Abstract
Background/Introduction: Aberrant expression of Toll like receptors (TLR) plays a vital role in pathogenesis of rheumatoid arthritis (RA). Micro RNAs (miRs) could play important role in the related signaling pathways. The present study was undertaken to establish the link between miR-147 and TLR-7 in rat macrophages (in vitro) and in pristane (PS) induced arthritic rats. Methodology: Dual luciferase assay was done to confirm the interaction between miR-147 and TLR-7. The effect of miR-147 on regulation of TLR-7 was done by RT-qPCR and Immunoblotting studies in rat macrophages (ATCC® CRL-2192TM) after treating them with miR-147 mimics and inhibitors. R-848 (Imiquimod) was used as TLR-7 stimulant, the mRNA and protein expression levels of IFN-β and TNF-α were recorded to determine the regulation of TLR-7. The levels of miR-147 and TLR-7 were evaluated during induction of rat bone marrow derived macrophage in the PS induced rat macrophages and spleens of methotrexate exposed rats. The miR-147 mimics was injected intraperitoneal to the PS treated rats and the severity of arthritis was studied. Results: The study confirmed TLR-7 mRNA as the potential target of miR-147 in rats. Alterations in miR-147 by transfecting mimics or inhibitors in ATCC® CRL-2192TM cells exhibited suppression and amelioration of TLR-7 and cytokine expression. The alteration in expression of miR-147 was inversely correlated with expression of TLR-7 during bone marrow derived macrophages induction in PS exposed cells and spleens. The abnormal expression was reversed in spleens of methotrexate treated arthritic rats. The treatment of miR-147 mimic caused suppression in expression of TLR-7 and improved the severity of arthritis in PS induced arthritic rats. Conclusions: MiR-147 inversely regulates the TLR-7 signaling by targeting TLR-7 itself both in vivo and in vitro. The study provides a novel approach for conditions involving abnormal TLR-7 expression in arthritis.
Keywords: Rheumatoid arthritis, toll like receptors, miR-147, TLR-7
Introduction
Rheumatoid arthritis (RA) is reported to be an autoimmune disorder involved with inflammatory response, the patient diagnosed with RA shows symptoms of pain in articular region, cartilage degeneration, loss of flexibility, space and narrowness in joints [1-3]. Toll-like receptors (TLRs) are group of proteins which recognize the conserved sequences in microbes and play a crucial role in the innate immune system. The stimulation of TLRs activates 2 distinct cascades namely MyD88 independent and dependent pathway. TLRs are mainly responsible for fighting against microbial pathogens [4]. Recent studies have confirmed about TLRs playing crucial role in development of inflammation associated immune disorders such as rheumatoid arthritis (RA), atherosclerosis, diabetes and asthma [4,5]. Our predictions for assuming role of TLRs in pathogenesis of rheumatoid arthritis arises from the studies showing TLR endogenous ligands, which are called as damage-associated molecular patterns (DAMPs) [6,7]. Previous reports have suggested that TLR-7 is over-expressed in rheumatoid arthritis synovial fibroblasts (RASFs) and monocytes derived dendritic cells [8]. TLR-7 has been found to mediate induction of IFN-α/β and -λ for immunity against the viruses in humans [9]. Literatures suggest that TLR-7 play an important role in disorders associated with viral infection and auto immunity specifically rheumatoid arthritis, in which RASFs has been found to show high levels of TLR-7, suggesting its involvement in rheumatoid arthritis [10,11]. The activation of TLR-7 cascade could lead to advancement of RASFs supporting activation of B cell in the synovium [12], also the suppression of TLR-7 regulates the severity of arthritis [10]. The findings confirm that over-expression of TLR-7 in synovial and macrophages cells could be contributing factor in development of arthritis. TLR-7, is among the family of TLRs found to be expressed by number of immune cells [13]. The studies clearly suggest that regulation of TLR-7 is vital in preventing the excessive generation of pro-inflammatory cytokines.
MiRNAs are class of non-coding RNAs which are responsible for regulation of expression of genes via binding 3’UTR region of target mRNAs [14]. Recent experiments have potentially directed studies on role of miRs in TLR-7 pathways instead of their role in altering the expression of TLR-7 [15-17]. In study EV-miR-21 was found to activate the TLR7 signaling cascade and resulted in neurotoxicity in simian immunodeficiency virus induced central nervous system disease induced in mice [16]. In a report by Muller et al. confirmed that miR-21 and miR-29a released by the cancer cells activated the TLR-7 and TLR-8 receptors in immune cells by binding them and caused TLR mediated activation of NF-κB and related inflammatory cytokines [17]. We presume that miRNAs are important regulators which can participates in orchestrating the gene expression relevant TLR-7 and its signal molecules. Studies so far on miRs have indicated the possibilities of their involvement in arthritis by altering the TLR signaling pathways. However, the studies involving interaction of miR and TLR-7 have been undervalued, and miR mediated regulation of TLR-7 and the associated pathways in RA remain unexplored. In the present study, we evaluated the involvement of miR which can potentially target TLR-7. We studied the expression of miR and TLR-7 in macrophages on PS stimulation and differentiation in PS induced arthritis rats, we also studied the changes in spleen and effects of miR-147 mimics on expression of TLR-7 for the severity of PS induced arthritis rats in rats.
