Abstract
Aim: To determine whether VKORC1 rs9923231, CYP2C9 rs1057910, CYP4F2 rs2108622 and ORM1 rs17650 genotypes contribute to warfarin therapy in patients during initiation and maintenance anticoagulation treatment after heart valve surgery. Methods: 287 Chinese patients with warfarin treatment more than three month after heart valve replacement operations were enrolled. Blood was collected from each subject for DNA extraction and genotyping. Analyzing the relationship between genotypes and warfarin curative effect. Results: Their mean age was 48.0 ± 10.5 years old. During the initiation phase, the growth rate of INR was partial correlated with VKORC1 rs9923231, CYP2C9 rs1057910 and ORM1 rs17650, respectively. Compared with AG or GG genotypes of VKORC1 c.-1639 carriers, patients with VKORC1 c.-1639AA reached target INR therapeutic range faster (P<0.001) and has a high risk of overanticoagulation (P<0.001). Carriers of at least one CYP2C9 *3 allele reached the target INR therapeutic range and supra-therapeutic INR were faster than CYP2C9 wild-type carriers (P=0.032, P=0.032, respectively). CYP4F2 rs2108622 could significantly influence on time to the target INR therapeutic range and time to INR above 3.0 after hierarchical analysis with VKORC1, CYP2C9 and ORM1 (P=0.011, P=0.044, respectively). VKORC1 rs9923231, CYP2C9 rs1057910 and ORM1 rs17650 were significantly influence the %TTR in three months (P=0.031, P=0.008, P=0.001, respectively). During the maintenance phase, VKORC1 c.-1639AA carriers spent more time at supra-therapeutic INRs (P<0.001). CYP2C9 rs1057910, CYP4F2 rs2108622 and ORM1 rs17650 gene variants did not affect outcome parameters in maintenance phase. Conclusions: This study found that genetic factors could significantly affected on warfarin therapy in Chinese. Meanwhile, genetic variations play a more important role in the initial phase than did in maintenance phase of warfarin therapy.
Keywords: Warfarin, VKORC1, CYP2C9, CYP4F2, ORM1
Introduction
Warfarin is one of the coumarins anticoagulants, which is used for prevent thromboembolic episodes [1]. However, Warfarin is characterized by a narrow therapeutic index and a wide interindividual variability in dose requirements [2,3]. Despite the warfarin dose is adjusted according to patients’ international normalized ratio (INR) and prothrombin time (PT), the patients still have a high risk of suffer from adverse event such as bleeding [4-6].
Genetic variations was confirmed the main factors for the warfarin therapy among various factors such as height, weight, age, sex, complicating disease, the intake of vitamin K and drug interactions [7,8]. CYP2C9 is the major enzyme responsible for metabolyzing S-warfarin to 7-hydroxywarfarin [4]. Warfarin exert its anticoagulant activity by inhibiting vitamin K epoxide reductase complex (VKORC1) directly [9,10]. Polymorphisms of VKORC1 rs9923231 and CYP2C9 rs1057910 can explain about 30% of warfarin dosage variation [11]. Cytochrome oxidase 4F2 (CYP4F2) is a metabolic enzyme of vitamin K1, previous studies had confirmed that CYP4F2 rs2108622 polymorphism could explain 2-7% variations of stable warfarin dose [12,13]. Acid glycoprotein, also called orosomucoid (ORM), as the major binding protein of warfarin. Our previous study has found that ORM1 rs17650 *S carriers required lower maintenance doses to achieved the considerable anticoagulation effect [14].
The adverse reactions of warfarin therapy are mainly happened in the first three months, especially in the first month with warfarin therapy [15-17]. Meanwhile, this period is also frequently for warfarin dose adjusted. Therefore, a scientific approach for shorten dose adjustment period and achieve a accurate warfarin initial dose is highly desirable. Pharmacogenomics may provide promising solutions to achieve this goal.
Some studies had confirmed that VKORC1 rs9923231 and CYP2C9 rs1057910 could significantly influence on the initiation and maintenance phases of phenprocoumon and warfarin therapy [18-21]. However, most of the studies were performed in Caucasians. Phenprocoumon is preferentially used in many European countries, however, warfarin is the most frequently prescribed anticoagulant in Chinese. Therefore, the purpose of this research was to evaluate the affect of VKORC1, CYP2C9, CYP4F2 and ORM1 gene variants on warfarin therapy during the initiation and maintenance phases in Chinese.
