10]. In as study Wei et al. [16] produced the first evidence confirming role of miRs in acute kidney injury by developing Dicer-knockout mice. Dicer is regarded as a key enzyme in synthesis of miRs, this enzyme was selectively deleted in the tubular cells of mice to produce Dicer-knockout strain of mice. The Dicer-knockout mice show normal kidney function and are resistant to acute kidney injury providing better survival compared to wild type mice involved in the experiment [16].
MiR-424 has been found to be associated in post-ischemic vascular remodeling and angiogenesis. It has also been reported that miR-424 is up-regulated in hypoxic endothelial cells and tissues [17]. However role of miR-424 in ischemia-reperfusion kidney injury is not investigated. In the current study we established the (Hypoxia Induced Factor-1) HIF-1 or the proximal tubule-specific p53 knockout mouse model. We examined the role of miR-424 in renal ischemia reperfusion injury both in vivo and in vitro. We found that the levels of miR-424 were up-regulated in mice subjected to ischemia-reperfusion injury (in vivo) and in renal tubular cells (in vitro). The up-regulation of miR-424 was arbitrated via p53 in tubular cells and upon up-regulation, miR-424 inhibited death receptor 6 thus preventing apoptosis of renal cells.
Material and methods
Animal model of ischemic renal injury
All the animal protocols were approved by ethical committee of Xinhua Hospital Affiliated to Medicine School of Shanghai Jiaotong University, Shanghai China, and the approval number was XHAMSS/DN/17-18/0411. The HIF-1α knockout or Proximal tubule-specific p53 mouse models were developed by performing cross of HIF1α flox/flox or p53flox/flox mice with the PEPCK-Cre as per the procedure described earlier [18]. The C57BL/6 mice were selected as normal mice. Both the mice types i.e transgenic and the normal were kept in polypropylene cages. The renal injury was induced in mice according to procedure described earlier [19] for the same the pedicles of kidney were clamped for blocking the blood flow which in turn caused ischemia reperfusion injury; the clamps were removed to allow reperfusion. The sham groups of mice were subjected to surgery with no clamping and exposing the kidney pedicles.
Delivery of miRNA mimic (in vivo)
The miR-424 mimic or miR-negative control (miR-NC) was delivered using Invivofectamine 3.0 reagent (ThermoFisher USA) as per supplier’s protocol. Briefly, miR-424 mimic or miR-NC was mixed with Invivofectamine reagent along with complextion buffer in the ratio (1:2:1). The mixture of all the three was incubated for 30 minutes at 50°C and diluted with phosphate buffer saline (PBS) with 10 volumes. The resulting complex was centrifuged at 4000 g for 30 min at 4°C. The complex was injected via tail vein at the rate of 30 μL/sec in mice 24 h prior to submitting them to surgery at a dose of 10 μL/g body weight.
Cell lines and induction of hypoxia
For in vitro studies, the proximal tubular kidney cell lines of rat were used. The cell lines along with dominant negative mutant (DN-p53) and wild type (WT) were obtained from Department of Nephrology, XinHua Hospital Affiliated to Medicine School of Shanghai Jiaotong University, Shanghai. The study required HIF-1α-null mouse embryonic fibroblasts (MEFs) and the (HIF-1α+/+ (Wild type) which were prepared as per the earlier procedure [19]. The stable proximal tubular kidney cells were transfected with DR6-shRNA (Origene Tech. USA) using Lipofectamine reagent (Thermo Fisher, USA). To confirm the knockdown, expression of DR-6 was done by western blot analysis using anti-DR6 antibody (Biocompare USA). To induce hypoxia, the proximal tubular kidney cells were subjected to plating for 12 h and then were incubated in a medium pre-equilibrated in a hypoxia chamber having 1% oxygen.
Evaluation of apoptosis by morphological analysis
The apoptosis was analyzed by subjecting the cells to Hoechst staining 33342 (10 μg/ml) for 3 minutes. Fluorescence microscopic analysis and phase-contrast was done evaluating the morphological characters of cells such as nuclear fragmentation and condensation along with cellular shrinkage. The morphological characters were analyzed by selecting 3 fields having approximately 100 to 150 cells, to find number apoptotic cells. Each experiment was repeated for at least 3 times.
Renal function analysis and histology
Renal function was assessed by studying BUN (ThermoFisher, USA) and serum creatinine (Abcam) using commercially available kits opting the procedure provided by suppliers. Briefly, the blood was collected and subjected to centrifugation at 10000 g for separating serum. Commercial BUN and serum creatinine measuring kits were used which were obtained from Thermo Fisher USA and Sigma Aldrich USA respectively. Kidney tissue damage was assessed by Hematoxylin-eosin staining (H&E), whereas apoptosis was examined by Terminal transferase-dUTP nick-end labeling (TUNEL) [20-23]. Briefly, the target tissues were fixed with paraformaldehyde (4%) and embedded in paraffin. Each tissue was sliced into sections of 4 μm and deparaffinized opting standard protocol followed by staining with standard H&E or TUNEL protocols. Tubular dilation was regarded as an indicator of renal tubular damage along with features such as loss of brush border; formation of protein/cell cast and degeneration of tubules. The samples were screened randomly to evaluate the percentage of tubular damage. For evaluation of apoptosis, 10-20 fields were selected randomly in each tissue section to count the number of cells positive for selected staining with the help of an Axioplan2 fluorescence microscope.
Extraction of total RNAs and real-time PCR analysis
From both kidney tissues and kidney tubular cell lines, the total RNAs were extracted using RNeasy Mini Kit (Qiagen USA). For reverse transcription a High-capacity cDNA reverse transcription kit (Applied Biosystems, USA) was used for reverse transcripting RNA into cDNA. The RT-PCR was carried by using Taqman probes with the help of Taqman miRNA assay kit (Thermo Fisher, USA). The fold changes recorded using 2-ΔΔCt values.