Material and methods
Animals and pristane-induced arthritis in rats
For the study Dark Agouti (DA) rats ageing 8 to 12 weeks were selected randomly, the rats were procured from the Laboratory Animal Center of the The second affiliated hospital of Xi’an medical university (Shaanxi, China). The animals were housed at temperatures ranging from 22±3°C, under controlled humidity of 40-70%, under 12-h dark/light cycle, with free access to water and food. All the animal studies were approved by the Animal ethics review committee of The second affiliated hospital of Xi’an medical university (Shaanxi, China) all the experiments were in accordance to guidelines provided by the animal ethical review board of hospital, the approval number was AEC/17-18/201RA. The rats were divided into three groups with each group having 8 rats. The arthritis model was created by injecting intra-dermal single dose of PS (150 μl) at the base of tail [18]. The PS treated rats were injected by intraperitoneal route with Methotrexate (MT) at a dose of 0.25 mg/kg in saline solution (200 μl) on 8th, 10th and 12th day, the rats were sacrificed on 20th day after induction of arthritis by PS [19], the rats were called MT-treated pristane group. The PS treated rats injected with saline (200 μl) were considered as saline-treated pristane group. The control group rats received no PS or MT. The progression of arthritis was supervised after every 2 to 4 days by studying the perimeters of foot pad using caliper, until the rats were sacrificed by injecting the overdose of pentobarbital intravenously. The spleens of rats were removed and freezed for isolation of RNA.
Pristane stimulation in rat macrophage cell line
NR8383 [AgC11x3A, NR8383.1] (ATCC® CRL-2192TM) (ATCC USA) the rat macrophage cells, were subjected to culture in Ham’s F-12K (Kaighn’s) Medium (Thermo Fisher USA) containing fetal bovine serum (15%) (Thermo Fisher USA). The cells were transfected with miR-147 mimic or inhibitor using Lipofectamine-2000 reagent (Invitrogen USA). The stimulation of PS (Sigma Aldrich USA) to the rat macrophages was done by a single exposure of cells to PS, for the same 5 × 106 cells/well were seeded for 24 hours and after that the wells were added with emulsion of PS (1 mM, 50 μl), the cells were harvested after exposing them for 24 hours. The NR8383 cells were also incubated along with miR-147 mimic or inhibitor for 24 hours prior to receiving activation from TLR-7, the cells were stimulated with PS or R-848 (Imiquimod, the TLR-7 stimulant) (100 nM, Sigma Aldrich USA) for another 24 h and were then collected for further study.
In silico bioinformatics analysis
The TLR-7 sequence of rat for mRNA was bought from UniProt (Hinxton Cambridge, UK). In in silico prediction of miRNAs in accordance to binding sites for seed region was done by Targetscan 7.0 (http://www.targetscan.org/) and miRanda 2010 computational algorithm. The obtained predictive outcome of the two data base was employed for further investigation.
Luciferase assay
The 3’UTR region of TLR-7 having putative binding site of miR147 (5’-GGTACC CCTTTCTGAAAAGTTCTGTGGAGCG-3’) was cloned for constructing the pMIR-TLR-7 vector, the cloning was done downstream of the Luciferase gene in the Hind-III and the Sac-I sites in the Luciferase vector. The mutated TLR-7 3’UTR fragment having the specific site mutations in the putative miR-147:TLR-7 seed region by PCR directed mutation, the same was cloned in the pMIR-TLR-7 vector, in the study pRL-TK was the control vector. Plasmid maxi kit (Thomas Sci. USA) was used for making plasmids. For the dual Luciferase assay, the Hela cells were cultured in high glucose containing DMEM medium (Sigma Aldrich USA) having FBS (10%) as per the supplied procedure. Briefly, the renilla pRL-TK vector (Promega) and firefly pMIR Luciferase (Promega) in the ratio 10:90 (10 ng:90 ng/well) were transfected in the Hella cells (2 × 106 cells/well) the cells were seeded for 24 hours before receiving the transfection, the cells were transfected simultaneously using Lipofecatmine 2000 reagent (Thermo Fisher USA) with miR-147 mimic/inhibitor (10 nM) or negative control (NC) in the culture plates. The activity of Luciferase was measured after 24 h of transfection using the Luciferase assay system-1000 (Thermo Fisher USA), the Luciferase activity was achieved against renilla as control.
Treatment of miR-147 mimics in pristane induced arthritic rats
miR-147 mimic was used in the study and was procured from Ambion, Fisher Scientific USA. The chemically-modified double-stranded microRNA (agomir) was used as NC while the saline was selected as control. The three groups of had eight DA rats in each, the rats were induced to arthritis as mentioned earlier, after injecting PS (day 0) the rats received treatment of miR-147 mimic, agomir (NC) and the saline (control) (12.0 nmol/kg in saline), the dose were given by intra peritoneal route (i.p.) 4 times on the 8th, 12th, 15th and the 19th day. The severity of PS induced arthritis was measured on alternate days via the documented scoring system until the animals were sacrificed [20]. The physical features such as circumference of foot pad, ankle along with body weight were recorded after every 4 days. The animals sacrificed on the 23rd day of the PS injection; the ankles were harvested and were subjected for H&E staining. The observed pathological alterations were destruction of joints, synovitis and the extent of repair, the score ranged from 0-3 for each of them. The plasma was collected and used further for studying the levels of TNF-α by ELISA analysis. The plasma nitric oxide (NO) was detected as per the reported method earlier [21]. The spleens were isolated and were cryo-freezed (-80°C) for studying the expression of proteins and RNA levels.
Real-time quantitative PCR (RT-qPCR) analysis
The total RNA was isolated (500 ng) using the Trizol reagent (Invitrogen), for reverse transcription reaction in miR specific stem loop 500 ng of RNA was used and 5 μg of RNA for the reverse transcription reaction using oligo primer. The cDNA synthesis kit (Sigma Aldrich USA) was used for synthesizing cDNA following supplied instructions. The RT-qPCR analysis was done using the Invitrogen Superscript IVTM One-Step RT-PCR System (Thermo Fisher USA). Each sample was analyzed at least three times (n=3), the expression of miR and gene was normalized against U6 snRNA and GAPDH respectively. The details of primer sequences are provided in Table 1.