Materials and methods
Patients
A total of 287 Chinese patients with operation of heart valve replacement and treated with warfarin during the whole follow-up period were recruited in this study, all patients signed informed consent. Demographics and clinical outcomes of patients were collected from medical records. Patients were recruited from Department of Cardiothoracic Surgery of the Second Xiangya Hospital and Department of Cardiothoracic Surgery of the Zhengzhou Central Hospital from October 2014 to December 2016. This study was approved by the ethics committee of the Institute of Clinical Pharmacology, Central South University and the ethics committee of Zhengzhou Central Hospital.
Study design
2 ml peripheral venous blood was collected from each subject for DNA extraction. Genotypes for VKORC1 rs9923231, CYP2C9 rs1057910, CYP4F2 rs2108622 and ORM1 rs17650 were determined by using standard pyrosequencing method. PCR primers were listed in our previous research [22]. All genotype results were confirmed by double-blind detection.
All the eligible patients were at least 18 years old with satisfactory hepatic and renal functions as well as platelet counts. The patients who continuous use warfarin more than 3 months (minimal period 91 days, maximal 382 days, median 194 days) were enrolled in our study. Patients were excluded from the study if they meet the exclusion criteria. INR values were determined every two days in the first week, every week in the first month, and then every month. The target INR was 2.0 to 3.0, the percentage of time spent in, above, and below the therapeutic INR range was calculated according to Rosendaal et al research [23].
Statistical analysis
Results are presented as mean ± SD or median (inter-quartile range) if it is not mentioned otherwise. Differences between groups were detected using the U-test of Mann-Whitney, or H-test of Kruskal and Wallis, as appropriate. Cox regression analyses were performed to deter mine hazard ratios (HR). SPSS 19.0 was used for data analysis (SPSS Inc, Chicago, Illinois). P<0.05 was considered to be statistically significant.
Results
Clinical characteristics
The demographics and clinical characteristics of the 287 patients were listed in Table 1. patients mean age was 48.0 ± 10.5 years old (18-73). 180 of them were female subjects (62.7%), whereas 107 of them were male subjects (37.3%). 278 (96.8%) of the patients with mechanical heart valve replacement and only 9 of the patients with biological valve implantation.
Table 1.
Population characteristics
| Variable | No (%) |
|---|---|
| Subjects - n | 287 |
| Female - n (%) | 180 (62.7) |
| Age | |
| x ± SD | 48.0±10.5 |
| Median | 48.0 |
| Range | 18.0-73.0 |
| Weight | |
| x ± SD | 56.6±10.0 |
| Median | 56.0 |
| Range | 35.5-95.0 |
| BSA | |
| x ± SD | 1.67±0.16 |
| Median | 1.65 |
| Range | 1.33-2.16 |
| Valve type | |
| Mechanical | 278 (96.8) |
| Biological | 9 (3.2) |
| Valves position | |
| Aortic | 34 (11.8) |
| Mitral/Tricuspid | 210 (73.2) |
| Both | 43 (15.0) |
| Atrial fibrillation (AF) | |
| Absent | 150 (52.3) |
| Present | 137 (47.7) |
| Coronary heart disease (CHD) | |
| Absent | 278 (96.9) |
| Present | 9 (3.1) |
Data are presented as the mean ± standard deviation (SD) with the range in parenthesis, or as the number with the percentage in parenthesis.
Impact of genotypes on warfarin initiation and maintenance therapy
As shown in Table 2, the growth rate of INR was partial correlated with VKORC1 rs9923231 from the second day on, and with CYP2C9 rs1057910 and ORM1 rs17650 from the fourth and the fifth day on, respectively. However, there was no correlation between CYP4F2 rs2108622 and the growth rate of INR. The impact of genotypes on the percentage of time INR values within the scope of the treatment (%TTR) in three months was shown in Table 3, VKORC1 rs9923231, CYP2C9 rs1057910 and ORM1 rs17650 were significantly influence %TTR. Compared with mutant-type carriers, the wild-type carriers of VKORC1 rs9923231, CYP2C9 rs1057910 and ORM1 rs17650 spent more time within target INR therapeutic range (P=0.031, P=0.008, P=0.001, respectively).
Table 2.
