Abstract
Long noncoding RNAs (lncRNAs) have been identified to be critical functional regulator in the human tumors, while the deepgoing mechanism by which lncRNAs modulates the endometrial carcinoma is still elusive. In this work, we found that lncRNA GAS5 was under-expressed in the endometrial carcinoma tissue specimens, especially these samples with type 2 diabetes mellitus. Besides, the aberrant under-expression of GAS5 was correlated with the advanced tumor stage as well as poor prognosis outcome. In cellular experiments, GAS5 was decreased in the cells exposed to the high glucose. Enforced GAS5 expression repressed the tumor phenotype of endometrial carcinoma cells, including proliferation and invasion. Molecular mechanism study further demonstrated that GAS5 functioned as a sponge for miR-222-3p, abrogating its ability of inhibiting p27 protein expression. In conclusion, these results confirmed the vital regulation of GAS5/miR-222-3p/p27 axis in the endometrial carcinoma tumorigenesis.
Keywords: Endometrial carcinoma, GAS5, high glucose, miR-222-3p, p27
Introduction
Endometrial carcinoma is one of the most common cancers of the female reproductive system, occurring1 in the endometrium of women with postmenopausal and perimenopausal period. Clinically, the mortality rate of endometrial carcinoma is second only to cervical cancer and ovarian cancer. The etiology of endometrial carcinoma is closely correlated to the somatic systemic disease. Emerging evidence, meta-analysis of cohort studies, have indicated the close relationship between diabetes mellitus and the increased risk of endometrial carcinoma incidence [1]. Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia. The long-standing hyperglycemia leads to chronic damage and dysfunction of various tissues, including eyes, kidneys, heart, blood vessels and nerves. Endometrial carcinoma complicated with type 2 diabetes mellitus has significant complex characteristics, which increases the difficulty of clinical treatment for endometrial carcinoma [2].
Long noncoding RNAs (lncRNAs) are an novel member of noncoding RNAs (ncRNAs), attracting great attention for their potential value in pathophysiological process [3-5]. Recent years, the technological advances of epigenetics have revealed the vital functions of lncRNAs in the human pathogenesis and a variety of biological processes, including immune response, cell differentiation, cell metabolism [6-8]. For instance, lncRNA GAS5 knock-down significantly decrease the airway hyper responsiveness in asthmatic rats and the GAS5/miR-10a/BDNF regulatory axis plays vital role for ASMCs proliferation of asthma [9]. In ovarian cancer, lower GAS5 expression is associated with larger tumor size and more advanced FIGO stage of ovarian cancer patients by targeting miR-196a-5p and thereby down-regulating HOXA5 expression [10].
The valuable findings suggest the vital role of lncRNA in the human pathogenesis by which lncRNA accelerate or impair the pathological process [11]. In this research, we find the ectopic expression of lncRNA GAS5 being capable of the modulation for the endometrial carcinoma. LncRNA GAS5 was closely correlated with the endometrial carcinoma with type 2 diabetes mellitus. In the simulative high glucose, GAS5 functioned as a sponge for miR-222-3p, abrogating its ability of inhibiting p27 protein expression, might function as promising interventional therapeutic targets for endometrial carcinoma.
Materials and methods
Clinical specimens
The tissue samples from endometrial carcinoma were collected who were undergoing surgery. These patients were diagnosed with or without diabetes mellitus. The tissue specimens were rapidly frozen in liquid nitrogen after surgical resection. All the informed consents were obtained before this study, and the scheme was approved by the Ethics Committee of The Metabolic Disease Hospital of Tianjin Medical University.
Cell culture
Human endometrial carcinoma cell lines (HEC1-B, HEC1-A and Ishikawa) were offered by the China Center for Type Culture Collection (CCTCC, Wuhan, China) and cultured in RPMI-1640 (HyClone, Logan, UT, USA) medium. Normal endometrial cell line (EMC) was purchased from Cell Resource Center, Shanghai Institutes for Biological Sciences (Shanghai, China). Medium was supplemented with penicillin/streptomycin (100 U/mL) and 10% fetal bovine serum (FBS). Cells were passaged in the environment of 5% CO2 incubator at 37°C.
Transfection
GAS5 sequence was subcloned into the pcDNA3.1 vector (Invitrogen) for the plasmid construction. Mimics for miR-222-3p and scrambled sequence were purchased from Transheep (Transheep, Shanghai, China). Vectors, mimics and controls were transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions. Sequences were found in the Table S1.
