Abstract
Endometriosis is a benign disease but manifests with malignant features and limited treatment options. Women with endometriosis should not be ignored or patronized by the medical profession and society. In this regard, a major cultural change and searching for the optimum therapeutic regimen from multiple perspectives is needed in China even in the whole world. Long non-coding RNAs are crucial for various human diseases while its potential functions and mechanisms are largely unknown in endometriosis. LINC00261 was significantly downregulated in endometriosis tissues and our study indicated that it suppresses proliferation and invasion of endometriosis cells functionally in vitro. Insights of the mechanism of competitive endogenous RNAs were obtained from bioinformatic analysis, RIP, RNA pull-down and luciferase assays, which further confirmed that LINC00261 functions as a molecular sponge to regulate BCL2L11 expression by binding to miR-132-3p directly. These data defined LINC00261/miR-132-3p/BCL2L11 regulatory networks may be a novel therapeutic target for endometriosis.
Keywords: Endometriosis, long non-coding RNA, LINC00261, miR-132-3p, BCL2L11
Introduction
Endometriosis is defined as the presence of endometrial glands and stroma outside the uterine cavity, predominantly, but not exclusively, in the pelvic compartment [1]. The prevalence of endometriotic disease seems to be 10-15%, with a peak between 25 years and 35 years of age. Although endometriosis is a benign disease, biological behaviors of endometriosis cells such as proliferation, invasion and relapse manifest with malignant features that debilitate women of reproductive age. Patients treated with drugs containing ingredients such as progestogen, short-acting contraceptive, gonadotropin releasing hormone agonist always lasting for long and cannot achieve satisfactory therapeutic effect according to clinical observation. Surgery is a basic method for treating endometriosis, especially operation under laparoscope benefits patients a lot. Thus, search for the optimum therapeutic regimen from multiple perspectives is the top priority in the field of endometriosis.
Long non-coding RNAs (lncRNAs) are a class of non-coding RNAs longer than 200 nucleotides. Advances in high-throughput sequencing platforms have enabled the rapid discovery and identification of lncRNAs as key regulatory molecules involved in various cellular processes and their dysregulation in various human diseases. LINC00261 is the abbreviation of long intergenic non-protein coding RNA 261, which is often used as a synonym for C20ORF56, DEANR1, HCCDR1 or NCRNA00261, shown to play pivotal roles in the tumor suppression. Recent studies revealed that LINC00261 inhibits gastric cancer cell proliferation, migration [2] and metastasis [3], sensitizes human colon cancer cells to cisplatin therapy [4], suppresses cell proliferation and invasion in hepatocellular carcinoma [5] and cholangiocarcinoma [6]. Decreased expression of LINC00261 is a prognostic marker for patients with non-small cell lung cancer [7] and maybe relative to T term degree and lymphamatic metastasis of laryngeal carcinoma [8] supported by preliminary studies. LINC00261 may contribute to the pathogenesis and development of endometriosis, especially capable of inhibiting cell growth and migration based on our previous study [9], however, the underlying mechanism remains to be illuminated. Through being like a molecular sponge or competitive endogenous RNA (ceRNA), lncRNAs could competitively interact with their same miRNA responsive elements and then regulate the protein expression [10]. LINC00261 is a relatively novel lncRNA, and little is known about its ‘sponge’ role for miRNAs.
In this paper, we report the role of LINC00261 in endometriosis and show that LINC00261 is downregulated in endometriosis tissues. Notably, we further confirmed that LINC00261 acts as a competing endogenous RNA to regulate BCL2L11 expression by sponging miR-132-3p. The LINC00261/miR-132-3p/BCL2L11 regulatory networks may be a novel therapeutic target for endometriosis.
Materials and methods
Tissue specimens and clinical data
Twenty paired endometriosis tissue samples and matched adjacent normal endometrium tissue samples were obtained from patients with endometriosis who had undergone surgery between October 2014 and September 2016 in First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China). All cases were reviewed by a pathologist and histologically confirmed as endometriosis. All samples were snap frozen in liquid nitrogen and stored at 80°C prior to RNA isolation. Informed consent was obtained from all patients. The data do not contain any information that could identify the patients. This study was approved by the Human Ethics Committee of First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China).