Outcome parameters in the initiation phase of warfarin therapy according to genotypes
| n | INR1-0/dose | INR2-1/dose | INR3-2/dose | INR4-3/dose | INR5-4/dose | INR6-5/dose | |
|---|---|---|---|---|---|---|---|
| VKORC1 | |||||||
| AA | 236 | 0.048 (0.024-0.097) | 0.044 (0.020-0.088) | 0.040 (0.020-0.088) | 0.052 (0.020-0.102) | 0.052 (0.012-0.104) | 0.044 (0.008-0.092) |
| GA | 47 | 0.044 (0.024-0.072) | 0.020 (0.000-0.048) | 0.016 (-0.004-0.046) | 0.022 (0.001-0.048) | 0.028 (0.002-0.050) | 0.012 (-0.004-0.044) |
| GG | 4 | 0.036 (0.024-0.060) | 0.032 (0.010-0.044) | 0.028 (0.002-0.048) | 0.010 (-0.005-0.022) | 0.008 (-0.007-0.014) | 0.006 (0.004-0.014) |
| P-value | 0.443 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| CYP2C9 | |||||||
| *1*1 | 268 | 0.044 (0.020-0.088) | 0.040 (0.016-0.078) | 0.036 (0.014-0.076) | 0.044 (0.016-0.088) | 0.044 (0.008-0.088) | 0.036 (0.000-0.076) |
| *1*3 | 18 | 0.048 (0.028-0.108) | 0.044 (0.012-0.108) | 0.044 (0.012-0.120 | 0.096 (0.026-0.206) | 0.080 (0.008-0.194) | 0.072 (0.005-0.196) |
| *3*3 | 1 | 0.020 | 0.012 | -0.028 | 0.048 | 0.144 | 0.144 |
| P-value* | 0.347 | 0.612 | 0.235 | 0.003 | 0.015 | 0.011 | |
| ORM1 | |||||||
| *F*F | 184 | 0.044 (0.020-0.084) | 0.040 (0.012-0.084) | 0.036 (0.012-0.080) | 0.044 (0.0150.088) | 0.044 (0.008-0.092) | 0.032 (0.000-0.076) |
| *F*S | 92 | 0.044 (0.017-0.092) | 0.036 (0.014-0.072) | 0.036 (0.012-0.073) | 0.048 (0.016-0.103) | 0.052 (0.012-0.108) | 0.048 (0.008-0.092) |
| *S*S | 11 | 0.052 (0.028-0.130) | 0.040 (0.024-0.106) | 0.040 (0.024-0.068) | 0.048 (0.020-0.116) | 0.032 (-0.002-0.060) | 0.012 (-0.021-0.055) |
| P-value | 0.215 | 0.477 | 0.444 | 0.610 | 0.015 | 0.005 | |
| CYP4F2 | |||||||
| CC | 217 | 0.052 (0.024-0.092) | 0.040 (0.016-0.084) | 0.040 (0.012-0.080) | 0.048 (0.016-0.092) | 0.044 (0.008-0.092) | 0.036 (0.000-0.076) |
| CT | 68 | 0.030 (0.010-0.074) | 0.032 (0.013-0.061) | 0.036 (0.016-0.068) | 0.041 (0.016-0.092) | 0.050 (0.012-0.100) | 0.040 (0.004-0.092) |
| TT | 2 | 0.065 (0.042-0.083) | 0.052 (0.021-0.105) | 0.028 (0.023-0.070) | 0.044 (0.012-0.207) | 0.010 (-0.010-0.080) | 0.041 (-0.026-0.034) |
| P-value | 0.000 | 0.208 | 0.825 | 0.858 | 0.182 | 0.119 | |
| Sex | |||||||
| Male | 107 | 0.040 (0.017-0.076) | 0.040 (0.016-0.076) | 0.036 (0.012-0.068) | 0.044 (0.015-0.081) | 0.042 (0.003-0.096) | 0.028 (0.000-0.076) |
| Female | 180 | 0.048 (0.024-0.092) | 0.040 (0.016-0.088) | 0.038 (0.013-0.088) | 0.048 (0.016-0.099) | 0.048 (0.012-0.096) | 0.040 (0.004-0.084) |
| P-value | 0.068 | 0.590 | 0.349 | 0.268 | 0.405 | 0.145 | |
| Total | 287 | 0.044 (0.020-0.088) | 0.040 (0.016-0.080) | 0.036 (0.012-0.076) | 0.044 (0.016-0.092) | 0.048 (0.008-0.096) | 0.038 (0.000-0.080) |
Values are given as median (inter-quartile range);
compared with *1*1 and *1*3.
Table 3.
Impact of genotypes on %TTR in three months
| n | %TTR | P-value | |
|---|---|---|---|
| VKORC1 | 0.031 | ||
| AA | 236 | 54.44 (33.89-82.77) | |
| AG | 47 | 43.33 (24.44-63.33) | |
| GG | 4 | 45.77 (16.66-64.80) | |
| CYP2C9 | 0.008 | ||
| *1*1 | 268 | 54.44 (32.77-80.00) | |
| *1*3 | 18 | 31.66 (13.06-52.50) | |
| *3*3 | 1 | 32.22 | |
| ORM1 | 0.001 | ||
| *F*F | 184 | 58.33 (38.88-84.44) | |
| *F*S | 92 | 41.11 (16.11-72.78) | |
| *S*S | 11 | 41.11 (17.24-65.00) | |
| CYP4F2 | 0.848 | ||
| CC | 217 | 52.22 (32.22-77.78) | |
| CT | 68 | 56.67 (28.88-83.33) | |
| TT | 2 | 42.22 (39.44-45.00) | |
| Total | 287 | 52.22 (32.22-77.78) |
Values are given as median (interquartile range).