Table 1.
Genes and primers used for the RT-qPCR study
| Gene | Sequence (5’ to 3’) |
|---|---|
| TLR-7 | GCCUUGAGGCCAACAACAUdTdT |
| GAPDH | CGGCAAGTTCAACGGCACAG |
| IFN-β | CTTGGGTGACATCCACGACTAC |
| TNF-α | TCAGCCTCTTCTCATTCTTGTCTT |
| miR-147 (miRBase) | GGTACC CCTTTCTGAAAAGTTCTGTGGAGCG-3’ |
Immunoblotting studies
For SDS/PAGE analysis, the total cell lysates were prepared and were analyzed as per the provided procedures (Invitrogen USA). For the analysis GAPDH was selected as loading control. The mouse anti-GAPDH antibody (Thermo Fisher USA) and rabbit anti-TLR-7 antibody (Abcam USA) were selected as Iry antibodies, the signals were measured using IIry antibody of goat anti-mouse immunoglobulin or goat anti-rabbit which was labeled using horseradish peroxidase (HRP). The signals were recorded using West Pico PLUS Chemiluminescent Substrate kit (Thermo Fisher USA).
Levels of TNF-α in rat plasma
For the study, the rat plasma and cell supernatants were collected followed by ELISA analysis for determining the levels of TNF-α as per the supplied instructions in kit (Thermo Fisher USA). Briefly, the cell supernatant or plasma (100 μl) was collected and was added to the plates pre-coated with antibodies for TNF-α followed by incubation of 2 hours at 25°C. The plates were added with biotin-conjugated detecting TNF-α antibody followed by incubation of 2 hours the plates were added with streptavidin-HRP, for development tetra-methylbenzidin was used. The values of optical density were recorded at wavelength of 450 nm using plate reader (Multiskan sky plate reader, Thermo Fisher USA). The concentration of TNF-α was computed using a standard calibration curve obtained from recording values from series of standard dilutions of TNF-α ranging from 0 pg/ml to 3500 pg/ml.
Statistics
All the data was expressed as mean ± % RSD (relative standard deviation), the differences between the groups was established statistically using the Mann-Whitney U-test. The differences having P values <0.05 were regarded as statistically significance.
Results
Dual luciferase assay confirmed TLR-7 was the potential target of miR-147
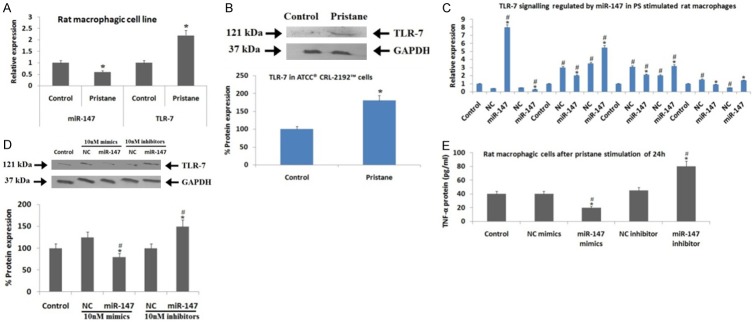
The in silico study for target prediction showed that miR-147 was the favorable targeting TLR-7 hence it was selected as target miR for further studies. To further confirm TLR-7 is the favorable target of miR-147, we performed the renilla and firefly dual Luciferase assay in the Hela cells (Figure 1A). When the Hela cells were transfected with miR-147 mimics and pMIR-TLR-7 vectors, resulted in suppression of Luciferase activity by 22% (P<0.05) compared to the NC mimics, or by 37% against the empty pMIR vector. But the transfection with miR-147 inhibitor resulted in a significant elevation in Luciferase activity (P<0.05) of pMIR-TLR-7 vector by about 72% compared to NC inhibitor or by 82% against the empty pMIR. We further verified the specific binding by constructing a mutated form of pMIR-TLR-7 vector bearing 3 nucleotide mutation on the putative binding site followed by transfection with miR-147 mimics and pRL-TK in the Hela cells (Figure 1B). It was observed that, after the transfection of WT pMIR-TLR3 vector and miR-147 mimics along with pRL-TK control there was a significant suppression in luciferase activity compared to transfection of pMIR-TLR-7 in Hela cells. The outcomes suggested that miR-147 binds particularly to the 3’UTR of TLR-7 mRNA of rats.
Figure 1.
Target relationship between TLR-7 and miR-147. A: Luciferase assay shows effect of miR-147 mimics and inhibitor on luciferase activity of pMIR-TLR-7 vector. The dual Luciferase assay was done to establish target relationship between TLR-7 mRNA 3’UTR and miR-147, The Hela cells were transfected with pMIR-TLR-7 or pMIR empty vectors, the pRL-TK vector was selected as an internal standard (control) and was normalized. pMIR-Report Luciferase vector was negative control (NC). *, P<0.05 against NC mIR; #, significant difference against vector (Mann-Whitney U-test). B: Luciferase activity of wild type (WT) or mutated pMIR-TLR-7 vector showing effect of miR-147 mimics. The TLR-7 mRNA 3’UTR fragment with binding site of miR-147 received transfection into the Hela cells. *, P<0.05 compared to mutant (Mann-Whitney U-test). The data shows relationship between miR-147 and WT or mutated pMIR-TLR-7 vector.