Cell culture
The human endometriosis primary cells and cell line CRL-7566 were used in this study. The primary cells were cultured as previously reported [11]. CRL-7566 was purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640 medium (Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco, USA). Cells were maintained at 37°C in a humidified incubator containing 5% CO2.
Cell transfection
The pcDNA-LINC00261 and the empty vector pcDNA-NC, pPG-miR-132-3p, pPG-anti-miR-132-3p and pPG-miR-NC were constructed and purchased from Sangon Biotech, Shanghai, China. Cells were cultured on six-well plates to confluency and transfected with plasmids containing LINC00261 or miR-132-3p using Lipofectamine 2000 transfection reagent (Invitrogen, CA, USA) to establish the overexpression of LINC00261, overexpression/knockdown of miR-132-3p in endometriosis primary cells and CRL-7566 cell line. After 48 h, the cells were harvested for subsequent experiments. Two cell lines were transfected with siRNA against LINC00261 or BCL2L11 to knock down respectively using Lipo2000. SiRNAs specifically targeting LINC00261 were designed and synthesized by Shanghai GenePharma Co, Ltd. The siRNAs sequences for LINC00261 are as follows: si-1: 5’-CAGTCGCTTGGTTTG AGCTCAAATA-3’, si-2: 5’-CCCTGTGACATAGGTGGATATTTAT-3’. BCL2L11 siRNA (si-BCL2L11-1) were purchased from Santa Cruz Biotechnology (USA; sc-29802), the second BCL2L11 siRNA (si-BCL2L11-2) was purchased from Cell Signaling Technology (USA; 6461). Briefly, the cells in the logarithmic phase were seeded in 6-well plates before transfection. The confluence of cells reached 70-90% and serum-free medium were used for transfection. At 6 h after transfection, the medium was changed into complete medium. After 48 h of transfection, knockdown efficiency of LINC00261 was confirmed by qRT-PCR.
RNA preparation, reverse transcription and quantitative real-time PCR (qRT-PCR)
RNA preparation and qRT-PCR were performed as previously described [9]. The primers used in this study were as follows: 5’-GTCAACGGATTTGGTCTGTATT-3’ (forward) and 5’-AGTCTTCTGGGTGGCAGTGAT-3’ (reverse) for GAPDH, 5’-GTCAGAAGGAAAGGCC GTGA-3’ (forward) and 5’-TGAGCCGAGATGAACAGGTG-3’ (reverse) for LINC00261, 5’-GCGCGCGTAACAGTCTACAGG-3’ (forward) and 5’-GTCGTATCCAGTGCAGGGTCC-3’ (reverse) for miR-132-3p, 5’-GCGCGCGTAACAGTCTACAGG-3’ (forward) and 5’-GTCGTATCCAGTGCAGGGTCC-3’ (reverse) for BCL2L11. The qRT-PCR reactions were performed by the ABI7500 system (Applied Biosystems, Carlsbad, CA, USA). The qRT-PCR amplification was performed in triplicate reactions beginning at 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, and 60°C for 60 s. Quantitative normalization of LINC00261 cDNA was performed in each sample using the expression of the GAPDH as an internal control. The relative level of LINC00261 transcripts to control GAPDH was determined by the 2-ΔΔCT method.
Colony formation assay
Endometriosis primary cells and cell line CRL-7566 were seeded into 6-well plates (approximately 1000 cells per well). After cultured for 10 days, cells were stained with 0.1% crystal violet solution and the number of colonies were assessed under microscope. The colonies containing at least 50 cells were counted and assessed at least three times independently.
Transwell invasion assay
Transwell invasion assay was performed using the Matrigel-coated (BD, Franklin Lakes, NJ, USA) filters in 24-well plates. Cells were trypsinized and seeded onto the upper chambers of the transwell (1×105 cells/well) in serum-free RPMI-1640 medium. The lower chambers were filled with the 1640 medium (including 10% FBS). The chambers were incubated at 37°C with 5% CO2 for 24 h. At the end of incubation, cells on the upper surface of the filter were removed by wiping with a cotton swab. Cells migrating through the filter to the lower surface were fixed with 4% paraformaldehyde for 10 min and stained with 0.1% crystal violet for 10 min. After washed in PBS for three times, cells were viewed and photographed under a phase-contrast microscope (Olympus, Tokyo, Japan) and counted from randomly chosen fields.