The time to target INR therapeutic range and time to INR above 3.0 were significantly associated with VKORC1 and CYP2C9 genotypes. Patients with AG or GG genotypes of VKORC1 c.-1639 had a prolonged initiation phase with the time to the target INR therapeutic range compared with VKORC1 c.-1639AA carriers (median/range: 14.50/7.30-25.00 days vs. 5.00/3.00-8.00 days, P<0.001; HR: 3.28, 95% CI 2.34-4.61, P<0.001; Figure 1A), and the time to INR above 3.0 has the same trend (median/range: 110.00/33.00-195.05 days vs. 25.00/12.00-126.00 days, P<0.001; HR: 3.09, 95% CI 1.85-5.18, P<0.001; Figure 2A). Carriers of CYP2C9 *3 allele reached the target INR therapeutic range faster compared to CYP2C9 wild-type carriers (median/range: 5.00/3.00-6.00 days vs. 6.00/4.00-10.00 days, P=0.032; HR: 2.80, 95% CI 1.28-6.15, P=0.010; Figure 1B) and patients carriers of at least one CYP2C9 *3 allele required fewer time to INR above 3.0 compared to CYP2C9 wild-type carriers (median/range: 13.00/7.00-108.50 days vs. 34.50/14.00-144.50 days, P=0.012; HR: 2.59, 95% CI 1.12-6.01, P=0.026; Figure 2B).
Figure 1.
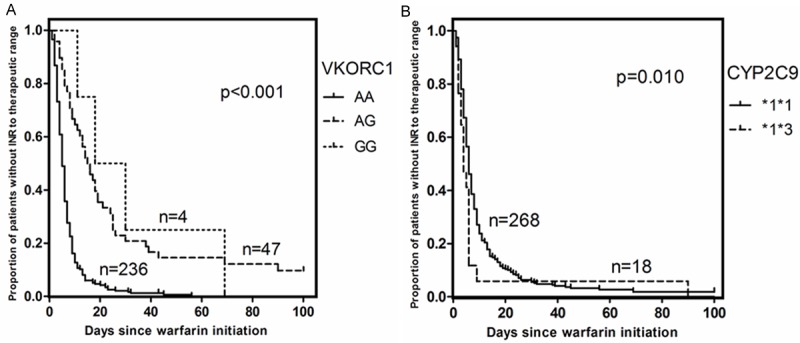
A. Time to the therapeutic range, plotted for VKORC1 c.-1639 gene variants. B. Time to the therapeutic range, plotted for CYP2C9 haplotypes.
Figure 2.
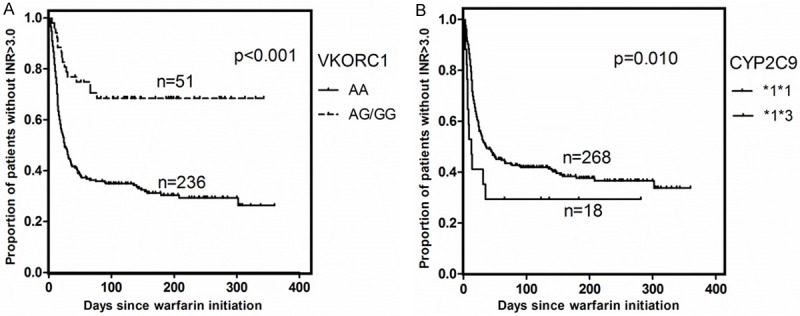
A. Time to INR above the therapeutic range (INR>3.0), plotted for VKORC1 c.-1639 gene variants. B. Time to INR above the therapeutic range (INR>3.0), plotted for CYP2C9 haplotypes.
Hierarchical analysis
After hierarchical analysis with VKORC1 9923231, CYP2C9 rs1057910, CYP4F2 rs2108622 and ORM1 rs17650, there was no significantly difference of analysis results of VKORC1 rs1057910, CYP2C9 *3 and ORM1 rs17650 between signal gene and hierarchical analysis. However, CYP4F2 rs2108622 could significantly influence on time to the target INR therapeutic range and time to INR above 3.0 after hierarchical analysis (Table 4, P=0.011, P=0.044, respectively).
Table 4.