miR-147 regulates expression of TLR-7 negatively by interfering its function in macrophages
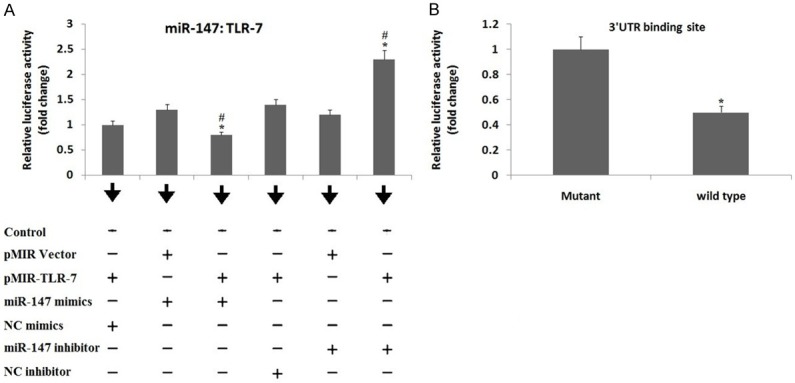
The ATCC® CRL-2192TM cells, a macrophagic cell line received transfection of miR-147 mimics, the cells showed significantly elevated levels of miR-147 compared to their negative control (NC) or control group (P<0.05) (Figure 2A). The results of TLR-7 mRNA expression showed that miR-147 mimics failed to affect the TLR-7 mRNA levels; however the transfection of miR147 inhibitor increased the expression levels of TLR-7 mRNA significantly compared to control or NC group (P<0.05) (Figure 2B). The outcomes of Immunoblotting suggested that, the exposure to miR-147 mimics suppressed the TLR-7 protein expression significantly compared to both control and NC (P<0.05), the transfection of miR-147 inhibitor caused a sharp increase in expression of TLR-7 protein expression compared to control and NC transfected cell group (P<0.05) (Figure 2C). Transfecting the ATCC® CRL-2192TM cells with increasing doses of miR-147 mimics confirmed the translational suppression in cells. The expression of TLR-7 protein showed a dose dependent inhibition by about 32%, 53% and 71% respectively versus the NC group (Figure 2D).
Figure 2.
Effects of miR-147 on expression of TLR-7 by suppression or gain of miR-147 function in Rat macrophagic cells. (A) Results of RT-qPCR for expression of miR-147. (B) RT-qPCR results for expression of TLR-7 mRNA and (C) Results are immunoblotting studies for protein expression of TLR-7 after miR-147 mimics or inhibitor was transfected into rat macrophagic cells for 48 h. (D) Immunoblotting results for TLR-7 protein expression after transfection with miR-147 mimics into rat macorphagic cells for 48 h. (E) Results of Immunoblotting studies for protein expression of TLR-7 and (F) Results of RT-qPCR for expression of mRNA expression of IFN-β and TNF-α in rat macrophagic cells and results of ELISA for protein expression of TNF-α in cell supernatant. (G) The cell supernatant incubated with with miR-147 mimics and inhibitors for 24 h and stimulation of R-848 (R-848, TLR-7 ligand) for next 24 h. GAPDH i.e glyceraldehyde-3-phosphate dehydrogenase was selected as loading standard control in RT-qPCR. *, P<0.05 compared to NC; #, P<0.05 compared to control (Mann-Whitney U-test).
To further investigate about whether miR-147 could alter the TLR-7 expression, the ATCC® CRL-2192TM cells were incubated along with miR-147 mimics (10 nM) or inhibitor for 24 hours before the activation of TLR-7 signaling by R-848 (Imiquimod) (TLR-7 stimulant) (100 nM, Sigma Aldrich USA) for 24 hours, the cells were than harvested for studying the expression. The protein and mRNA expression levels of TNF-α and IFN-β which are the two downstream cytokines were studied after the TLR-7 signaling was activated by its activating ligand i.e. R-848. The outcomes clearly showed that, miR-147 mimics resulted in a significant decrease, whereas the inhibitors caused an approximately two fold increase in levels of TLR-7 protein compared to NC and control group (Figure 2E). The miR-147 mimics resulted in approximately 61% and 32% decrease in expression levels of IFN-β mRNA against the NC (P<0.05) or control group (P<0.05), whereas an absolute reduction of TNF-α mRNA levels was seen compared to NC (P<0.05). The miR-147 inhibitor resulted in about 58% elevation in levels of IFN-β mRNA compared to control and NC group (P<0.05), whereas an absolute (approx 100%) increase in levels of TNF-α mRNA levels was observed compared to negative control group (P<0.05) (Figure 2F). The results of ELISA demonstrated that protein levels of TNF-α in obtained cell supernatant was also decreased significantly after transfection with miR-147 mimic compared to NC (P<0.05), whereas the levels were elevated after the transfection with inhibitor compared to control and NC group (P<0.05) (Figure 2G).