In situ hybridization (ISH) of miRNA
The ISH was done using an In Situ Hybridization Kit (ISH), BioAssay (TM) as per supplied instructions. Briefly. The kidneys of mice were removed and tissue sections of 5 μm were prepared, the sections were stored in paraformaldehyde (4%) at room temperature for 15 min. The process of de-proteination was carried using proteinase-K (10 μg/ml) at room temperature for 15 min. The sections were subjected to prehybridization using the Biochan prehybridizing solution for 3 hours initially and then for 12 hours. The sections were washed for 15 minutes using 1× SSC at room temperature. The washed sections were subjected to incubation along with blocking solution at 37°C for 60 minutes and again incubated with an anti-DIG antibody (1:100) in the blocking solution at 4°C for 12 hours. Finally the sections were rinsed once using phosphate buffer saline followed by detection using 1-Step™ NBT/BCIP Substrate Solution (Thermo Fisher USA).
Chromatin IP assay of P53 binding to miR-424 promoter region
Chromatin immune precipitation assay (ChIP assay) was done to identify the binding of p53 to miR-424 promoter region ChIP kit (Merck USA) following the supplied instructions. Briefly, the cultured cells were fixed using formaldehyde (1%) the chromatin and cell lysate were collected by sonication. The resultants were subjected to immunoprecipitation along with anti-p53 antibody. The complex was submitted to RT-PCR and amplified using specific primers for p53 binding sequence and control gene p21.
Luciferase assay
The miR-424 binding site in DR6 3’UTR along with both the forward and reverse sequences were normalized and introduced into luciferase miRNA reporter vector obtained from Applied Biosystems USA at specified sites. The Human embryonic kidney cells 293 were transfected with control vector or cloned construct along with miR-424 mimic or scrambled-sequence oligonucleotides. For creating transfection normalization we used miRNA Expression Reporter Vector System (Thermo Fisher USA). The cell lysate was obtained 24 hours after transfection followed by measurement of luciferase activity.
In silico bioinformatics analysis and potential target of miRNA
In silico prediction of miR-424 targets was done by Tragetscan7.0 and miRanda 2010. Another computational algorithm JASPAR CORE was utilized to identify the possible binding sites of p53 to miR-424.
Western blot analysis for expression of proteins
For western blot analysis, cell lysates were collected employing SDS-PAGE buffer (2%). The proteins (30 μg) were resolved using SDS-PAGE gel followed by transfer to PVDF membrane (Thermo Fischer, USA). The blots were incubated along with primary and secondary antibodies but before were blocked using non-fat milk (5%) for 1 hour. The antibodies used for the study were anti-p53, anti-DR6, anti-caspase-3, anti-phopsho-p53 from Santa Cruz Biotech and cyclophilin B and anti-β-actin from Cell Signaling Tech.
Statistical analysis
All the values expressed are mean ± SD, the statistical analysis was done using GraphPad Prism version 3. The statistical significance was established using student’s t-test, the p values <0.05 was regarded as statistically significance.
Results
Expression of miR-424 is elevated in ischemic renal injury mice (in vivo) and in hypoxia induced proximal tubular kidney cells (in vitro)
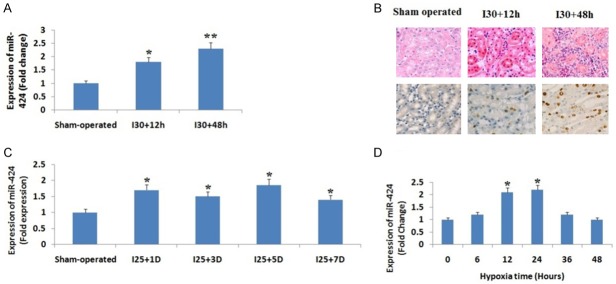
The mice were subjected to 25 and 30 minutes of renal ischemia followed by reperfusion for creating moderate and severe ischemic renal injury. The expression of miR-424 in renal tissues was determined using qRT-PCR. We observed that the mice induced with severe renal ischemic injury, the level of miR-424 increased significantly (P<0.05) compared to sham operated group of mice after 12 hours, the levels increased more significantly (P<0.01) after 48 hours compared to sham group of mice (Figure 1A). In situ hybridization was done for confirming the localization of miR-424, we found that the mice induced with severe ischemia (i.e. for 30 minutes) followed by perfusion after 48 hours showed significantly higher presence of miR-424 compared to sham operated group of mice which showed a weak presence of miR-424 (Figure 1B). In the mice subjected to moderate renal perfusion injury (25 minutes), the expression of miR-424 was detectable only after 24 hours (1 day) which remained elevated for the whole 7 days of study (Figure 1C). For in vitro analysis cultured proximal tubular kidney cells of rat were subjected to hypoxia (1% oxygen), we found that the hypoxic condition barely elevated the levels of miR-424 after 6 hours, but the levels elevated significantly after 12 hours of hypoxia (Figure 1D). The experiment hence established that ischemic renal injury in renal tissues of mice (In vivo) and hypoxic condition in cultured proximal tubular kidney cells (In vitro) are associated with elevated levels of miR-424.
Figure 1.
miR-424 is over-expressed in ischemic renal injury in vivo and under hypoxic conditions in vitro. A: miR-424 was over-expressed in ischemic renal injury. The RNA samples extracted from kidney cortex of mice induced to ischemic renal injury of 30 min followed by 12 h or 48 h reperfusion, or sham operated mice. The levels of miR-424 were assessed by qRT-PCR and were up-regulated significantly in mice with 30 min IRI and reperfusion of 48 h. *P<0.05 compared to sham group mice. B: In situ hybridization study for expression of miR-424 in vivo. The mice were induced to renal ischemia of 30 minutes followed by reperfusion of 48 h (I30/48 h) or sham operated mice considered as control for obtaining sections of kidney tissues. The images show significant over-expression of miR-424 in renal tubules. C: miR-424 in up-regulated in moderate ischemic renal injury mice. The total RNA extracted from the kidney cortex of mice induced with 25 minutes of renal ischemia followed by reperfusion of 1 days (I2/1D), 3 days (I25/3D), 5 days (I25/5D), and 7 days (I25/7D), or sham operated (sham). *P<0.05 compared to sham group. D: miR-542 is up-regulated in cultured proximal tubular kidney cells subjected to hypoxia. The tubular cells were cultured under hypoxic (1% oxygen) and a non-hypoxic condition for 6 to 48 hours, qRT-PCR analysis was done which showed a significant up-regulation of miR-424 at 24 h post hypoxia. *P<0.05 compared to expression at 0 hours.