Outcome parameters of warfarin therapy according to CYP4F2 after hierarchical analysis
| CYP4F2 | P Value | |||
|---|---|---|---|---|
|
| ||||
| AA*1*1CC*F*F (n=104) | AA*1*1CT*F*F (n=42) | AA*1*1TT*F*F (n=1) | ||
| Time to the target INR therapeutic range | 5.00 (4.00-8.00) | 7.00 (4.50-13.30) | 8.00 | 0.011 |
| %TTR | 53.48 (38.27-77.21) | 64.39 (39.30-80.53) | 65.24 | 0.243 |
| Time to INR above 3.0 | 28.50 (13.30-134.00) | 55.00 (22.80-191.50) | 15.00 | 0.044 |
| Time above INR 3.0 (%) | 5.50 (0.00-18.58) | 1.57 (0.00-14.39) | 14.59 | 0.200 |
Values are given as median (inter-quartile range).
Impact of genotypes on warfarin maintenance therapy
During the maintenance phase, the %TTR, sub-therapeutic and supra-therapeutic INR ranges was 54.22%, 41.52%, and 4.26%, respectively. The time above INR 3.0 (%) was significantly associated with the VKORC1 rs9923231. Patients with the VKORC1 c.-1639AA genotype spent significantly more time above the therapeutic range (median/IQR: 5.95/0.00-17.86%) than did patients with the VKORC1 c.-1639GG (median/IQR: 0.00/0.00-2.95%) and VKORC1 c.-1639AG (median/IQR: 0.00/0.00-5.67%) genotypes (Table 5, P<0.001). Others gene and clinical factors did not significantly influence %TTR and the time above INR 3.0 (%) in the maintenance phase.
Table 5.
Outcome parameters of warfarin therapy during the maintenance phase
| n | %TTR | Time above INR 3.0 (%) | |
|---|---|---|---|
| VKORC1 | |||
| AA | 236 | 54.55 (34.74-74.72) | 5.95 (0.00-17.86) |
| AG | 47 | 54.38 (25.72-75.55) | 0.00 (0.00-5.67) |
| GG | 4 | 40.96 (11.44-67.98) | 0.00 (0.00-2.95) |
| P-value | 0.502 | 0.000 | |
| CYP2C9 | |||
| *1*1 | 268 | 54.58 (34.90-74.96) | 4.07 (0.00-14.60) |
| *1*3 | 18 | 45.28 (23.64-70.77) | 6.88 (0.00-13.01) |
| *3*3 | 1 | 38.32 | 18.69 |
| P-value* | 0.145 | 0.414 | |
| ORM1 | |||
| *F*F | 184 | 55.77 (35.91-76.27) | 4.06 (0.00-14.36) |
| *F*S | 92 | 50.64 (29.70-71.85) | 4.32 (0.00-16.10) |
| *S*S | 11 | 52.69 (33.17-80.10) | 8.44 (0.00-32.64) |
| P-value | 0.295 | 0.474 | |
| CYP4F2 | |||
| CC | 217 | 52.58 (34.16-72.78) | 4.45 (0.00-14.69) |
| CT | 68 | 63.27 (33.99-76.02) | 2.98 (0.00-14.29) |
| TT | 2 | 68.40 (65.24-71.56) | 8.86 (3.13-14.59) |
| P-value | 0.088 | 0.607 | |
| Sex | |||
| Male | 107 | 54.99 (35.00-74.73) | 5.57 (0.00-18.31) |
| Female | 180 | 53.29 (33.99-74.72) | 4.07 (0.00-13.98) |
| P-value | 0.680 | 0.540 | |
| Valves | |||
| Mechanical | 278 | 54.55 (33.99-75.31) | 4.08 (0.00-14.58) |
| Biological | 9 | 46.55 (34.71-58.87) | 16.33 (1.29-27.98) |
| P-value | 0.240 | 0.167 | |
| Age | |||
| P-value | 0.105 | 0.409 | |
| BSA | |||
| P-value | 0.411 | 0.181 | |
| Total | 287 | 54.22 (34.24-74.72) | 4.26 (0.00-14.59) |
Values are given as median (inter-quartile range);
compared with *1*1 and *1*3.
Discussion
There were most significant ethnic differences in warfarin therapy between Chinese and Caucasians. The initial warfarin dose and maintenance warfarin dose of Chinese were significantly lower than Caucasians as the difference in frequency of alleles. Although there were some studies had confirmed the impact of gene polymorphisms on the initiation phase and maintenance phase of warfarin treatment in Caucasians, the similar research in Chinese population was scarcely.