Protein extraction and western blot analysis
Cells were lysed in RIPA buffer containing fresh protease and phosphatase inhibitor cocktails (Sigma) by incubating for 15 min on the ice. Protein concentration were measured using the BCA assay (Beyotime Biotechnology, China) according to manufacturer’s instructions. Whole protein extracts were resolved on a 10% SDS polyacrylamide gel and electrotransferred to polyvinylidene fluoride membranes (Millipore, US). The blot was blocked in 5% skim milk for 2 h at room temperature and then incubated on a shaker overnight at 4°C in the specific primary antibodies. GAPDH (Cell Signaling Technology, CST #5174) was used as an internal control. The primary antibody against BCL2L11 (Santa Cruz #374358), E-cadherin (CST #3195), Claudin-1 (CST #4933), Snail (CST #3879), TWIST1 (CST #46702), Vimentin (CST #5741). After washed in TBST (Tris Buffered Saline, with Tween-20) buffer, the blot was incubated in Horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000, CST) for 1 h at room temperature. Blots were developed using enhanced chemiluminescence detection reagents and scanned with a Molecular Imager system (Bio-Rad).
Biotin-labelled miRNA pull-down assay
Endometriosis primary cells were transfected with biotinylated miR-132-3p (Bio-miR-132-3p), Bio-miR-132-3p-mutant and Bio-NC (GenePharma, China). Cells were collected at 48 h. The cell lysates were incubated with M-280 streptavidin magnetic beads (Invitrogen, USA). To prevent non-specific binding of RNA and protein complexes, the beads were coated with RNase-free bovine serum albumin and yeast tRNA (both from Sigma-Aldrich). The beads were incubated at 48°C for 3 h and washed three times with ice-cold lysis buffer and once with high salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0 and 500 mM NaCl). The bound RNAs were purified using TRIzol for the analysis.
RNA-binding protein immunoprecipitation assay
RNA immunoprecipitation (RIP) assays were performed using the EZ-Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, USA) according to the manufacturer’s protocol. Endometriosis primary cells were lysed in complete RIP lysis buffer and cleared lysates were then incubated with RIP buffer containing magnetic beads conjugated to human anti-Ago2 antibody (Proteintech, China). The NC was normal mouse IgG (Beyotime, China), and the positive control was SNRNP70 (Millipore, USA). The coprecipitated RNAs were isolated by TRIzol reagent (TaKaRa, China) and were detected by qRT-PCR.
Luciferase reporter assay
Primary cells were grown in a 24-well plate and co-transfected with 1.25 μg of empty pmiR-GLo-NC, pmiR-GLo-LINC00261-Wild type, pmiR-GLo-LINC00261-Mutant type, pmiR-GLo-BCL2L11-Wild type or pmiR-GLo-BCL2L11-Mutant type (Sangon Biotech, Shanghai, China) with 50 nM of a pPG-miR-132-3p or pPG-miR-NC into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA). The luciferase activities were assessed using a dual-luciferase reporter assay kit (Promega, USA) according to the manufacturer’s protocol. The relative luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL). Experimental results were evaluated using the two-tailed Student t test. All values were expressed as mean ± SD. Statistical significance was noted at P<0.05. Three independent triplicated experiments were performed for cell biological assays, unless otherwise stated.
Results
LINC00261 is significantly downregulated in endometriosis tissues
To explore the potential role of LINC00261 in human endometriosis, we performed qRT-PCR to assess the expression levels of LINC00261 in twenty pairs of human endometriosis tissue samples and paired adjacent normal tissue samples. As shown in Figure 1A, LINC00261 levels were significantly down-regulated in endometriosis tissue samples compared to those of adjacent normal tissue.
Figure 1.
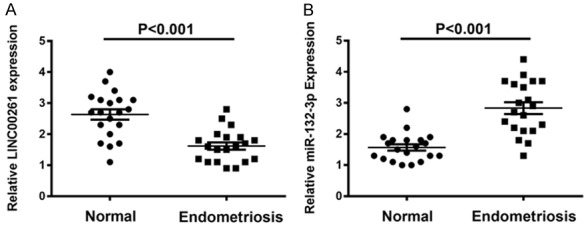
Expression levels of LINC00261 and miR-132-3p in endometriosis tissues. A. LINC00261 levels were significantly down-regulated in twenty endometriosis tissue samples compared to those of adjacent normal tissues. B. Expression level of miR-132-3p presented with upregulation in endometriosis tissues.