Transfection of miR-424 mimic attenuated hypoxia mediated apoptosis in cultured proximal tubular kidney cells (In vitro)
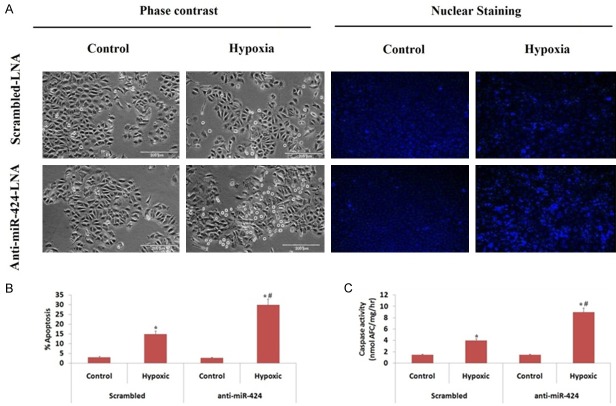
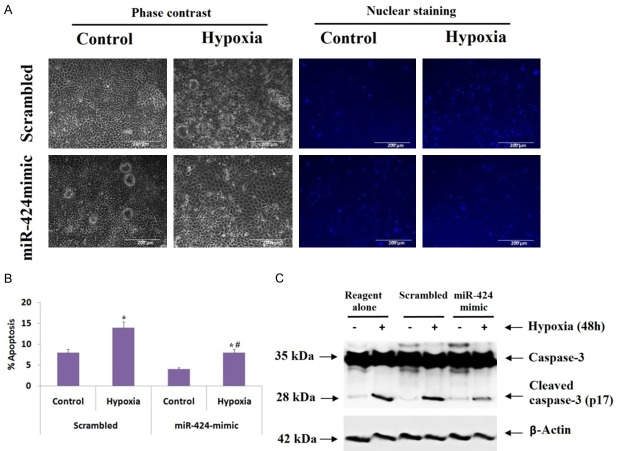
We evaluated the protective role of miR-424 in hypoxic injury of cultured proximal tubular kidney cells (In vitro), for the same we used anti miR-locked nucleic antigen-424 (anti-miR-424 LNA). The cultured proximal tubular kidney cells were transfected with scrambled sequence LNA followed by hypoxia, which resulted an increase in cell apoptosis (Figure 2A). We found that the transfection of anti-miR-424 LNA caused a two fold increase in apoptosis in hypoxic kidney cells (Figure 2A, 2B), which was confirmed by measuring the caspase activity (Figure 2C). Upon transfecting the cells with miR-424 mimic caused a significant reduction in apoptosis mediated by hypoxia as evidenced by morphological study as well as by measurement of caspase-3 activity (Figure 3A-C). The outcome of experiment hence suggested that increase in levels of miR-424 during ischemic renal injury may play a protective role against the renal injury and associated tissue damage.
Figure 2.
Anti-miR-424 LNA aggravates renal cell injury under hypoxic conditions. The proximal tubular kidney cells received transfection of anti-miR-424 LNA or scrambled LNA followed by incubation under normal conditions (Control) or hypoxic conditions (1% oxygen) for 48 h. A: The image shows cellular morphology and nuclear staining of proximal tubular kidney cells. B: The data shows percentage of proximal tubular kidney cells apoptosis via morphological evaluation. C: Caspase activity was assessed for proximal tubular kidney cells under normal and hypoxic conditions. *P<0.05 compared to control (non-hypoxic), #P<0.05 compared to cells transfected with Scrambled LNA under hypoxia.
Figure 3.
miR-424 mimic inhibits apoptosis in tubular cells under hypoxic condition. Proximal tubular kidney cells received transfection of miR-424 mimic or scrambled oligonucleotides followed by incubation under normal conditions (control) or hypoxic conditions for 48 h. A: The images show the morphological and nuclear staining of proximal tubular kidney cells. B: Extent of apoptosis observed after morphological evaluation, *P<0.05 compared to control cells (non-hypoxic), # P<0.05 compared to scrambled transfected under hypoxic condition. C: Western blot analysis of cleaved caspase-3. The cell lysates of proximal tubular kidney cells with miR-424 mimic or scrambled oligonucleotides or reagent transfected under normal and hypoxic condition, cleaved caspase-3 was inhibited significantly by miR-424 transfection. The results were normalized against loading control β-actin.