Our result showed that the genetic variation of VKORC1 strongly modulated the initiation phase of warfarin treatment. Patients with VKORC1 c.-1639AA reached the target INR therapeutic range faster than VKORC1 c.-1639G allele carriers, which means these patients could achieve the proper anticoagulation effect faster. Meanwhile, patients with VKORC1 c.-1639AA showed a high risk of over-anticoagulation in the initiation phase of warfarin therapy. Compared to VKORC1 c.-1639GG and VKORC1 c.-1639AG carriers, the risk of INR above 3.0 was 3.09 times with the VKORC1 c.-1639AA carriers (Figure 2A). Beate Luxembourg et al also found that VKORC1 c.-1639AA and GG carriers showed a prolonged initiation phase with a large number of anticoagulation visits and a high risk of over-anticoagulation in the initiation phase of phenprocoumon therapy (HR 3.06) [18]. The main reason of the inconsistent result was the difference in frequency of alleles between Chinese and Caucasians. The frequency of alleles VKORC1 c.-1639G was 64.8% in Beate Luxembourg study, however, the frequency of alleles VKORC1 c.-1639G was 9.6% in our study. Warfarin exerts its anticoagulant activity by inhibit the activity of VKORC1 and which is a key enzyme response to recycling of vitamin K, an essential component of several coagulation factors. VKORC1 rs9923231 impaired the inhibitory activity of warfarin and led to more dosage to maintain warfarin therapy [24]. We found that VKORC1 rs9923231 could significantly affected the growth rate of INR. Patients with a VKORC1 c.-1639G allele were obvious insensitive to warfarin, the growth rate of INR of these patients was significantly lower than the VKORC1 c.-1639AA carriers from the second day with warfarin therapy. This might also be an explanation for the fact that the patients with VKORC1 c.-1639GA and VKORC1 c.-1639GG need more dosage to maintain warfarin therapy.
Warfarin is mainly metabolized by CYP2C9 in vivo, and the mutation of CYP2C9 rs1057910 significantly impaired the activity of CYP2C9. In our study, the growth rate of INR of patients with CYP2C9 *3 allele was 2 times compared to the wild carriers from the fourth day with warfarin therapy. During the initial 10 day with warfarin therapy, 50% patients with CYP2C9 *3 allele encountered at least one time of INR above 3.0, this data was just 14.6% in CYP2C9 *1*1 carriers. Since, patients with CYP2C9 *3 allele more easily reached the therapeutic range and had a high risk of overanticoagulation. Schalekamp et al also found that CYP2C9 *2 or *3 carriers showed an increased risk of overanticoagulation during the initial weeks of phenprocoumon treatment [25]. Beate Luxembourg et al also found that CYP2C9 *2 or *3 carriers reached stable INRs significantly faster compared to wildtype carriers [18].
The anticoagulation cycle is a complex process. Excepting VKORC1 and CYP2C9, genetic variation of other components involved in the vitamin K cycle also could affected warfarin therapy. CYP4F2 is a metabolic enzyme of vitamin K1, which play a vital role of the anticoagulant action of warfarin [26]. We found that CYP4F2 rs2108622 did not significantly influence on the growth rate of INR during the initial week of warfarin therapy. However, after hierarchical analysis, patients with AA*1*1CC*F*F genotypes of CYP4F2 rs2108622 reached the target INR therapeutic range faster compared to CYP4F2 rs2108622 AA*1*1CT*F*F carriers (median/range: 5.00/4.00-8.00 days vs. 7.00/4.50-13.30 days, p=0.032; HR: 1.95, 95% CI 1.32-2.89, P=0.001). Meanwhile, patients with AA*1*1CC*F*F genotypes increased the risk of overanticoagulation. Our data testified that CYP4F2 had a moderate but statistically significant association with the variation of warfarin dose.
ORM1 was the main binding protein of warfarin. The binding affinity of ORM1 rs17650 two polymorphic variants were reported differed many times [27,28]. Wang LS et al study found that ORM1 rs17650 could significantly influence on warfarin maintain dose, ORM1 rs17650 *S carriers required lower maintenance doses to achieved the considerable anticoagulation effect [14]. Our early study found that patients with ORM1 rs17650 *S genotypes were more sensitive to warfarin, and the polymorphism could account for 1.2% variation in the effect index of warfarin [22]. Although we found that ORM1 rs17650 could influence on the growth rate of INR during the initial stage. However, there was no significantly difference of time to the target INR therapeutic range and time to INR above 3.0 between various genotypes. Even so, patients with ORM1 *S genotypes has the trend of increased risk of overanticoagulation.
Our study also take into account non-genetic factors such as weight, body surface area (BSA), sex, age, valve type, complicating disease. We found that patients with biological valve replacement were easier reached the therapeutic range and had a high risk of overanticoagulation compared to mechanical valve replacement. As previous studies observed, weight could affected warfarin maintain dose, we found that BSA could affected warfarin therapy during the initial stage.