Expression of MiR-147 was suppressed and TLR-7 was elevated during the bone marrow derived macrophage induction in rats
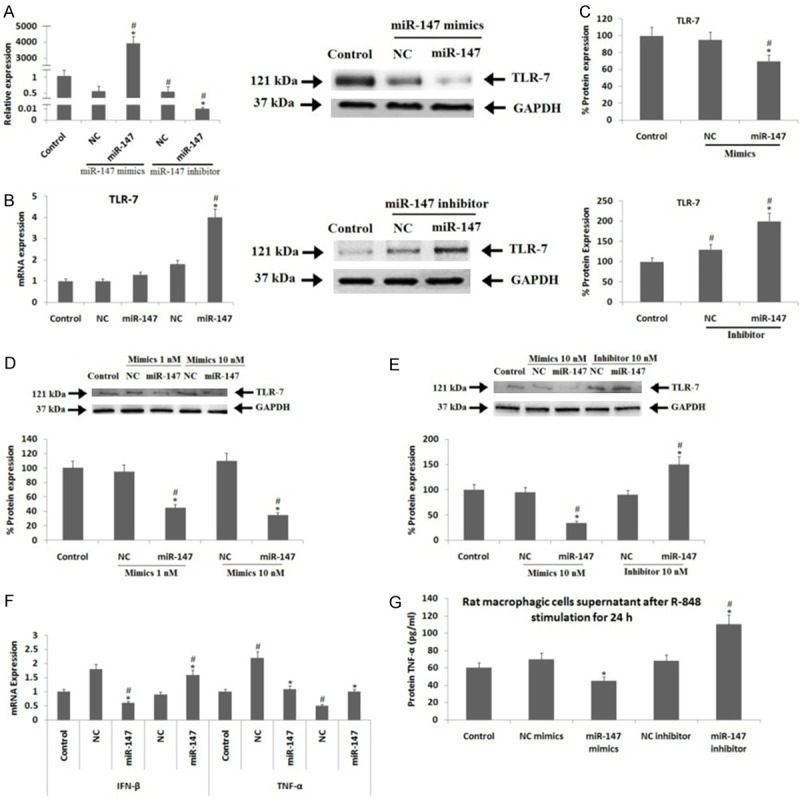
The expression levels of miR-147 and TLR-7 were studied after the rats were subjected to the bone marrow derived macrophage induction for 0, 3 and 6th day. During the induction of macrophage, the mRNA levels of TLR-7 were elevated by 5 and 8 times on 3rd day and 6th day respectively whereas, the levels of miR-147 decreased by 62% and 71% on the 3rd and 6th day respectively compared to the day 0 of macrophage induction (Figure 3A). The outcomes also showed that the protein expression of TLR-7 was up-regulated by 3 times upon macrophage induction on the 3rd and 6th day (Figure 3B).
Figure 3.
Expression of miR-147 and TLR-7 during induction of bone marrow-derived macrophage (BMDM). (A) Results of RT-qPCR for expression of TLR-7 mRNA and miR-147 and (B) Results of Immunoblotting studies of for protein expression of TLR-7 on day-0, day-2 and day-3 during induction of BMDM. The bone marrows were harvested from the DA rats (n=3). GAPDH i.e glyceraldehyde-3-phosphate dehydrogenase was selected as loading standard control in RT-qPCR. *, P<0.05 compared to expression on day-0 (Mann-Whitney U-test).
Pristane stimulation caused an in inverse expression of miR-147 and TLR-7 in rat macrophages
The PS treatment in ATCC® CRL-2192TM cells (rat macrophage cells) resulted in up-regulation of mRNA levels of TLR-7 significantly (P<0.05) and protein levels by two times compared to the control (media incubated only), whereas, the miR-147 levels were on significantly lower side (P<0.05) after the macrophages were exposed for 24 hours to PS stimulation (Figure 4A and 4B). The macrophages were incubated with miR-147 mimics or inhibitors for 24 hours followed by stimulation with PS for next 24 hours in process to confirm the suppression of TLR-7 signaling by miR-147 in rat macrophages. The qRT-PCR analysis for expression of miR-147 confirmed the success of transfection; the outcomes demonstrated that alterations of miR-147 levels could result in regulation of TLR-7 signaling after the macrophages were subjected to PS stimulation. Both, the miR-147 mimics and inhibitors caused 30% and 40% suppression respectively in mRNA levels of TLR-7 (P<0.05), 30% suppression and 60% elevation in expression of IFN-β mRNA (P<0.05), about 40% reduction and twice increase in TNF-α mRNA (P<0.05) compared to negative control (Figure 4C). The mimics and inhibitors of miR-147 or the negative control activated the mRNA levels of TLR-7 and IFN-β compared to control (P<0.05). The negative control mimics elevated, whereas the inhibitors suppressed the mRNA levels of TNF-α significantly (P<0.05). The miR-147 mimics showed decrease in protein expression of TLR-7 by 40% compared to negative control and 25% against the control group, the miR-147 inhibitors elevated the expression of TLR-7 by up to 1.5 times compared to both negative control or control group (Figure 4D).
Figure 4.
Expression of miR-147 and TLR-7 in rat macrophages after stimulation of PS in vitro. (A) Results RT-qPCR for expression of TLR-7 mRNA and miR-147 and (B) Results of imunoblotting studies for protein expression of TLR-7 in rat macrophagic cells after stimulation with by PS (1 mM) for 24 h. (C) Results of RT-qPCR for expression of miR-147, TLR-7, IFN-β and TNF-α mRNA, (D) Results of Immunoblotting studies for protein expression of TLR-7 in rat macrophages and (E) Results of ELISA for protein expression of TNF-α in the cell supernatant post incubating with miR-147 mimics and inhibitors for time period of 24 h followed by stimulation of PS for next 24 h. GAPDH i.e glyceraldehyde-3-phosphate dehydrogenase was used as loading standard control in RT-qPCR. (A and B) *, P<0.05 compared with control (media). (C and D) *, P<0.05 compared with the NC miRNA group; #, P<0.05 compared to Control group (Mann-Whitney U-test, P<0.05).
The TNF-α expression in cell supernatant was estimated by ELISA, the outcomes showed that the expression was suppressed significantly when treated with miR-147 mimics and elevated when treated with inhibitor compared to control or negative control group (Figure 4E). The study of spleens from PS induced arthritis rats showed a clear implication of miR-147. The arthritis score and perimeters of foot pad (Figure 5A and 5B) in saline treated arthritis rats were different from control or MT treated PS induced arthritic rats, however there was no observable difference between the MT treated and control rats, indicating that MT could abolish PS induced arthritis in rats. The levels of TLR-7 and miR-147 in spleens of PS induced arthritic rats showed that mRNA levels of TLR-7 were significantly unregulated (P<0.01), on contrary the miR-147 expression levels decreased significantly (P<0.01). However, the treatment of MT in control rats recovered the up-regulation of TLR-7 and suppression of miR-147 (Figure 5C).