MiR-424 mimic protects against ischemic acute kidney injury in mice
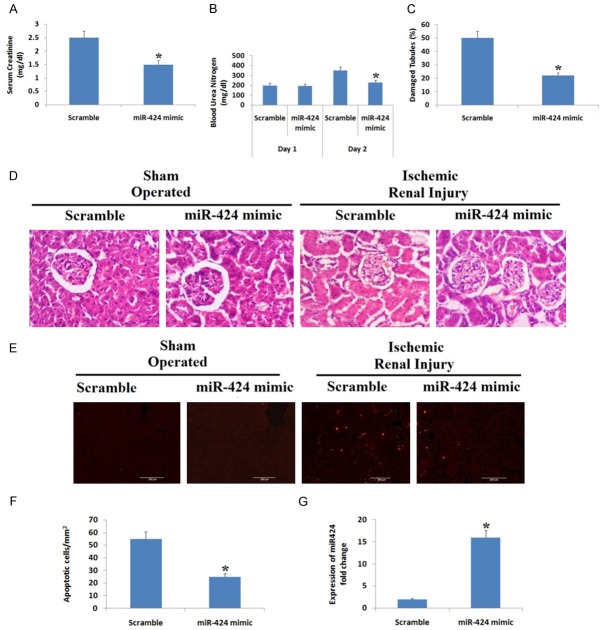
We further evaluated the role of miR-424 mimic in mice, for the same the mice were injected with miR-424 mimic or scramble sequence oligo followed by ischemic renal injury or sham operation. We found that mice injected with miR-424 mimic showed significant reduction in levels of serum creatinine and blood urea nitrogen (BUN) compared to mice treated with scrambled oligo (Figure 4A, 4B). The extent of renal tissue damage was assessed by H&E or TUNEL staining. The H&E stained slides showed that the kidneys of sham operated group showed no signs of injury (Figure 4C and 4D), whereas mice injected with scramble oligo and subjected to ischemia and reperfusion showed about 50% tubular damage. Similar results were seen with TUNEL staining (Figure 4E). However, mice injected with miR-424 mimic showed a significant reduction in tubular damage i.e 20% after ischemic renal injury, the mice also demonstrated significantly lower number of apoptotic cells/mm2 of kidney tissue (25/mm2) compared to mice injected with scrambled oligo (55/mm2) (Figure 4F). The expression of miR-424 was significantly higher in miR-424 mimic treated mice compared to scrambled oligo group (Figure 4G). The findings suggest protective role of miR-424 in ischemia mediated acute kidney injury.
Figure 4.
miR-424 mimic protects against ischemic kidney injury in mice. Mice received injection of miR-424 mimic or scramble sequence oligo followed by ischemic renal injury of 30 min and 48 h reperfusion or sham operation. A: Serum creatinine levels. B: Blood urea nitrogen. C: Percentage of damaged tubules post 30 minutes of ischemia followed by reperfusion of 48 h. P<0.05 compared to scrambled oligo group. D: Hematoxylin and eosin staining indicating renal tissue damage in sham operated and ischemic renal injured mice. E: Images showing TUNEL staining indicating apoptosis in renal tissues. F: Number of apoptotic cells per mm square of isolated renal tissue *P<0.05 compared to scrambled sequence oligo receiving group. G: Expression of miR-424 in scrambled and miR-424 mimic treated groups. *P<0.05 compared to oiligo group.
miR-424 protects kidney cells and tissues via restricting death receptor 6 (DR6) in vivo and in vitro
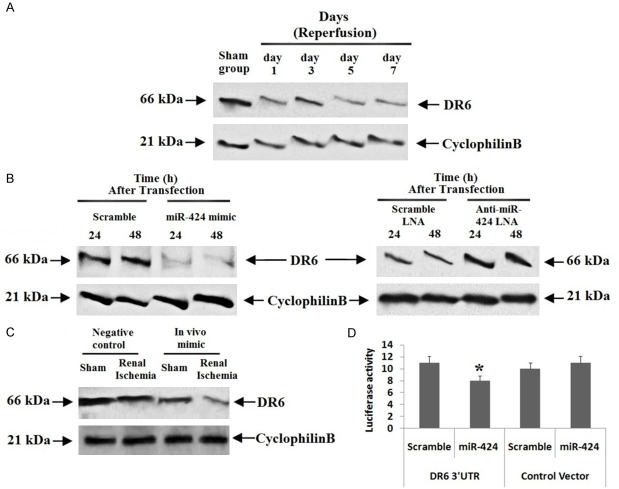
To identify the favorable target for miR-424, we verified two data bases named TragetScan and miRanda. The study of data base by TragetScan (http://www.targetscan.org) and miRanda repeatedly predicted DR6 as target gene of miR-424. DR6 is a member of tumor necrosis factor super family proteins, 78 to 450 bases long and is found on the 6th chromosome. The in silico analysis suggested conserved binding sites for miR-424 in 3’UTR DR6 of mice, rats and human species. We found that, the expression of DR6 decreased significantly in cortical tissues of kidney after subjecting the animals to ischemia of 25 minutes followed by 1-7 days of perfusion (Figure 5A) whereas the levels of miR-424 were increased (Figure 1B). In cultured proximal tubular kidney cells, the levels of DR6 decreased after transfecting them with miR-424 mimics, whereas transfection with anti-miR-424-LNA increased the levels of DR6 (Figure 5B). For in vivo confirmation, we transfected mice with miR-424 mimic for 24 h, which decreased the expression of DR6 (Figure 5C).
Figure 5.
Death Receptor 6 was the favorable target of miR-424 during ischemic renal injury and hypoxic conditions of proximal tubular kidney cell line. A: Western blot analysis of DR6 in renal tissue mice. The renal cortical tissues of mice were extracted for analyzing levels of DR6 employing western blot analysis, cyclophilin B was selected as loading control. B: Western blot analysis for DR6 in proximal tubular kidney cells transfected with miR-424 mimic or anti-424-LNA for which scrambled RNA or LNA was selected as control respectively, cyclophilin B was selected as loading control. C: Western blot analysis was done for expression of DR6 in mice injected with miR-424 mimic or NC oligo. The mice were subjected to ischemic renal injury followed by perfusion of 48 h or sham operated, cyclophilin B was selected as loading control. D: The HEK cells were transfected with Luciferase-DR6-3’-UTR construct or with the Luciferase empty vector combined with miR-424 mimic or scrambled oligonucleotides. The Luciferase activity was done to demonstrate the inhibitory effect of miR-424. *P<0.05 compared to scrambled sequence.