RNA isolation and reverse transcription quantitative polymerase chain reaction (RT-PCR)
RNA extraction was performed with the Trizol LS reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol for the total RNA. Total RNA was reversely transcribed into cDNA using PrimeScript RT Reagent Kit (Dalian, China). qRT-PCR reactions were carried out on ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). All primer sequences are listed in Table S1.
Cell proliferation assay
HEC1-A and Ishikawa cells, 2 × 103 cells per well, were seeded in 96-well plates 24 h for further experiment. After being transfected with vectors, cells were measured using the Cell Counting Kit-8 (CCK-8) kit (Dojindo, Japan) according to the manufacturer’s protocol.
Colony formation assay
Cells were transfected and suspended in medium with 10% Fetal Bovine Serum and seeded in 6-well plates 1 × 103 cells/well. After two weeks, cell colonies were washed by the PBS and fixed with methanol and stained with 0.1% crystal purple. Clone number was calculated as the clone quantity more than 450 cells.
Invasion assay
For the migration assay, Transwell chambers (8 µm pore) (Corning, Corning, NY. USA) with 24-well plate were pre-applied with 50 uL Matrigel (dilution 1:2; BD Biosciences, Franklin Lakes, NJ, USA). Medium (600 µl) with 10% FBS was added to the lower chamber to attract cells, and the serum-free RPMI-1640 medium (200 µl) with 2 × 104 cells was added to top chamber. After incubation of 24 h, the invaded cells were stained and counted.
Western blotting assay
Cells were lysed with the RIPA buffer (Sigma-Aldrich, St. Louis, MO, USA) supplemented with protease inhibitor and phosphatase inhibitor (Diagnostics, Mannheim, Germany). Then, the BCA protein assay kit (Thermo Scientific, USA) was used for the protein concentration. Primary anti-p15 antibody was purchased from Abcam LTD.
Subcellular fractionation
The nuclear and cytoplasmic fractions were separated from the cells. Cells were suspended in the cell fraction buffer and incubated under the ice. After centrifugation, the extracted RNA was precipitated in the lower with the nuclear pellet.
Luciferase reporter assay
The sequences matched with miR-222-3p (wild type, mutant) in the GAS5 and p27 gene 3’-UTR were sub-cloned into the downstream of the luciferase reporter gene to construct the plasmids. For the transfection, the miR-222-3p mimics and controls were co-transfected with the GAS5 wild and mutant type, p27 wild and mutant type respectively. After 48 h, the binding specificity was measured using the luciferase assay kit (Promega, Madison, WI, USA) normalized to Renilla fluorescence.
Statistical analysis
All data were expressed as mean ± standard deviation (SD) and calculated by SPSS 19.0 software with the ANOVA analysis and Student’s t-test. The statistical significance difference was set as the threshold value less 0.05 and 0.01.
Results
Long noncoding RNA GAS5 is decreased in the endometrial carcinoma tissue specimens with type 2 diabetes mellitus
To discovery the differently expressed level of lncRNA GAS5, RT-PCR was carried out in theses collected endometrial carcinoma tissue specimens. Data revealed that lncRNA GAS5 was markedly under-expressed in the endometrial carcinoma tissue (Figure 1A). Moreover, the intra-group analysis revealed that lncRNA GAS5 was lower in the endometrial carcinoma tissue compared with the normal adjacent tissue (Figure 1B). These recruited endometrial carcinoma patients was divided into two group based on the complication of diabetes mellitus, including endometrial carcinoma group (EC) and endometrial carcinoma group with diabetes mellitus (EC/DM) (Figure 1C). The long term prognosis of endometrial carcinoma patients with high- or lower-GAS5 expression was analyzed by the Kaplan-Meier analysis, showing the low survival rate of endometrial carcinoma patients with high GAS5 level (Figure 1D). Together, data from this clinical research demonstrate that lncRNA GAS5 is decreased in the endometrial carcinoma tissue specimens with type 2 diabetes mellitus.
Figure 1.