Figure 5.
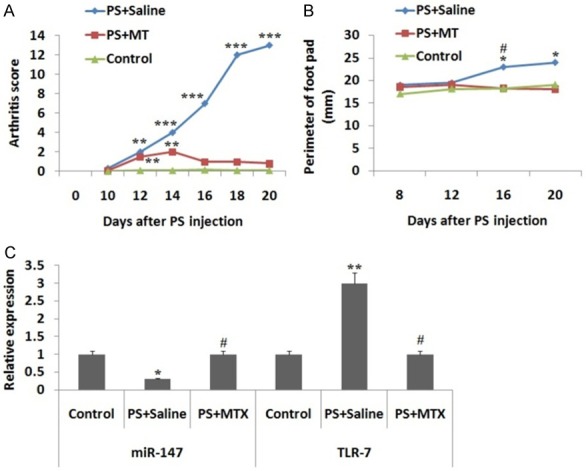
Expression of miR-147 and TLR-7 in PS induced arthritis rats (PIA) with or without receiving treatment of methotrexate (MT). (A) Score for arthritis and (B) Perimeter of rat foot-pad (mm) in PIA rats with or without treatment of MT. (C) Results of RT-qPCR for expression of TLR-7 mRNA and miR-147 in rat spleens rats of control group, saline-treated PS and MT treated PIA rats group on day 20 after PS injection. GAPDH i.e. glyceraldehyde-3-phosphate dehydrogenase was used as loading control standard *, P<0.05 compared to control, #, P<0.05 compared to PS + MT treated rats, **, P<0.01 and ***, P<0.001 compared to control group rats.
MiR-147 mimic improves the pristane induced arthritis in rats by controlling the expression of TLR-7
To confirm whether over-expression of miR-147 in rats can affect the severity of arthritis, the PS induced arthritis rats were exposed to miR-147 mimic, negative control mimics and saline (4 times). The scores of arthritis suggested that miR-147 failed to avoid the occurrence of arthritis from the origin, but resisted the severity of arthritis significantly after receiving the 3rd injection on the 15th day until the rats were subjected to sacrifice on the last day i.e the 23rd day (Figure 6A). The ankle circumference (Figure 6B) and foot-pad (Figure 6C) in PS induced + miR-147 treated group was on a significantly lower side compared to PS induced + saline treated or PS induced negative control rats on the 23rd day, suggesting that decrease in extent of swelling in joints after treatment of mir-147 mimic. The study of body weight suggested, loss of weight after induction of arthritis. The review of ratio of organ/body weights for heart, lymph nodes, spleen, liver, kidney and lungs suggested no significant variation. We then evaluated the main pathological measures for arthritis in experimental rats such as, synovitis, extent of destruction of joints and joint repair, the outcomes suggested that miR-147 mimics can bring down synovitis in PS induced + miR-147 group of rats compared to saline treated group (Figure 6D). The results suggested no significant variation in the pathological measures. Simultaneously, the spleens were isolated and expression of RNA and protein was studied. The miR-147 levels in isolated spleens from PS induced + miR-147 group of rats was about 3 folds higher compared to the negative control group of rats (Figure 6E). The protein expression of TLR-7 in the spleen tissue was down regulated significantly in PS induced + miR-147 group of rats compared to negative control or the saline treated control group (Figure 6F). The results of ELISA suggested that the plasma levels of TNF-α in PS induced + miR-147 rats were down-regulated compared to the PS induced + saline treated rats (Figure 6G), nevertheless, no significant variation in levels of nitric oxide was observed in groups. The findings of the experiment suggested that, miR-147 mimic controlled the expression of TLR-7 and corrected the severity of arthritis in PS induced arthritic rats.
Figure 6.
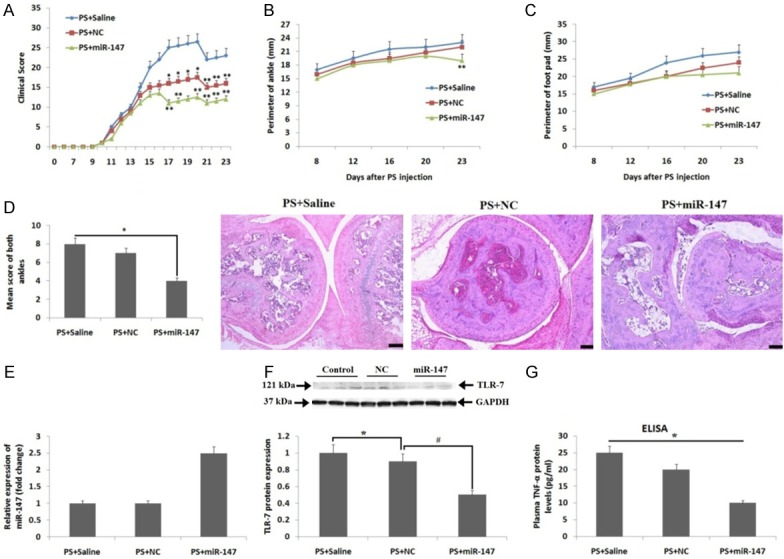
Effect of treatment of miR-147 mimic in PS induced arthritic rats. (A) Clinical score curve for arthritis in rats from 0th-day to 23rd-day. (B) Results of ankle perimeter (mean, mm) and (C) foot-pad perimeter (mean, mm) of rats. (D) Data showing pathological scores suggesting synovitis of arthritis (left figure) and the H&E staining of ankle from each group of rats (right figure), (E) Expression of miR-147 evaluated by qRT-PCR in harvested spleens of miR-147-treated rats. (F) Protein expression of TLR-7 by immunoblotting studies. GAPDH i.e glyceraldehyde-3-phosphate dehydrogenase was used as loading control. (G) Protein expression of TNF-α analyzed by ELISA in plasma of rats belonging to all groups. *, P<0.05 compared to saline treated control; #, P<0.05 compared to NC: **, P<0.05 compared to saline treated control (Mann-Whitney U-test).