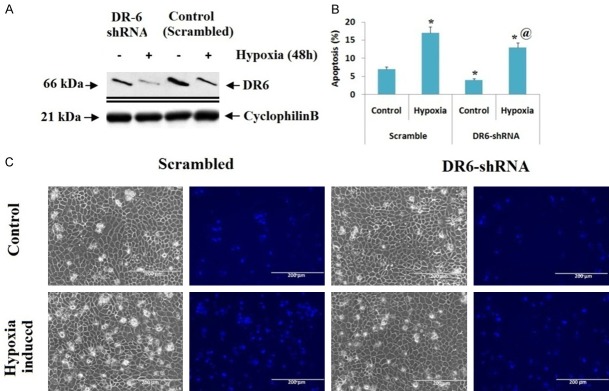
Luciferase assay was done to find whether miR-424 targets directly to the 3’UTR of DR6. The human embryonic kidney cells were transfected with Luciferase-DR6-3’-UTR construct or with the Luciferase empty vector combined with miR-424 mimic or scrambled oligonucleotide. We found that, miR-424 mimic suppressed the expression of Luciferase in cells transfected with Luciferase-miR-424-DR6 and remained unchanged in scrambled oligonucleotide (Figure 5D), suggesting miR-424 targets DR6-3’-UTR directly. Altogether, the results confirmed that miR-424 exerts an attenuating effect in ischemia mediated renal cell injury via suppressing DR6. To further confirm the involvement of DR6 in miR-424 attributed renal protective effect, the proximal tubular kidney cells were transfected with shRNAs to knockdown the DR6 in them. We found that the cells transfected with DR6-shRNA showed a significant suppression of DR6 compared to cells receiving transfection of shRNAs (Figure 6A). We also noticed that, after subjecting the cells to hypoxia for 48 hours, the extent of apoptosis in scrambled oligonucleotides transfected cells was 15% whereas it was 11% in cells knockdown for DR6 (Figure 6C). The outcomes of experiment indicated that DR6 plays a pro-apoptotic role in ischemia mediated renal injury and miR-424 provides protective effect in kidney injury by suppressing DR6.
Figure 6.
Suppression of DR6 protects hypoxia-mediated apoptosis in renal cells. A: Immunoblotting analysis showed that, proximal tubular kidney cells transfected with DR6-shRNA had lower expression of DR6 under hypoxic conditions compared to control (shRNA transfected, control). B: The DR6 knockdown and scrambled proximal tubular kidney cells were incubated under non-hypoxic or hypoxic conditions for 48 hours. The apoptosis (%) was calculated by morphological study. *P<0.05 compared to normal control, @P<0.05 compared to control. C: The images show cell morphology and nuclear staining.
Increased levels of miR-424 during hypoxia and ischemia are not mediated by hypoxia induced factor-1 (HIF-1)
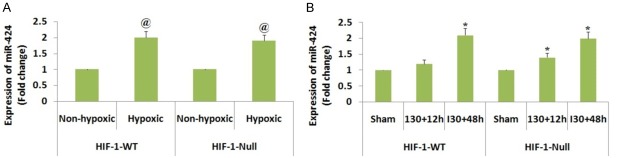
We in our experiments confirmed that DR6 was the favorable target of miR-424, we further extended our study to identify the upstream mechanism involved for enhanced expression of miR-424 in ischemic renal injury. Literature has confirmed that hypoxia induced factor-1 (HIF-1) as the main transcription factor which regulates gene expression in hypoxic and ischemic conditions [24]. A report earlier has suggested role of HIF-miR-424 expression in endothelial cells which promotes angiogenesis [17]. Looking into the role of HIF-1 in hypoxia and ischemia, we decided to evaluate involvement of HIF-1 along with miR-424 in ischemic renal injury mice and hypoxia induced proximal renal tubular cells. To evaluate the same, we studied the levels of miR-424 by hypoxia in HIF-1 null and wild type cells in vitro. We found that the levels of miR-424 increased (Figure 7A) in both HIF-1 null and wild type cells which ruled out the involvement of HIF-1 in hypoxia mediated rise in expression of miR-424. Further we tested the involvement of HIF-1 in vivo using HIF-1 knockout mice, we evidenced that the levels of miR-424 were elevated in proximal tubule of both HIF-1 null and wild type mice, regardless of the HIF-1 status (Figure 7B). The experiment hence denies role of hypoxia induced factor-1 for increased levels of miR-424 mediated by ischemia renal injury in vivo and hypoxia in vitro.
Figure 7.
Hypoxia induced factor-1 is not involved for increased levels of miR-424 during ischemic renal injury in vivo and hypoxia in vitro. A: Expression of miR-424 during hypoxia in both HIF-1-WT and HIF-1 null cells. The cells subjected to hypoxic and non-hypoxic conditions 24 hours for isolation of RNA followed by RT-PCR analysis of miR-424. @ P<0.05 compared to non hypoxic control. B: The levels of miR-424 were elevated in proximal tubule of both HIF-1 null and wild type mice. The mice induced to ischemic renal injury of 30 minutes followed by reperfusion of 12 or 48 hours. The RT-PCR study was done for RNA samples extracted from the renal tissues. *P<0.05 compared to sham operated mice.
p53 binds to miR-424 promoter gene and elevates the expression during hypoxia of renal tubular cells (in vitro)
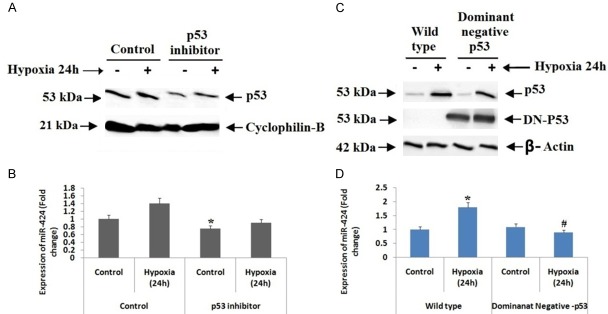
P53 has been identified to play a crucial role in ischemic renal injury [25,26]. To identify the involvement of p53 in regulation of miR-424, we evaluated the levels of p53 in hypoxia induced proximal renal tubular cells. We found that levels of both p53 and its phosphorylated form were elevated after the cells were subjected to 6 to 48 hours of hypoxia (Figure 8A). Later we carried in silico analysis with the help of JASPAR CORE 2016 database to find the promoter region in miR-424. The bioinformatics analysis suggested that miR-424 had a putative binding site for p53. In our experiment we performed chromatin immunoprecipitation (ChIP) assay to find the binding of p53 to the promoter region of miR-424, for the same we selected p21 as positive control which is known target p53 target gene. The results showed higher binding of p53 in hypoxia induced proximal renal tubular cells (Figure 8B). The outcomes suggest that p53 may arbitrate miR-424 levels in hypoxic conditions via directly binding in its promoter region.