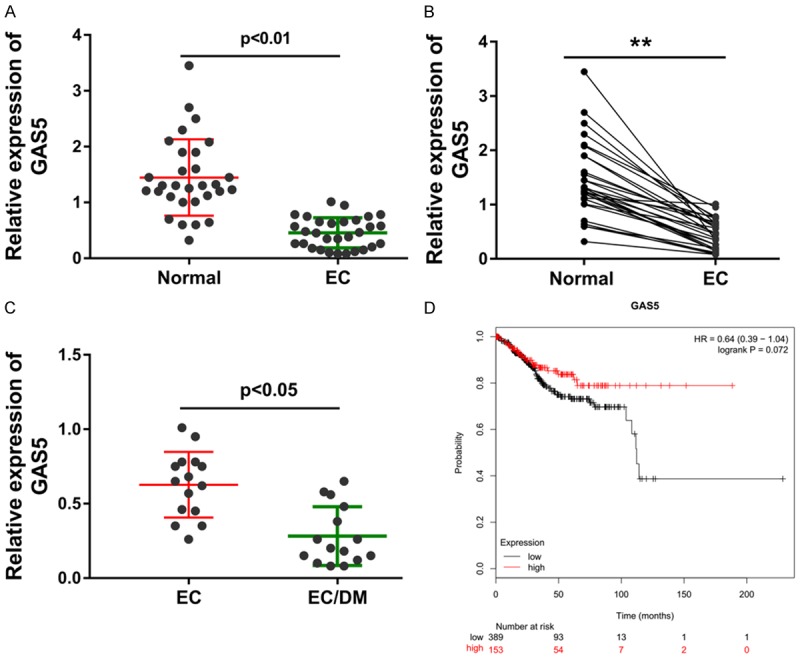
LncRNA GAS5 is decreased in the endometrial carcinoma tissue specimens with type 2 diabetes mellitus. A. Data from RT-PCR revealed the expression level of lncRNA GAS5 in the endometrial carcinoma (EC) tissue and adjacent normal tissue. B. Intra-group analysis revealed the correspondence of lncRNA GAS5 in the endometrial carcinoma tissue (EC) compared with the normal adjacent tissue. C. LncRNA GAS5 level in the endometrial carcinoma group without diabetes mellitus (EC) and endometrial carcinoma group with diabetes mellitus (EC/DM). D. Kaplan-Meier analysis revealed the long term prognosis of endometrial carcinoma patients with high- or lower-GAS5 expression. **presets the p-value less than 0.01.
LncRNA GAS5 inhibited the proliferation and invasion of endometrial carcinoma cells
In the cellular assays, RT-PCR revealed that lncRNA GAS5 was under-expressed in the endometrial carcinoma cells (Figure 2A). In the high glucose induced endometrial carcinoma cells (HEC1-A, Ishikawa), we found that lncRNA GAS5 was low-expressed with the concentration increasing, indicating the suppression of high glucose for the endometrial carcinoma cells (Figure 2B). Proliferative CCK-8 assay revealed that the high glucose administration could increase the proliferation, while the enhanced GAS5 expression markedly suppressed the proliferative absorbance (Figure 2C, 2D). Colony formation assay revealed that the high glucose administration could increase the clone number, while the enhanced GAS5 expression markedly suppressed the clone (Figure 2E, 2F). The transwell invasion assay also get the similar result (Figure 2G, 2H). Therefore, these data illustrated that the high glucose administration could increase the tumor phenotype of endometrial carcinoma cells, while lncRNA GAS5 suppressed these.
Figure 2.