There was no consistent conclusion about whether patients could benefit from genetic testing. A clinical research performed by EU-PACT found that the treatment efficacy of genotype-guided group was superior to the standard dose group, in the first 12 weeks, %TTR increased by 7%, and %TTR of INR 4 decreased by 69%, time to achieve therapeutic INR reduced by 28% [20]. However, COAG clinical study found that there was no significantly difference of %TTR between genotype-guided group and clinically guided group [21]. In our study, we found that VKORC1 rs9923231, CYP2C9 rs1057910 and ORM1 rs17650 were significantly influence the %TTR in three months. However, all the genetic factors and non-genetic factors had no significantly influence on %TTR during the maintenance phase. Meanwhile, only VKORC1 rs9923231 could significantly influence on the time above INR 3.0(%) during the maintenance phase.
In summary, this study found that VKORC1 9923231, CYP2C9 rs1057910, CYP4F2 rs2108622 and ORM1 rs17650 could affect warfarin therapy during the initial stage in Chinese. However, the impact of gene polymorphisms on warfarin therapy during the maintenance phase was limited. Meanwhile, we found that there was a different way of the impact of gene polymorphisms on warfarin therapy between Chinese and Caucasians. A major limitation of our study is stronger endpoints such as bleeding, thrombosis, or death were not evaluated in our study. However, the patients were recruited continuously, more data will be collected for validate our findings.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grants 81072707 and 81472031), the Medical Science Research Project of Henan Province (Grants 2018020765).
Disclosure of conflict of interest
None.
References
- 1.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists. Chest. 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 2.Tatarunas V, Lesauskaite V, Veikutiene A, Grybauskas P, Jakuska P, Jankauskiene L, Bartuseviciute R, Benetis R. The effect of CYP2C9, VKORC1 and CYP4F2 polymorphism and of clinical factors on warfarin dosage during initiation and long-term treatment after heart valve surgery. J Thromb Thrombolysis, 2014;37:177–85. doi: 10.1007/s11239-013-0940-x. [DOI] [PubMed] [Google Scholar]
- 3.Moreau C, Loriot MA, Siguret V. Vitamin K antagonists: from discovery to pharmacogenetics. Ann Biol Clin. 2012;70:539–551. doi: 10.1684/abc.2012.0740. [DOI] [PubMed] [Google Scholar]
- 4.Shil AB, Strohm MP. Strohm, warfarin pharmacogenetics. N Engl J Med. 2009;360:2474–5. [PubMed] [Google Scholar]
- 5.Gage BF, Lesko LJ. Pharmacogenetics of warfarin: regulatory, scientific, and clinical issues. J Thromb Thrombolysis. 2008;25:45–51. doi: 10.1007/s11239-007-0104-y. [DOI] [PubMed] [Google Scholar]
- 6.Bodin L, Perdu J, Diry M, Horellou MH, Loriot MA. Multiple genetic alterations in vitamin K epoxide reductase complex subunit 1 gene (VKORC1) can explain the high dose requirement during oral anticoagulation in humans. J Thromb Haemost. 2008;6:1436–9. doi: 10.1111/j.1538-7836.2008.03049.x. [DOI] [PubMed] [Google Scholar]
- 7.International Warfarin Pharmacogenetics Consortium. Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, Limdi NA, Page D, Roden DM, Wagner MJ, Caldwell MD, Johnson JA. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–64. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesko LJ. The critical path of warfarin dosing: finding an optimal dosing strategy using pharmacogenetics. Clin Pharmacol Ther. 2008;84:301–3. doi: 10.1038/clpt.2008.133. [DOI] [PubMed] [Google Scholar]
- 9.Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hörtnagel K, Pelz HJ, Lappegard K, Seifried E, Scharrer I, Tuddenham EG, Müller CR, Strom TM, Oldenburg J. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–41. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 10.Sadler JE. Medicine: K is for koagulation. Nature. 2004;427:493–4. doi: 10.1038/427493a. [DOI] [PubMed] [Google Scholar]
- 11.Wen MS, Lee M, Chen JJ, Chuang HP, Lu LS, Chen CH, Lee TH, Kuo CT, Sun FM, Chang YJ, Kuan PL, Chen YF, Charng MJ, Ray CY, Wu JY, Chen YT. Prospective study of warfarin dosage requirements based on CYP2C9 and VKORC1 genotypes. Clin Pharmacol Ther. 2008;84:83–89. doi: 10.1038/sj.clpt.6100453. [DOI] [PubMed] [Google Scholar]