Figure 8.
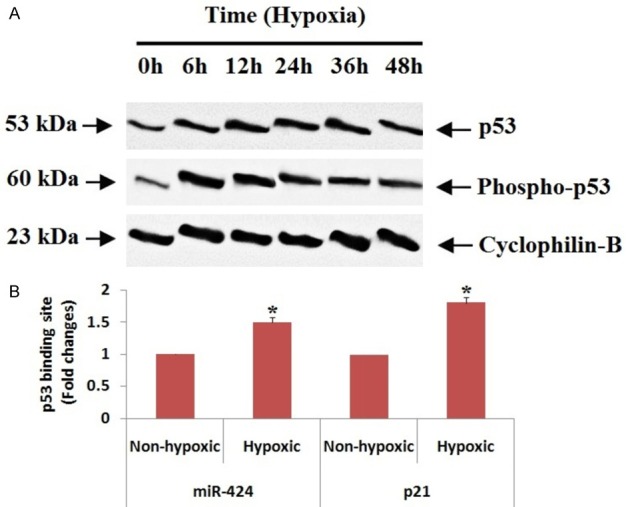
p53 arbitrates miR-424 levels in hypoxic conditions via directly binding in its promoter region. A: Western blot analysis for p53 and phospo-p53 was done, cyclophilin B was selected as control. B: The RT-PCR analysis of miR-424 showed hypoxic conditions elevated the binding site for p53 significantly compared to non-hypoxic condition. *P<0.05 compared to non-hypoxic cells.
To confirm whether p53 is involved in increased expression of miR-424 under hypoxic conditions in renal cells, we evaluated the inhibitory consequences of pifithrin-α (p53 inhibitor) renal cells (Figure 9A). Real time PCR demonstrated that pifithrin-α caused inhibition of miR-424 expression during hypoxic condition of renal cells (Figure 9B). In order to further confirm these RT-PCR findings, we evaluated stable renal cells (Not subjected to hypoxia) transfected with p53 dominant negative mutant along with hypoxia induced renal wild-type cells. We found that (Figure 9D), the levels of miR-424 were inhibited in p53 dominant negative mutant cells whereas the levels increased in wild-type cells (Figure 9C, 9D). The experiment clearly confirms that p53 is involved in increased expression of miR-424 in hypoxic renal cells.
Figure 9.
p53 is involved in increased expression of miR-424 in hypoxic renal cells. A: The proximal tubular kidney cells were cultured in non-hypoxic and hypoxic conditions along with or without p53 inhibitor, followed by western blot study using cyclophilin-B as internal control. B: Effect of p53 inhibitor on expression of miR-424. *P<0.05 compared to control. C: The proximal tubular kidney cells having p53 dominant negative or wild type were cultured under non hypoxic or hypoxic conditions, β-actin was selected as loading control. D: RT-PCR analysis to study effect of dominant negative-p53 on levels of miR-424. *P<0.05 compared to non hypoxic cells, # P<0.05 compared to wild type cells.
p53 leads to increased expression of miR-424 during ischemia in renal injury mice
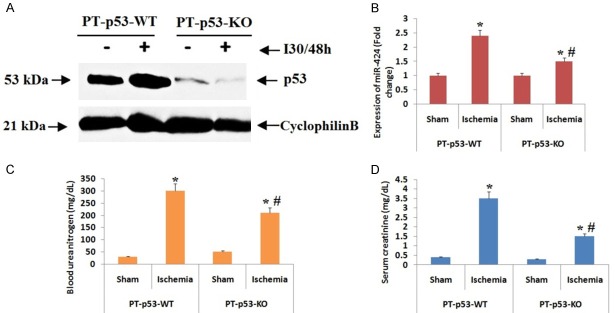
We in this experiment evaluated if over-expression of miR-424 during ischemia mediated renal injury is related to p53. To confirm the same, we selected conditional knockout mice which are specifically ablated for p53 from the proximal tubules of kidney (PT-p53-KO) we also used wild-type mice of similar mice called to as PT-p53-WT [27]. We found that, the PT-p53-KO mice exhibited lower expression of p53 versus PT-p53-WT in the kidney tissues irrespective of ischemic renal injury (Figure 10A). We also found that, submitting mice to 30 minutes of renal ischemia followed by 48 hours of reperfusion caused at least 2 fold elevation in expression levels of miR-424, which was suppressed to about 1 fold in kidney tissues of PT-p53-KO mice (Figure 10B). The findings of blood urea nitrogen and serum creatinine suggested that the PT-p53-KO mice were protected from ischemic renal injury not wholly but partially (Figure 10C, 10D).
Figure 10.
P53 leads to elevation in levels of miR424 during ischemia in renal injured mice. A: The proximal tubule p53 wild-type (PT-P53-WT) and proximal tubule p53 knockout (PT-P53-KO) mice were induced to renal ischemia of 30 minutes and then reperfusion of 48 hours. Western blot analysis for expression of p53 was done using tissue lysates from renal tissues. B: The expression of miR-424 was significantly increased after ischemic renal injury which was down regulated in PT-P53-KO mice. C: The levels of blood urea nitrogen were significantly elevated under ischemic condition in mice. *P<0.05 compared to PT-P53-WT, # P<0.05 compared to PT-P53-wild type ischemia. D: Levels of serum creatinine were found to be elevated significantly under ischemia. *P<0.05 compared to PT-P53-WT, # P<0.05 compared to PT-P53-wild type ischemia.