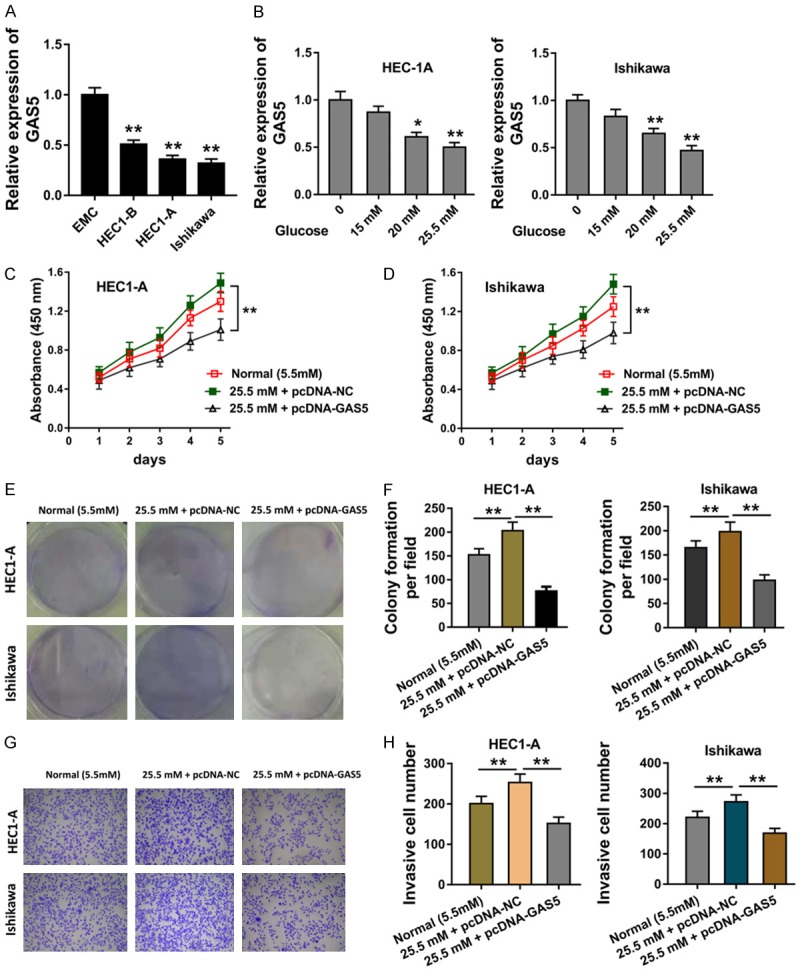
LncRNA GAS5 inhibited the proliferation and invasion of endometrial carcinoma cells. A. RT-PCR revealed the lncRNA GAS5 expressed quantity in the endometrial carcinoma cells and normal cells. B. LncRNA GAS5 level in the glucose (15 mM, 20 mM, 25.5 mM) induced endometrial carcinoma cells (HEC1-A, Ishikawa). C, D. Proliferative CCK-8 assay revealed the proliferative absorbance of endometrial carcinoma cells (HEC1-A, Ishikawa) treated with the normal or high glucose and enhanced GAS5 expression plasmid. E, F. Colony formation assay revealed the clone number in the high glucose administration and enhanced GAS5 expression plasmid. G, H. Transwell invasion assay for the invaded cell number in the high glucose administration and enhanced GAS5 expression plasmid. **presets the p-value less than 0.01. *presets the p-value less than 0.05.
LncRNA GAS5 functions as the sponge of miR-222-3p
For the cellular mechanism of GAS5, we analyzed the subcellular location of GAS5 in Ishikawa cells, indicating the major distribution of GAS5 in the cytoplasm (Figure 3A). Bioinformatics online tools revealed the possible binding sites within GAS5 3’-UTR and miR-222-3p, which was validated using the luciferase reporter assay (Figure 3B). Expression of miR-222-3p in the HEC1-A and Ishikawa cells was over-expressed comparing to the normal endometrial cell line (EMC) (Figure 3C). In the endometrial carcinoma cells induced with high glucose, expression of miR-222-3p was increased with the concentration gradient (Figure 3D). Interestingly, the expression of miR-222-3p was opposite with that of GAS5 (Figure 3E). In the HEC1-A and Ishikawa cells transfected with enhanced GAS5 plasmid (pcDNA-GAS5), miR-222-3p level was markedly decreased (Figure 3F). Thus, all these results demonstrated that lncRNA GAS5 functions as the sponge of miR-222-3p.
Figure 3.