Overexpression of LINC00261 inhibits endometriosis cells proliferation and invasion
We then conducted colony formation and transwell assays to explore the effect of LINC00261 on the proliferation and invasion behaviors of endometriosis cells. After respectively transfecting CRL-7566 and endometriosis primary cells with pcDNA-LINC00261, we found that the expression levels of LINC00261 were both significantly increased (Figure 2A, 2B). And the numbers of colony formation in pcDNA-LINC00261 group were obviously decreased compared with NC group, suggesting that LINC00261 could suppress the proliferation ability of endometriosis cells (Figure 2C, 2D). Next, transwell invasion assay in endometriosis primary cells and CRL-7566 cell line demonstrated that pcDNA-LINC00261 significantly decreased cell invasion ability compared with control (Figure 2E, 2F). Together, these results demonstrated that LINC00261 could inhibit endometriosis cells proliferation and invasion in vitro.
Figure 2.
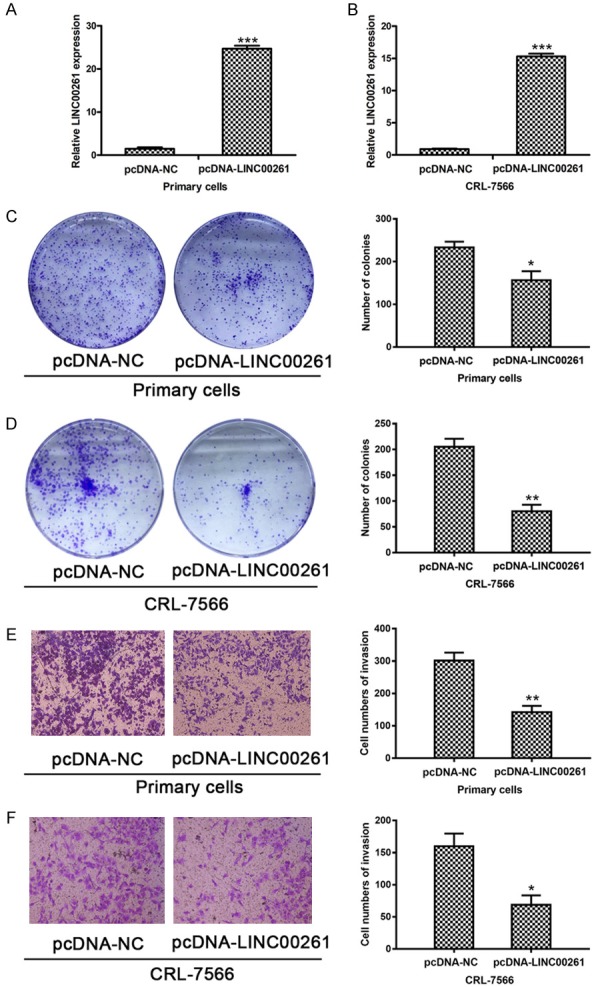
Overexpression of LINC00261 inhibits endometriosis cells proliferation and invasion. A, B. The expression levels of LINC00261 significantly increased after transfecting CRL-7566 and endometriosis primary cells with pcDNA-LINC00261 respectively. C, D. The numbers of colony formation in pcDNA-LINC00261 group were obviously decreased compared with NC group, suggesting that LINC00261 could suppress the proliferation ability of endometriosis cells. E, F. Transwell invasion assay results demonstrated that pcDNA-LINC00261 significantly decreased cell invasion ability compared with control in both cell lines. (Statistical significance indicated as *P<0.05, **P<0.01 and ***P<0.001).