Discussion
In the present study, we predicted that miR-147 could specifically target TLR-7 in rats. The correlation between miR-147 and TLR-7 was investigated and confirmed by performing dual reporter assay. We found that, miR-147 negatively regulated the levels of TLR-7 by targeting TLR-7 itself in rat macrophages, modulation of miR-147 resulted in suppression of TLR-7 and attenuation of RA. In bone marrow derived macrophage induction and PS stimulated ATCC® CRL-2192TM cells (rat macrophage cells), the down regulation of miR-147 resulted in up-regulation of TLR-7 in rat macrophages. The levels of miR-147 were suppressed as expression of TLR-7 was down-regulated in spleens of PS induced arthritic rats; this was reversed after the rats were treated with MT. The PS induced arthritic rats were treated with miR-147 mimic, and the outcomes showed that the expression of TLR-7 protein was down-regulated and the arthritis was ameliorated significantly. The results of our study suggested association of miR-147 expression with levels of TLR-7 and confirms a novel pathway associated with abnormal up-regulation of TLR-7 in arthritic experimental rats.
MicroRNAs are small non-coding RNAs which bind with the 3’UTR fragment of target genes by forming complementary pairs, causing degradation of mRNA or there repression [22]. Studies recently have demonstrated that, miRs are regulated selectively in the various phases of inflammation by suppressing the chemokines, pro-inflammatory cytokines, Toll-like receptors also called to be TLRs and various transcription factors [23-25]. Studies recently have established that upon administrating exogenous miRs mimics corrected the lung injury against the altered response of stress and inflammation [26]. The expression profile and associated acting pathways of miR-147 are less-studied, recently, only one report showed that miR-147b was involved in regulating endothelial barrier function [27]. miR-147 is found to be the murine homologue of miR-147b, In a report earlier, the expression of miR-147 was up-regulated via various TLR agonists, TLR-4 was the major one and the receptor for bacterial lipopolysaccharide, miR-147 also played a role of negative regulator of bacterial lipopolysaccharide-evoked pathway in macrophages. However a study reported, inhibition of miR-147 caused a significant elevation in expression of TLR mediated stimulation of cytokine [25]. miR-147 has been confirmed to be as an anti-inflammatory micro RNA acting on macrophages involving TLR, however there are no reports available regarding its target and function in Arthritis. Thus, the novel and unparalleled aspect of our research was to confirm TLR-7 as the potential target of miR-147 in PS induced arthritic rats.
The outcomes of In silico bioinformatics study suggested that TLR-7 was the potential target of miR-147 in rats, rabbits and mouse; however, the affinity of TLR-7-miR-147 was not seen in the human genome having 2 nucleotide mutations in the seed region against the genome of rat. The prediction algorithm also suggested that miR-147 targets TLR-7 in humans, horse and chimpanzee. This profile of miR-147 was a complementary among the available data base of vertebrates. Moreover, TLR-7 or TLR-8 signaling have ability to target IRF-5, for which MYD88, receptor associated kinase-1, interleukin and TNF-6 are necessary for activating IRF-5 in the TLR-7 signaling cascade [28]. According to our study, we confirmed that regulation of TLR-7 cascade mediators and TLR-7 itself by micro RNAs play a vital role TLR-7 signaling, controlling the pro-inflammatory events. It has been reported that TLR-7 is expressed in rat macrophages, thus, in the present work we selected ATCC® CRL-2192TM rat macrophage cells for studying the regulation and expression of TLR-7 after transfecting them with miR-147 mimics or inhibitors. The in-active ATCC® CRL-2192TM rat macrophage cells showed a negative regulation of TLR-7 gene from miR-147, the same pattern was observed upon submitting the cells to bone marrow derived macrophage induction and after PS stimulation, confirming that miR-147 have the ability to govern the TLR-7 signaling in rat macrophages. However, miR-147 mimics failed to affect the mRNA levels of TLR-7 but suppressed the protein levels significantly, whereas with the inhibitors the mRNA and protein levels of TLR-7 were up-regulated significantly compared to negative control. We found that the treatment of miR-147 inhibitors had a potential effect on TLR-7 compared to mimics. However, after activation of TLR-7 signaling, the inverse regulation mediated by miR-147 seems to be exaggerated. We observed that, after subjecting to PS, the miR-147 mimics suppressed the mRNA and protein levels of TLR-7 and the inhibitors elevated the mRNA and protein levels of TLR-7 significantly. We believe that, the endogenous miR-147 may acts as buffer in regulating the fluctuations in expression of TLR-7 in the macrophages due to which the miR-147 inhibitors are exhibiting a potential role compared to the mimics. In addition to this, down regulation of miR-147 and up-regulation of TLR-7 in spleens of PS induced arthritic rats treated with Methotrexate compared to saline treated rats suggested the role of miR-147 in arthritis induced rats. All these findings support the potential of miRs in the ongoing biological reactions [30].
Conclusion
In conclusion, the treatment of miR-147 in PS induced arthritic rats showed that over-expression of miR-147 can suppress the protein expression of TLR-7 in rats. Treatment of miR-147 mimic could suppress expression of TLR-7 and improve the arthritic condition in PS induced arthritic rats. The outcomes suggest that miR-147 regulates the TLR-7 cascade via governing the expression of TLR-7, and confirm miR-147 as a drug target for suppressing inflammation in arthritic therapy. The study confirms the therapeutic potential of micro RNA in inflammatory conditions involving over-expression of toll like receptors.