- 12.Namazi S, Azarpira N, Hendijani F, Khorshid MB, Vessal G, Mehdipour AR. The impact of genetic polymorphisms and patient characteristics on warfarin dose requirements: a cross-sectional study in Iran. Clin Ther. 2010;32:1050–60. doi: 10.1016/j.clinthera.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Cavallari LH, Langaee TY, Momary KM, Shapiro NL, Nutescu EA, Coty WA, Viana MA, Patel SR, Johnson JA. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin Pharmacol Ther. 2010;87:459–64. doi: 10.1038/clpt.2009.223. [DOI] [PubMed] [Google Scholar]
- 14.Wang LS, Shang JJ, Shi SY, Zhang YQ, Lin J, Guo ZH, Wang YC, Tang J, Liu J, Liu YZ, Li Z, Tan ZR, Zhou HH, Jiang HH, Xie HT. Influence of ORM1 polymorphisms on the maintenance stable warfarin dosage. Eur J Clin Pharmacol. 2013;69:1113–20. doi: 10.1007/s00228-012-1448-6. [DOI] [PubMed] [Google Scholar]
- 15.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 16.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–96. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 17.Douketis JD, Foster GA, Crowther MA, Prins MH, Ginsberg JS. Clinical risk factors and timing of recurrent venous thromboembolism during the initial 3 months of anticoagulant therapy. Arch Intern Med. 2000;160:3431–6. doi: 10.1001/archinte.160.22.3431. [DOI] [PubMed] [Google Scholar]
- 18.Luxembourg B, Schneider K, Sittinger K, Toennes SW, Seifried E, Lindhoff-Last E, Oldenburg J, Geisen C. Impact of pharmacokinetic (CYP2C9) and pharmacodynamic (VKORC1, F7, GGCX, CALU, EPHX1) gene variants on the initiation and maintenance phases of phenprocoumon therapy. Thromb Haemost. 2011;105:169–80. doi: 10.1160/TH10-03-0194. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, Kahn SF, May HT, Samuelson KM, Muhlestein JB, Carlquist JF Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116:2563–70. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 20.Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, Kesteven P, Christersson C, Wahlström B, Stafberg C, Zhang JE, Leathart JB, Kohnke H, Maitland-van der Zee AH, Williamson PR, Daly AK, Avery P, Kamali F, Wadelius M EU-PACT Group. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013;369:2294–303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 21.Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF, Rosenberg YD, Eby CS, Madigan RA, McBane RB, Abdel-Rahman SZ, Stevens SM, Yale S, Mohler ER 3rd, Fang MC, Shah V, Horenstein RB, Limdi NA, Muldowney JA 3rd, Gujral J, Delafontaine P, Desnick RJ, Ortel TL, Billett HH, Pendleton RC, Geller NL, Halperin JL, Goldhaber SZ, Caldwell MD, Califf RM, Ellenberg JH COAG Investigators. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med. 2013;369:2283–93. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Jiang HH, Wu DK, Zhou YX, Ye HM, Li X, Luo ZY, Guo Z, Zhang YL, Wang YC, Zhang W, Zhou HH, Wang LS. Effect of gene polymorphims on the warfarin treatment at initial stage. Pharmacogenomics J. 2017;17:47–52. doi: 10.1038/tpj.2015.81. [DOI] [PubMed] [Google Scholar]
- 23.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–9. [PubMed] [Google Scholar]
- 24.Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 25.Schalekamp T, Oosterhof M, van Meegen E, van Der Meer FJ, Conemans J, Hermans M, Meijerman I, Boer A. Effects of cytochrome P450 2C9 polymorphisms on phenprocoumon anticoagulation status. Clin Pharmacol Ther. 2004;76:409–417. doi: 10.1016/j.clpt.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 26.McDonald MG, Rieder MJ, Nakano M, Hsia CK, Rettie AE. CYP4F2 is a vitamin K1 oxidase: an explanation for altered warfarin dose in carriers of the V433M variant. Mol Pharmacol. 2009;75:1337–1346. doi: 10.1124/mol.109.054833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kishino S, Nomura A, Di ZS, Sugawara M, Iseki K, Kakinoki S, Kitabatake A, Miyazaki K. Alpha-1-acid glycoprotein concentration and the protein binding of disopyramide in healthy subjects. J Clin Pharmacol. 1995;35:510–514. doi: 10.1002/j.1552-4604.1995.tb04096.x. [DOI] [PubMed] [Google Scholar]
- 28.Eap CB, Cuendet C, Baumann P. Binding of amitriptyline to alpha 1-acid glycoprotein and its variants. J Pharm Pharmacol. 1988;40:767–770. doi: 10.1111/j.2042-7158.1988.tb05169.x. [DOI] [PubMed] [Google Scholar]