LINC00261 acts as a sponge directly binds to miR-132-3p in endometriosis cells
Inspired by the ‘ceRNA’ regulatory network, we hypothesized that LINC00261 could function as a molecular sponge and modulate miRNAs in cytoplasm. Through prediction in online databases (miRcode; http://www.mircode.org/), we found that there are putative complementary sequences between LINC00261 and miR-132-3p. First, we performed qRT-PCR to assess the expression levels of miR-132-3p in twenty pairs of endometriosis samples with adjacent normal tissues and confirmed its upregulation in endometriosis tissue samples (Figure 1B). Moreover, we detected the expression levels of miR-132-3p in pcDNA-LINC00261 transfected primary cells and CRL-7566 by qRT-PCR. As we expected, the miR-132-3p showed obviously decrease in pcDNA-LINC00261 than NC (Figure 3A, 3B). After transfecting two cell lines with two siRNAs against LINC00261, si-1 and si-2 could efficiently knockdown the endogenous LINC00261 and dramatically increase the relative level of miR-132-3p (Figure 3C-E). To further determine whether miR-132-3p is capable of negatively regulating LINC00261, we transfected primary cells and CRL-7566 with pPG-miR-132-3p, pPG-anti-miR-132-3p or pPG-miR-NC. The qRT-PCR results showed that pPG-miR-132-3p significantly increased miR-132-3p expression and reduced LINC00261 expression, while pPG-anti-miR-132-3p dramatically suppressed the miR-132-3p expression and augmented LINC00261 expression in two cell lines (Figure 3F-I).
Figure 3.
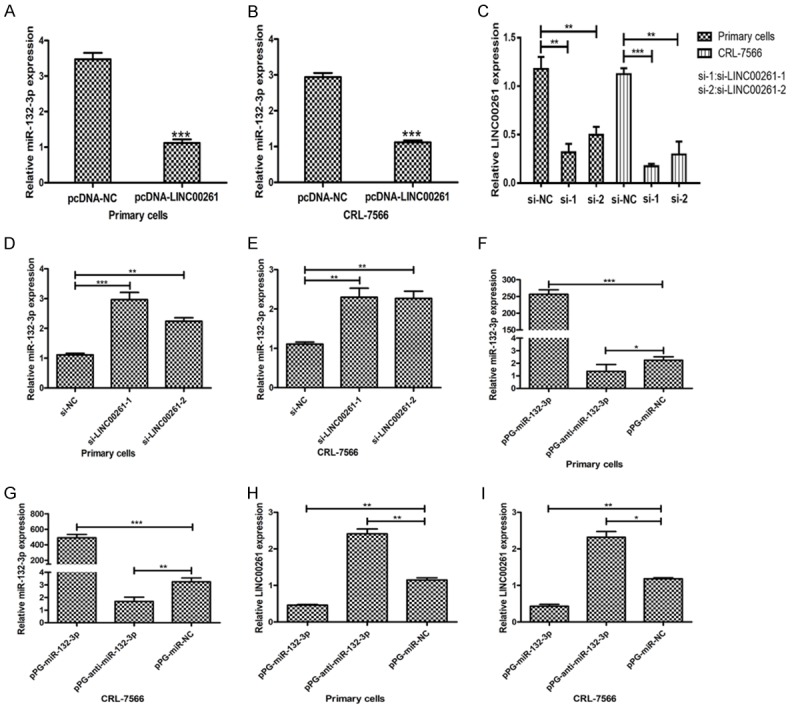
Negative regulation between LINC00261 and miR-132-3p. A, B. The expression levels of miR-132-3p in pcDNA-LINC00261 transfected primary cells and CRL-7566 were detected by qRT-PCR and results showed that miR-132-3p levels obviously decreased in pcDNA-LINC00261 than NC. C-E. The knockdown efficiency of two siRNAs against LINC00261, si-1 and si-2 were verified, and the relative levels of miR-132-3p were dramatically increased when endogenous LINC00261 being knockdown. F-I. Primary cells and CRL-7566 were transfected with pPG-miR-132-3p, pPG-anti-miR-132-3p or pPG-miR-NC. The qRT-PCR results showed that pPG-miR-132-3p significantly increased miR-132-3p expression and reduced LINC00261 expression, while pPG-anti-miR-132-3p dramatically suppressed the miR-132-3p expression and augmented LINC00261 expression in two cell lines. (Statistical significance indicated as *P<0.05, **P<0.01 and ***P<0.001).