Discussion
Micro RNAs also called as miRs are potential post-transcriptional governors of gene expression responsible for number of cellular processes required for homeostasis via altering target genes. Here, in the present study, we provide the first attest suggesting regulating role of miR-424 in ischemia mediated acute kidney injury. We found that miR-424 was over-expressed both in vitro (i.e proximal renal tubular cells subjected to hypoxia) and in vivo (i.e ischemia reperfusion kidney injured mice). The over-expression of miR-424 was found to be mediated by p53 directly and not by Hypoxia Induced Factor-1. Over-expression of miR-424 may contribute for its cyto-protective role in survival of kidney cells by suppressing DR6.
MiR-424 has been confirmed to be associated in post-ischemic vascular remodeling and angiogenesis. It has also been evidenced that miR-424 is up-regulated in hypoxic endothelial cells and tissues [17]. Though miR-424 is involved with hypoxia associated with human endothelial cells of vascular tissues, its role in ischemia-reperfusion kidney injury is not investigated. Here we investigated involvement of miR-424 in renal ischemia reperfusion injury both in vivo and in vitro. We evidenced that expression levels of miR-424 were up-regulated in mice subjected to ischemia-reperfusion injury (in vivo) and in renal tubular cells (in vitro) subjected to hypoxia. The up-regulation of miR-424 was arbitrated via p53 in tubular cells and upon up-regulation, miR-424 inhibited death receptor 6 thus preventing apoptosis of renal cells.
To confirm the up-regulation of miR-424 and its role in ischemic acute kidney injury, we performed qRT-PCR with the help of Taqman miRNA assay kit. The outcomes of qRT-PCR suggested up-regulation of miR-424 during ischemic renal injury (Figure 1). On further analysis by inhibiting anti-miR-424-LNA caused increase in apoptosis under hypoxic condition, miR-424 mimic suppressed apoptosis (Figure 2) whereas miR-424-LNA treated cells remained unaffected. Looking into the outcomes, we focused our study on function and regulation of miR-424 in ischemic renal injury. Ultimately, we established that miR-424-mimic inhibited whereas anti-miR-424-LNA increased apoptosis, confirming the protective function of miR-424. The findings were parallel to a study associated with ischemic injury of cardiac tissues and hypoxia induced cardiac cells [28].
MiR-424 have been identified to target number of downstream genes under varied pathological conditions which include, cysteine-rich secretory protein LCCL domain-containing 2 (CRISPLD2) pathway [28], PTEN/PI3K/Akt pathway in lung cancer cells [29] and Chk1 in cervical cancer [30] and targeting CYLD in human pancreatic cancer [31]. In the present study, we identified Death receptor-6 (DR-6) as favorable target of miR-424 using various analytical procedures. To our survey, this is the first study confirming DR-6 as favorable target of miR-424. Death receptor-6, has been reported to initiate survival or apoptotic signals [32]. DR-6 and DR-3 together may activate NF-κB which is responsible the expression of multiple survival genes [33]. Reports recently, have suggested about increased levels of DR-6 under hypoxic conditions could worsen the damage of inflamed nerve cells, whereas the suppression of DR-6 could shield the neuronal cells from injury after ischemia [34,35]. Consistent to these findings, in this study, shRNA mediated knockdown of DR-6 imparted protection to proximal renal tubular cells against apoptosis, indicating that DR-6 promotes death in hypoxia induced kidney cells. Inhibition of DR-6 halted apoptosis in control cells, which is feature of TNF group of proteins (DR-6 being a member of TNF proteins). It was remarkable that up-regulation of miR-424 was observed from the 1st day and lasted up to 7 days after ischemic renal injury, which was associated with inhibition of DR-6 (Figures 1, 5). These longer expressions of miR-424 may lead to better cell survival even after initial injury along with better chances of fixing renal tissues.
Hypoxia induced factor-1 and p53 are two main factors triggered during ischemic renal injury they are also responsible for up-regulation of certain genes including miRs. It has been found that, number of HIF-1 related genes conform to ischemia or hypoxic injury. HIF-1 have also been reported to be responsible for up-regulation of some deleterious genes [26]. In, a study earlier, HIF-1 have been found to regulate miR-424 in endothelial cells [17]. However, in the present report deficiency of HIF-1 did not affect the expression of miR-424 both in vivo and in vitro (Figure 7). Alternatively, we confirmed that up-regulation of miR-424 in ischemic injury mice in vivo and hypoxia induced renal cells in vitro is p53 dependent (Figures 9, 10). The CHIP analysis, demonstrated binding of p53 on the gene promoter of miR-424 (Figure 7). P53 is regarded as one of the important factor in ischemia mediated renal injury, it regulates the myriad genes which are associated with arrest of cell cycle, cell death along with renal inflammation [25,36]. We confirmed that miR-424 as the favorable target of p53 in ischemic renal injury; hence our study provides a new path suggesting involvement of p53 in ischemia mediated renal injury. The up-regulation of miR-424 in ischemic renal injury was stronger, but was weak in PT-p53-KO mice (Figure 10B). The findings of our study suggested that p53/miR-424/DR6 as a protective cascade during ischemic renal injury. The finding was unparallel to earlier studies which have confirmed p53 as a contributing factor in death of tubular cells further causing tissue damage [25,36]. However, p53 is also found to be a stress responsive transcription factor, among the target genes of p53, some genes promotes cell death whereas some promote survival for cells. p21 is a well studied gene which is transcripted by p53, p21 is up-regulated in acute kidney injury and has been found to protect the renal tissues [37]. In the present study, we have evidenced miR-424 as a new protective gene regulated via p53. We found that, during renal injury, miR-424 is up-regulated via p53, miR-424 enhances cell survival and inhibits apoptosis during ischemic renal injury possibly by suppressing Death receptor 6.
Conclusion
Altogether, the study suggests up-regulation of miR-424 in ischemic renal injured mice and hypoxia induced renal tubular cells. The up-regulation resulted in better survival of cells both in vivo and in vitro. The study confirmed p53/miR-424/DR6 as a protective cascade during ischemic renal injury.