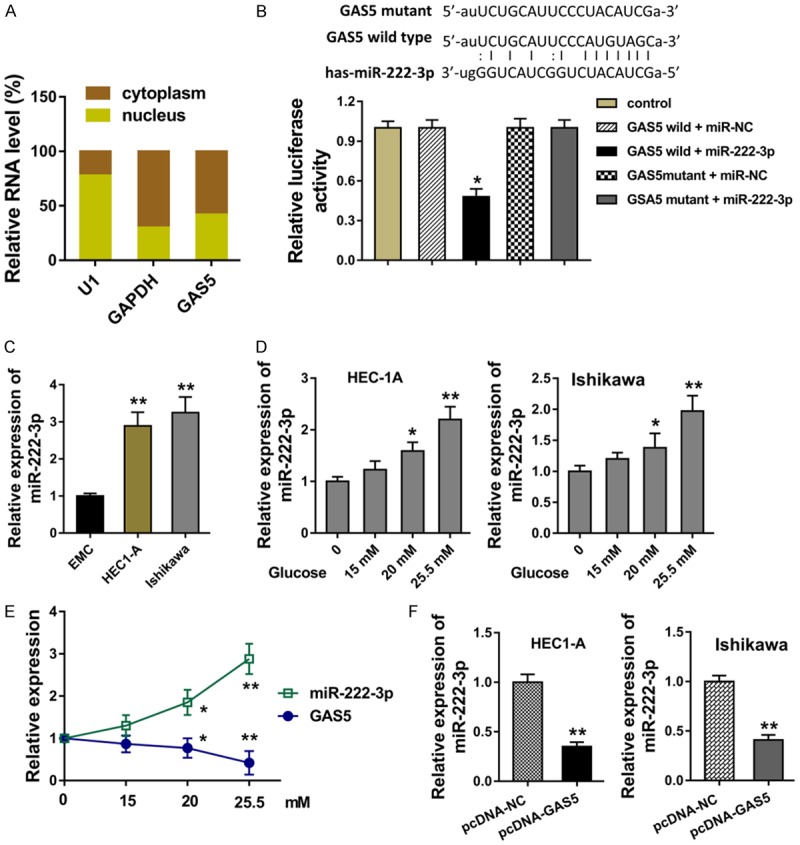
LncRNA GAS5 functions as the sponge of miR-222-3p. A. Subcellular location of GAS5 in Ishikawa cells for the distribution in the nuclear and cytoplasm. B. The possible binding sites within GAS5 3’-UTR and miR-222-3p was predicted using bioinformatics online tools. The binding site was validated using the luciferase reporter assay. C. Expression of miR-222-3p in the HEC1-A and Ishikawa cells and the normal endometrial cell line (EMC). D. Expression of miR-222-3p in the endometrial carcinoma cells induced with high glucose. E. Expression of miR-222-3p and GAS5 in the glucose induced cells. F. RT-PCR revealed the miR-222-3p level in the HEC1-A and Ishikawa cells transfected with enhanced GAS5 plasmid (pcDNA-GAS5). **presets the p-value less than 0.01. *presets the p-value less than 0.05.
p27 acts as the target of miR-222-3p in endometrial carcinoma cells
For the further mechanism regulation of GAS5/miR-222-3p in the endometrial carcinoma carcinogenesis, we discovered the functional effector of them. Bioinformatics tools predicted that miR-222-3p targeted the 3’-UTR of CDKN1B gene, moreover, there were two predictive sites within miR-222-3p and CDKN1B, including the 201-208 and 274-281 of CDKN1B 3’-UTR (Figure 4A). Then, the interaction within CDKN1B and miR-222-3p was tested using the luciferase reporter assay (Figure 4B). In the HEC1-A and Ishikawa cells, the transcript mRNA of the CDKN1B, p27 mRNA, was found to be decreased (Figure 4C). In the endometrial carcinoma cells induced with high glucose, expression of p27 mRNA was decreased with the concentration gradient (Figure 4D). Moreover, when the lncRNA GAS5 level was enforced in the Ishikawa cells, p27 mRNA was consistently enhanced (Figure 4E). Western blot demonstrated that p27 protein was boosted in the GAS5 plasmid transfection, while it was impaired in the miR-222-3p mimics transfection (Figure 4F, 4G). These results indicate that p27 acts as the target of miR-222-3p in endometrial carcinoma cells.
Figure 4.