Furthermore, we performed miRNA pull-down assays by transfecting Bio-miR-132-3p, Bio-miR-132-3p-mut or Bio-NC into primary cells. It could be observed that LINC00261 could be pulled down by miR-132-3p (Figure 4A). MiRNAs exert their miRNA-mediated gene silencing function by binding to Ago2, a core component of the RNA-induced silencing complex (RISC) [12]. Thus, we performed RIP assays with Ago2 antibody to isolate the RNA from RISC. The results indicated that LINC00261 was preferentially enriched in Ago2-containing beads in endometriosis primary cells (Figure 4B). Summing up the above, we inferred that LINC00261 could directly bind to miR-132-3p.
Figure 4.
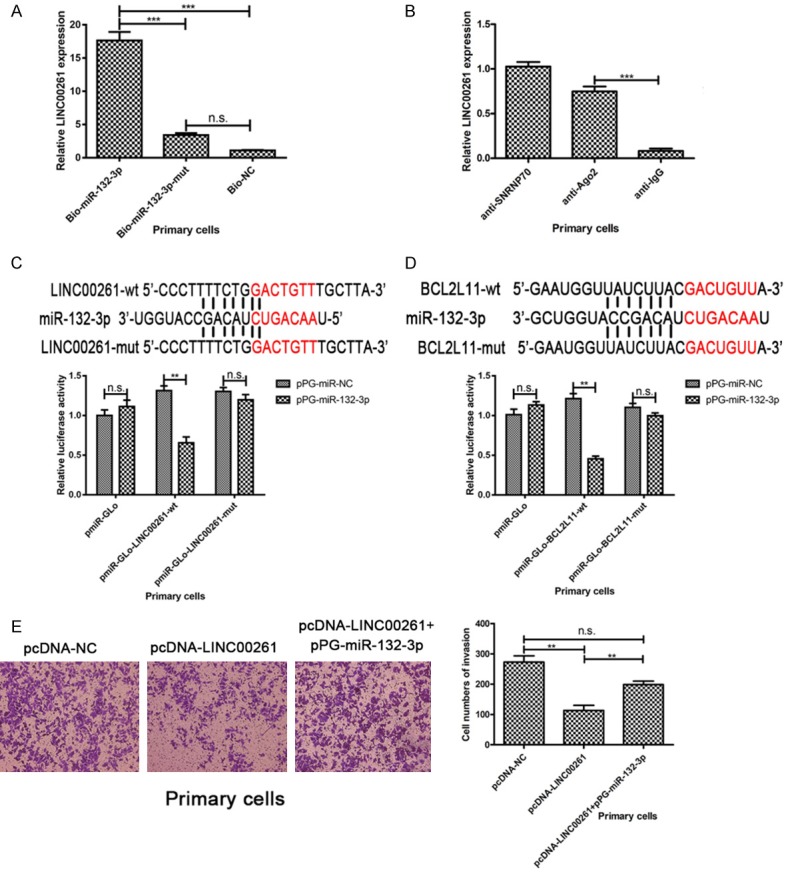
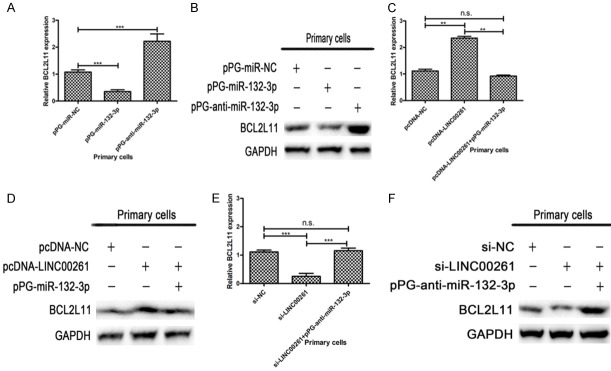
LINC00261 acts as a sponge directly binds to miR-132-3p in endometriosis cells. A. MiRNA pull-down assays identified that LINC00261 could be pulled down by miR-132-3p. B. Result of RIP assays indicated that LINC00261 was preferentially enriched in Ago2-containing beads in endometriosis primary cells. C. The putative binding site between LINC00261 and miR-132-3p. Dual-luciferase reporter assay in endometriosis primary cells showed that the luciferase activity was reduced in primary cells co-transfected with pmiR-GLo-LINC00261-wt but not in cells co-transfected with pmiR-GLo empty vector or pmiR-GLo-LINC00261-mut. D. The putative binding site between miR-132-3p and BCL2L11. The results of luciferase reporter assays confirmed that there is a direct interaction between miR-132-3p and the 3’-untranslated regions (3’-UTR) of BCL2L11 in primary cells. E. pPG-miR-132-3p rescued the invasion ability inhibition induced by pcDNA-LINC00261. (Statistical significance indicated as **P<0.01 and ***P<0.001; n.s., not statistically significant).