Disclosure of conflict of interest
None.
References
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26:1765–1776. doi: 10.1681/ASN.2015010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4:20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manne ND, Arvapalli R, Nepal N, Shokuhfar T, Rice KM, Asano S, Blough ER. Cerium oxide nanoparticles attenuate acute kidney injury induced by intra-abdominal infection in Sprague-Dawley rats. J Nanobiotechnol. 2015;13:1–11. doi: 10.1186/s12951-015-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS. Messengers without borders: mediators of systemic inflammatory response in AKI. J Am Soc Nephrol. 2013;24:529–536. doi: 10.1681/ASN.2012060633. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalmay T. MicroRNAs and cancer. J Intern Med. 2008;263:366–375. doi: 10.1111/j.1365-2796.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 9.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation bymicroRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 10.Aguado-Fraile E, Ramos E, Conde E, Rodríguez M, Martín-Gómez L, Lietor A, Candela Á, Ponte B, Liaño F, García-Bermejo ML. A pilot study identifying a set of microRNAs as precise diagnostic biomarkers of acute kidney injury. PLoS One. 2015;10:e0127175. doi: 10.1371/journal.pone.0127175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaucsar T, Révész C, Godó M, Krenács T, Albert M, Szalay CI, Rosivall L, Benyó Z, Bátkai S, Thum T, Szénási G, Hamar P. Activation of the miR-17 family and miR-21 during murine kidney ischemia-reperfusion injury. Nucleic Acid Ther. 2013;23:344–54. doi: 10.1089/nat.2013.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du J, Cao X, Zou L, Chen Y, Guo J, Chen Z, Hu S, Zheng Z. MicroRNA-21 and risk of severe acute kidney injury and poor outcomes after adult cardiac surgery. PLoS One. 2013;8:e63390. doi: 10.1371/journal.pone.0063390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran K, Saikumar J, Bijol V, Koyner JL, Qian J, Betensky RA, Waikar SS, Vaidya VS. Human miRNome profiling identifies microRNAs differentially present in the urine after kidney injury. Clin Chem. 2013;59:1742–52. doi: 10.1373/clinchem.2013.210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hübner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, Kölling M, Sörensen I, Hinz H, Heineke J, van Rooij E, Haller H, Thum T. MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol. 2014;25:2717–29. doi: 10.1681/ASN.2013121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang S, Wang W, Gou X. MicroRNA 26a modulates regulatory T cells expansion and attenuates renal ischemia-reperfusion injury. Mol Immunol. 2015;65:321–7. doi: 10.1016/j.molimm.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Wei Q, Bhatt K, He HZ, Mi QS, Haase VH, Dong Z. Targeted deletion of Dicer from proximal tubules protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2010;21:756–61. doi: 10.1681/ASN.2009070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, Ramakrishnan S. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J Clin Invest. 2010;120:4141–54. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol. 2014;25:2278–2289. doi: 10.1681/ASN.2013080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt K, Wei Q, Pabla N, Dong G, Mi QS, Liang M, Mei C, Dong Z. MicroRNA-687 induced by hypoxia-inducible factor-1 targets phosphatase and tensin homolog in renal ischemia-reperfusion injury. J Am Soc Nephrol. 2015;26:1588–96. doi: 10.1681/ASN.2014050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei Q, Dong G, Franklin J, Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007;72:53–62. doi: 10.1038/sj.ki.5002256. [DOI] [PubMed] [Google Scholar]
- 21.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol. 2007;293:F1282–1291. doi: 10.1152/ajprenal.00230.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Q, Hill WD, Su Y, Huang S, Dong Z. Heme oxygenase-1 induction contributes to renoprotection by G-CSF during rhabdomyolysis-associated acute kidney injury. Am J Physiol Renal Physiol. 2011;301:F162–170. doi: 10.1152/ajprenal.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC. P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. J Am Soc Nephrol. 2003;14:128–138. doi: 10.1097/01.asn.0000040596.23073.01. [DOI] [PubMed] [Google Scholar]
- 26.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate AKI. J Am Soc Nephrol. 2014;25:2278–2289. doi: 10.1681/ASN.2013080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lou Y, Wang S, Qu J, Zheng J, Jiang W, Lin Z, Zhang S. miR-424 promotes cardiac ischemia/reperfusion injury by direct targeting of CRISPLD2 and regulating cardiomyocyte pyroptosis. Int J Clin Exp Pathol. 2018;11:3222–3235. [PMC free article] [PubMed] [Google Scholar]
- 29.Lu C, Wang H, Chen S, Yang R, Li H, Zhang G. Baicalein inhibits cell growth and increases cisplatin sensitivity of A549 and H460 cells via miR-424-3p and targeting PTEN/PI3K/Akt pathway. J Cell Mol Med. 2018;22:2478–2487. doi: 10.1111/jcmm.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F, Xie X, Zhou C, Lu W. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32:976–87. doi: 10.1038/onc.2012.121. [DOI] [PubMed] [Google Scholar]
- 31.Xu XY, Yan WJ, Jiang WM, Wang J, Yang J, Lv W, Li CM, Zhou DH. MiR-424-5p promotes cell invasion and migration by targeting CYLD in human pancreatic cancer. Int J Clin Exp Med. 2016;9:5960–5968. [Google Scholar]
- 32.Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118:265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 33.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 34.Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Mi S, Lee X, Hu Y, Ji B, Shao Z, Yang W, Huang G, Walus L, Rhodes K, Gong BJ, Miller RH, Pepinsky RB. Death receptor 6 negatively regulates oligodendrocyte survival, maturation and myelination. Nat Med. 2011;17:816–821. doi: 10.1038/nm.2373. [DOI] [PubMed] [Google Scholar]
- 36.Ying Y, Kim J, Westphal SN, Long KE, Padanilam BJ. Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J Am Soc Nephrol. 2014;25:2707–2716. doi: 10.1681/ASN.2013121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1998;101:777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]