Disclosure of conflict of interest
None.
References
- 1.Mertsch S, Schmitz N, Jeibmann A, Geng JG, Paulus W, Senner V. Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. J Neurooncol. 2008;87:1–7. doi: 10.1007/s11060-007-9484-2. [DOI] [PubMed] [Google Scholar]
- 2.Challal S, Minichiello E, Boissier MC, Semerano L. Cachexia and adiposity in rheumatoid arthritis. Relevance for disease management and clinical outcomes. Joint Bone Spine. 2016;83:127–133. doi: 10.1016/j.jbspin.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Ji L, Geng Y, Zhou W, Zhang Z. A study on relationship among apoptosis rates, number of peripheral T cell subtypes and disease activity in rheumatoid arthritis. Int J Rheum Dis. 2016;19:167–171. doi: 10.1111/1756-185X.12211. [DOI] [PubMed] [Google Scholar]
- 4.Keogh B, Parker AE. Toll-like receptors as targets for immune disorders. Trends Pharmacol Sci. 2011;32:435–442. doi: 10.1016/j.tips.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, Wijmenga C, O’Neill LA. Genetic variation in toll-like receptors and disease susceptibility. Nat Immunol. 2012;13:535–542. doi: 10.1038/ni.2284. [DOI] [PubMed] [Google Scholar]
- 6.Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–64. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3:382–90. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 8.Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, Van Lieshout AW, Sprong T, Van den Hoogen FH, Van den Berg WB, Radstake TR. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–22. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 9.Yang K, Puel A, Zhang S, Eidenschenk C, Ku CL, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S, Al-Hajjar S, Al-Ghonaium A, Maródi L, Davidson D, Speert D, Roifman C, Garty BZ, Ozinsky A, Barrat FJ, Coffman RL, Miller RL, Li X, Lebon P, Rodriguez-Gallego C, Chapel H, Geissmann F, Jouanguy E, Casanova JL. Human TLR-7-, -8-, and -9-mediated induction of IFN-α/β and -λ is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crozat K, Beutler B. TLR7: a new sensor of viral infection. Proc Natl Acad Sci U S A. 2004;101:6835–6836. doi: 10.1073/pnas.0401347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlain ND, Kim SJ, Vila OM, Volin MV, Volkov S, Pope RM, Arami S, Mandelin AM, Shahrara S. Ligation of TLR7 by rheumatoid arthritis synovial fluid single strand RNA induces transcription of TNF-α in monocytes. Ann Rheum Dis. 2013;72:418–426. doi: 10.1136/annrheumdis-2011-201203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, van Lieshout AW, Sprong T, van den Hoogen FH, van den Berg WB, Radstake TR. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–22. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 13.Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, Pienkowski C, Chaumeil J, Mejía JE, Guéry JC. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 14.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Wang H, Liu Y, Song Y, Lai L, Han Q, Cao X, Wang Q. Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1beta production in macrophages by targeting STAT3. PLoS One. 2012;7:e42971. doi: 10.1371/journal.pone.0042971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yelamanchili SV, Lamberty BG, Rennard DA, Morsey BM, Hochfelder CG, Meays BM, Levy E, Fox HS. MiR-21 in extracellular vesicles leads to neurotoxicity via TLR7 signaling in SIV neurological disease. PLoS Pathog. 2018;14:e1007068. doi: 10.1371/journal.ppat.1007068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vingsbo C, Sahlstrand P, Brun JG, Jonsson R, Saxne T, Holmdahl R. Pristane-induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996;149:1675–1683. [PMC free article] [PubMed] [Google Scholar]
- 19.Hultqvist M, Olofsson P, Gelderman KA, Holmberg J, Holmdahl R. A new arthritis therapy with oxidative burst inducers. PLoS Med. 2006;3:e348. doi: 10.1371/journal.pmed.0030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vingsbo C, Sahlstrand P, Brun JG, Jonsson R, Saxne T, Holmdahl R. Pristane-induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996;149:1675–1683. [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Sun Q, Asim MB, Jiang X, Zhong B, Shahzad M, Zhang F, Han Y, Lu S. Nitric oxide in both bronchoalveolar lavage fluid and serum is associated with pathogenesis and severity of antigen-induced pulmonary inflammation in rats. J Asthma. 2010;47:135–144. doi: 10.3109/02770900903483808. [DOI] [PubMed] [Google Scholar]
- 22.Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 23.Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci U S A. 2009;106:15819–15824. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adyshev DM, Moldobaeva N, Mapes B, Elangovan V, Garcia JG. MicroRNA regulation of nonmuscle myosin light chain kinase expression in human lung endothelium. Am J Respir Cell Mol Biol. 2013;49:58–66. doi: 10.1165/rcmb.2012-0397OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee V, Beard RS Jr, Reynolds JJ, Haines R, Guo M, Rubin M, Guido J, Wu MH, Yuan SY. MicroRNA-147b regulates vascular endothelial barrier function by targeting ADAM15 expression. PLoS One. 2014;9:e110286. doi: 10.1371/journal.pone.0110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N, Pitha PM, Fitzgeraldand KA, Golenbock DT. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem. 2005;280:17005–12. doi: 10.1074/jbc.M412584200. [DOI] [PubMed] [Google Scholar]
- 29.Celhar T, Pereira-Lopes S, Thornhill SI, Lee HY, Dhillon MK, Poidinger M, Connolly JE, Lim LH, Biswas SK, Fairhurst AM. TLR7 and TLR9 ligands regulate antigen presentation by macrophages. Int Immunol. 2016;28:223–232. doi: 10.1093/intimm/dxv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]