The putative binding site between LINC00261 and miR-132-3p according to the prediction in online databases is presented in Figure 4C. To further explore whether the binding site is functional, a dual-luciferase reporter assay in endometriosis primary cells was carried out. We constructed a luciferase reporter containing the wild-type LINC00261 and a mutant LINC00261 with mutations of the predicted miR-132-3p binding sites. The luciferase activity was reduced in primary cells co-transfected with pmiR-GLo-LINC00261-wt but not in cells co-transfected with pmiR-GLo empty vector or pmiR-GLo-LINC00261-mut (Figure 4C). These data suggested that the binding site is essential for the reciprocal repression of LINC00261 and miR-132-3p. As shown in Figure 4E, 4F, pPG-miR-132-3p rescued the invasion ability inhibition induced by pcDNA-LINC00261.
LINC00261 regulates BCL2L11 level via influencing the level of miR-132-3p
The study of Cai et al. [13] provided supportive evidence that miR-132-3p plays a role in TSC-AMLs (TSC patients developing angiomyolipomas) through the regulation of BCL2L11. Here we observed the similar phenomenon of mRNA and protein level changes in endometriosis primary cells. Primary cells transfected with pPG-miR-132-3p obviously reduced the expression of BCL2L11 in both mRNA and protein levels, and vice versa (Figure 5A, 5B). The putative binding site between miR-132-3p and BCL2L11 is presented in Figure 4D. Furthermore, we constructed wild-type and mutated luciferase reporters of BCL2L11. And the results of luciferase reporter assays confirmed that there is a direct interaction between miR-132-3p and the 3’-untranslated regions (3’-UTR) of BCL2L11 in primary cells (Figure 4D).
Figure 5.
LINC00261 regulates BCL2L11 level via influencing the level of miR-132-3p. A, B. Expression of BCL2L11 were obviously reduced in both mRNA and protein levels when primary cells transfected with pPG-miR-132-3p, whereas transfected with pPG-anti-miR-132-3p obviously increased the expression of BCL2L11. C, D. According to qRT-PCR and western blot results, overexpression of LINC00261 significantly increased BCL2L11 mRNA and protein expression levels while adding pPG-miR-132-3p reversed these effects. E, F. The knockdown of LINC00261 in primary cells resulted in the significantly decreasing of BCL2L11 mRNA and protein expression levels, and pPG-anti-miR-132-3p reversed these effects. (Statistical significance indicated as **P<0.01 and ***P<0.001; n.s., not statistically significant).
Then we explored whether LINC00261 could regulate BCL2L11 by competing with miR-132-3p in endometriosis primary cells. Overexpression of LINC00261 significantly increased BCL2L11 mRNA and protein expression levels while adding pPG-miR-132-3p reversed these effects (Figure 5C, 5D). Conversely, when we knockdown LINC00261 expression of primary cells, the BCL2L11 mRNA and protein expression levels were significantly decreased, and pPG-anti-miR-132-3p reversed these effects (Figure 5E, 5F). These data indicated that LINC00261 regulates BCL2L11 level via binding to miR-132-3p and then influencing its level.
BCL2L11 exerts its invasion-suppressive effect in endometriosis through EMT
To identify the function of BCL2L11 in endometriosis, two siRNAs targeted to BCL2L11 were designed and synthesized. The knockdown efficacy of siRNAs was verified at both, mRNA and protein levels (Figure 6A, 6B). Our research showed that BCL2L11 suppresses tumor invasion and epithelial-mesenchymal transitions (EMT) process in endometriosis primary cells. Apparently, the protein expression levels of E-Cadherin and Claudin-1 were reduced when primary cells treating with si-BCL2L11, whereas SNAIL, TWIST1 and Vimentin protein levels presented with the tendency of increasing by western blot (Figure 6C). Integrating above changes in EMT markers with the results of invasion ability in primary cells transfected with si-BCL2L11, it can be inferred that BCL2L11 exerts its invasion-suppressive effect in endometriosis through promoting EMT (Figure 6D). Collectively, our results provide the promising evidence that LINC00261 suppresses proliferation and invasion of endometriosis cells in vitro. We further confirmed that LINC00261 functions as a competing endogenous RNA to regulate BCL2L11 expression by binding to miR-132-3p directly. The LINC00261/miR-132-3p/BCL2L11 regulatory networks may be a novel therapeutic target for endometriosis.