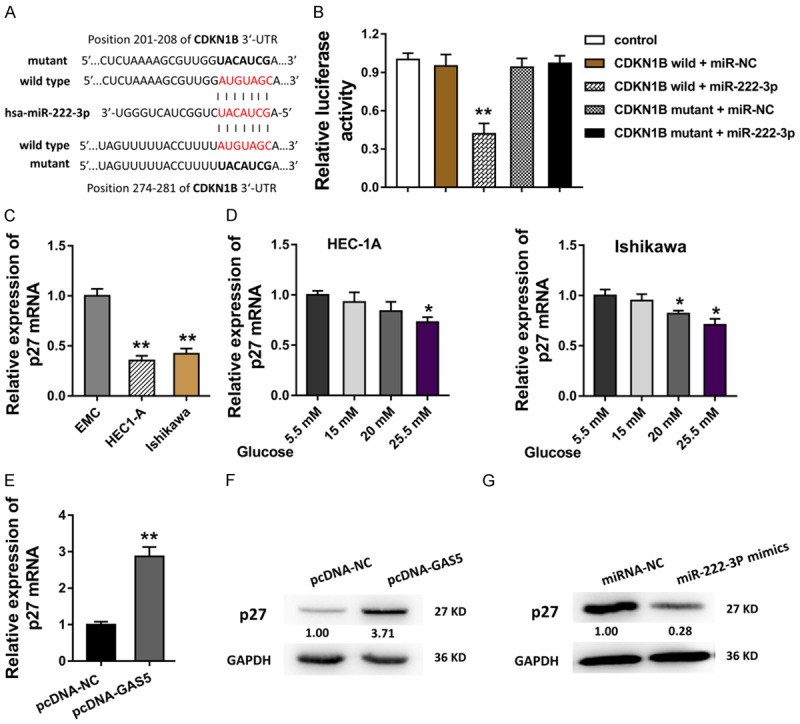
p27 acts as the target of miR-222-3p in endometrial carcinoma cells. A. Bioinformatics tools predicted that miR-222-3p targeted the 3’-UTR of CDKN1B gene with two predictive sites, including the 201-208 and 274-281 of CDKN1B 3’-UTR. B. The interaction within CDKN1B and miR-222-3p was tested using the luciferase reporter assay. C. In the HEC1-A and Ishikawa cells, the transcript mRNA of the CDKN1B, p27 mRNA, was measured using the RT-PCR. D. p27 mRNA was measured using RT-PCR in the endometrial carcinoma cells induced with the concentration gradient. E. p27 mRNA level in the Ishikawa cells with the GAS5 enhanced plasmid transfection. F, G. Western blot demonstrated the p27 protein in the GAS5 plasmid transfection and the miR-222-3p mimics transfection. **presets the p-value less than 0.01. *presets the p-value less than 0.05.
Discussion
In this field of epigenetics, long noncoding RNAs (lncRNAs) are a major group of the noncoding (ncRNA) in the human cancers [12]. Lots of research work have been done to assess the various novel identified ncRNAs in tumorigenesis. However, the research regarding endometrial carcinoma is lesser, and the evidence of these studies is limited as the mechanism used hardly illustrate the pathogenesis. LncRNAs could function as the vital accelerator or inhibitor in the cancer carcinogenesis.
Recent studies indicate that lncRNAs might be potential regulator for endometrial carcinoma [13,14]. For instance, lncRNA MIR22HG could act as the tumor suppressor for endometrial carcinoma by inhibiting cells proliferation, inducing EC cells apoptosis, and arresting EC cells in G0/G1 phase, via regulating miR-141-3p/DAPK1 axis [15]. LncRNA ABHD11-AS1 is significantly overexpressed in endometrial carcinoma compared to normal endometrial tissue, which functions as an oncogene as promoting the cell proliferation and invasion and inhibiting apoptosis [16]. This present work revealed that lncRNA GAS5 was markedly decreased in the endometrial carcinoma tissue and cells. Interestingly, the expression of GAS5 was lower in these patients with type 2 diabetes mellitus or these cells administrated with high glucose. This phenomenon indicated that the GAS5 might be correlated with the diabetes mellitus characteristic and its typical high glucose.
To examine the role of GAS5 in endometrial carcinoma, GAS5 expression was enforced by plasmid transfection. In the cellular assay, the high glucose (25.5 mM) administration could promote the proliferation and invasion of endometrial carcinoma cells. Then, the enhanced GAS5 expression could significantly suppress the tumour phenotype, including proliferation and invasion. To further explore the underlying molecular mechanisms by which GAS5 regulated downstream effectors in endometrial carcinoma cells, the subcellular localization of GAS5 was identified. Data reported the cytoplasmic location of GAS5 in endometrial carcinoma cell and its function might focus on the post-transcriptional regulation given that lncRNA functions are dependent on its subcellular localization.
The mechanisms underlying the regulation of GAS5 remain to be elucidated [17-19]. Then, miR-222-3p is identified to be the downstream of GAS5, which is confirmed by luciferase reporter assay. Moreover, the significant downregulation of p27 mRNA and protein expression levels was observed. The post-transcriptional regulation axis is the GAS5/miR-222-3p/p27 in the endometrial carcinoma cells.
Emerging evidence have confirmed that p27 (CDKN1B) is identified as a negative regulator controlling the cycle progression at G1/S phase [20-22]. The function by which p27 protein regulate cancers is the regulation by post-translational modifications, especially phosphorylation of particular amino acid, to alter the cellular localization and the degradation [23-25]. In the endometrial carcinoma, the loss of p27 associated with the risk [26]. Besides, the nuclear p27 expression correlated with stage and produced near-significant results in univariate survival analysis in endometrial endometrioid adenocarcinoma [27].
Therefore, the GAS5/miR-222-3p/p27 axis could inhibit the diabetes mellitus related tumorigenesis in the endometrial carcinoma cells. In the present study, we explored the effect of lncRNA GAS5 in the endometrial carcinoma. The realization that GAS5 can act as tumor suppressor for endometrial carcinoma, which occurred in the post-transcriptional regulation, innovated the fields of competing endogenous RNA (ceRNA) and the therapeutic methods in future studies.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Zhang ZH, Su PY, Hao JH, Sun YH. The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: a meta-analysis of prospective cohort studies. Int J Gynecol Cancer. 2013;23:294–303. doi: 10.1097/IGC.0b013e31827b8430. [DOI] [PubMed] [Google Scholar]
- 2.Friberg E, Orsini N, Mantzoros CS, Wolk A. Diabetes mellitus and risk of endometrial cancer: a meta-analysis. Diabetologia. 2007;50:1365–1374. doi: 10.1007/s00125-007-0681-5. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y. The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med. 2018;22:5768–5775. doi: 10.1111/jcmm.13866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botti G, De Chiara A, Di Bonito M, Cerrone M, Malzone MG, Collina F, Cantile M. Noncoding RNAs within the HOX gene network in tumor pathogenesis and progression. J Cell Physiol. 2018;234:395–413. doi: 10.1002/jcp.27036. [DOI] [PubMed] [Google Scholar]
- 5.Lei L, Chen J, Huang J, Lu J, Pei S, Ding S, Kang L, Xiao R, Zeng Q. Functions and regulatory mechanisms of metastasis-associated lung adenocarcinoma transcript 1. J Cell Physiol. 2018;234:134–151. doi: 10.1002/jcp.26759. [DOI] [PubMed] [Google Scholar]
- 6.Salehi S, Taheri MN, Azarpira N, Zare A, Behzad-Behbahani A. State of the art technologies to explore long non-coding RNAs in cancer. J Cell Mol Med. 2017;21:3120–3140. doi: 10.1111/jcmm.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Zheng H, Chan MT, Wu WK. HULC: an oncogenic long non-coding RNA in human cancer. J Cell Mol Med. 2017;21:410–417. doi: 10.1111/jcmm.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mousavi MJ, Jamshidi A, Chopra A, Aslani S, Akhlaghi M, Mahmoudi M. Implications of the noncoding RNAs in rheumatoid arthritis pathogenesis. J Cell Physiol. 2018;234:335–347. doi: 10.1002/jcp.26911. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XY, Tang XY, Li N, Zhao LM, Guo YL, Li XS, Tian CJ, Cheng DJ, Chen ZC, Zhang LX. GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR-10a/BDNF signaling pathway. Life Sci. 2018;212:93–101. doi: 10.1016/j.lfs.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Yu H, Zheng J, Ning N, Tang F, Yang Y, Wang Y. Lowly-expressed lncRNA GAS5 facilitates progression of ovarian cancer through targeting miR-196-5p and thereby regulating HOXA5. Gynecol Oncol. 2018;151:345–355. doi: 10.1016/j.ygyno.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Yu WD, Wang H, He QF, Xu Y, Wang XC. Long noncoding RNAs in cancer-immunity cycle. J Cell Physiol. 2018;233:6518–6523. doi: 10.1002/jcp.26568. [DOI] [PubMed] [Google Scholar]
- 12.Yang CH, Zhang XY, Zhou LN, Wan Y, Song LL, Gu WL, Liu R, Ma YN, Meng HR, Tian YL, Zhang Y. LncRNA SNHG8 participates in the development of endometrial carcinoma through regulating c-MET expression by miR-152. Eur Rev Med Pharmacol Sci. 2018;22:1629–1637. doi: 10.26355/eurrev_201803_14698. [DOI] [PubMed] [Google Scholar]
- 13.Zhou YX, Wang C, Mao LW, Wang YL, Xia LQ, Zhao W, Shen J, Chen J. Long noncoding RNA HOTAIR mediates the estrogen-induced metastasis of endometrial cancer cells via the miR-646/NPM1 axis. Am J Physiol Cell Physiol. 2018;314:C690–C701. doi: 10.1152/ajpcell.00222.2017. [DOI] [PubMed] [Google Scholar]
- 14.Sun KX, Wu DD, Chen S, Zhao Y, Zong ZH. LncRNA MEG3 inhibit endometrial carcinoma tumorigenesis and progression through PI3K pathway. Apoptosis. 2017;22:1543–1552. doi: 10.1007/s10495-017-1426-7. [DOI] [PubMed] [Google Scholar]
- 15.Cui Z, An X, Li J, Liu Q, Liu W. LncRNA MIR22HG negatively regulates miR-141-3p to enhance DAPK1 expression and inhibits endometrial carcinoma cells proliferation. Biomed Pharmacother. 2018;104:223–228. doi: 10.1016/j.biopha.2018.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Wang LL, Chen S, Zong ZH, Guan X, Zhao Y. LncRNA ABHD11-AS1 promotes the development of endometrial carcinoma by targeting cyclin D1. J Cell Mol Med. 2018;23:9854–9864. doi: 10.1111/jcmm.13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan J, Zhang N, Zheng Y, Chen YD, Liu J, Yang M. LncRNA GAS5 indel genetic polymorphism contributes to glioma risk through interfering binding of transcriptional factor TFAP2A. DNA Cell Biol. 2018;37:750–757. doi: 10.1089/dna.2018.4215. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Wang Y, Zhang X, Huang Q, Diao Y, Yin H, Liu H. Long non-coding RNA HOXD-AS1 in cancer. Clin Chim Acta. 2018;487:197–201. doi: 10.1016/j.cca.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Arshi A, Sharifi FS, Khorramian Ghahfarokhi M, Faghih Z, Doosti A, Ostovari S, Mahmoudi Maymand E, Ghahramani Seno MM. Expression analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in breast cancer tissues from young women and women over 45 years of age. Mol Ther Nucleic Acids. 2018;12:751–757. doi: 10.1016/j.omtn.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue ZX, Gao RQ, Gao C, Liu SG, Zhao XX, Xing TY, Niu J, Li ZG, Zheng HY, Ding W. The prognostic potential of coilin in association with p27 expression in pediatric acute lymphoblastic leukemia for disease relapse. Cancer Cell Int. 2018;18:106. doi: 10.1186/s12935-018-0600-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou W, Wang J, Qi Q, Feng Z, Huang B, Chen A, Zhang D, Li W, Zhang Q, Bjerkvig R, Li X, Wang J. Matrine induces senescence of human glioblastoma cells through suppression of the IGF1/PI3K/AKT/p27 signaling pathway. Cancer Med. 2018;7:4729–4743. doi: 10.1002/cam4.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie M, Ji Z, Bao Y, Zhu Y, Xu Y, Wang L, Gao S, Liu Z, Tian Z, Meng Q, Shi H. PHAP1 promotes glioma cell proliferation by regulating the Akt/p27/stathmin pathway. J Cell Mol Med. 2018;22:3595–3604. doi: 10.1111/jcmm.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbastabar M, Kheyrollah M, Azizian K, Bagherlou N, Tehrani SS, Maniati M, Karimian A. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: a double-edged sword protein. DNA Repair (Amst) 2018;69:63–72. doi: 10.1016/j.dnarep.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Zeng J, Sun F, Tong X, Meng G, Wu C, Ding X, Liu L, Han M, Lu C, Dai F. p27 inhibits CDK6/CCND1 complex formation resulting in cell cycle arrest and inhibition of cell proliferation. Cell Cycle. 2018;17:2335–2348. doi: 10.1080/15384101.2018.1526598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu R, Huang KJ, Kuo CH, Chen SH, Lin CY, Lee YR. Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J Cell Mol Med. 2018;22:1894–1908. doi: 10.1111/jcmm.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q, Al-Hendy A. The emerging role of p27 in development of diseases. Cancer Stud Mol Med. 2018;4:e1–e3. doi: 10.17140/CSMMOJ-4-e006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santala S, Talvensaari-Mattila A, Soini Y, Kuvaja P, Santala M. Cyclins A, B, E and p27 in endometrial endometrioid adenocarcinoma. Anticancer Res. 2016;36:6467–6473. doi: 10.21873/anticanres.11245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