Figure 6.
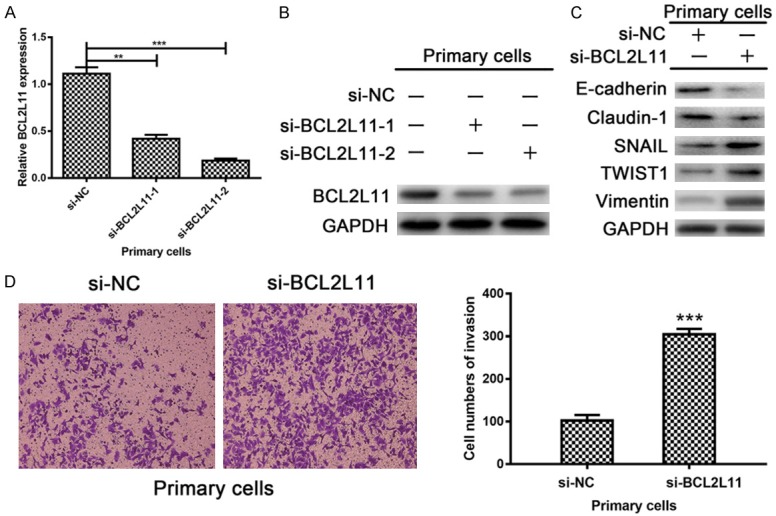
BCL2L11 exerts its invasion-suppressive effect in endometriosis through EMT. A, B. The knockdown efficacy of two siRNAs targeted to BCL2L11 were verified at both mRNA and protein levels. C. Western blot showed that the protein expression levels of E-Cadherin and Claudin-1 were reduced when treated with si-BCL2L11 in primary cells, whereas SNAIL, TWIST1 and Vimentin protein levels presented with the tendency of increasing. D. The cell numbers of invasion were obviously multiplied in si-BCL2L11 group from the results of transwell invasion assay. (Statistical significance indicated as **P<0.01 and ***P<0.001).
Discussion
Although much progress and evidence-based consensus or guideline have been made in the diagnosis and treatment of endometriosis, early-detection methods and effective remedies are waiting to be explored and utilized. The complexity of treating endometriosis stems from our poor understanding of the pathophysiological mechanisms and heterogeneity of the disease [14]. LINC00261 is a relatively novel lncRNA and our previous study proved that LINC00261 is capable of inhibiting cell growth and migration in endometriosis [9]. CeRNA network, as the most mature mechanism between lncRNAs and miRNAs, often presents as reciprocal repression model. In post-transcriptionally level, miRNAs regulate gene expression by inhibiting mRNA translation or inhibiting mRNA and protein degradation, more precisely, miRNAs suppress gene expression through sequence-specific binding with the 3’-UTR of a target mRNA [15].
Cumulating evidence has identified that miR-132-3p dysregulation might be one of the leading factors in tumorigenesis, such as pancreatic carcinoma [16], breast cancer [17], glioblastoma [18], prostate cancer [19], and be of great significance in nervous system diseases [20] such as Alzhimer [21,22], Huntington’s Disease [23], pituitary tumor [24], and may be dispensable for immune response [25], biological clock [26], etc. The role of miR-132-3p has not been identified in endometriosis. Our study showed that miR-132-3p is upregulated in endometriosis tissue samples and fortunately, reciprocal repression between LINC00261 and miR-132-3p was observed in present study.
BCL2L11 (also known as BIM) is a member of BCL-2 family and is located in the outer membrane of mitochondria, where this protein acts as an important regulator that mediates apoptosis [27-29] and as an dual-agent that regulates autophagy in drug resistance [30]. The anti-apoptotic BCL2 members have multiple domains; while the pro-apoptotic members of BCL2 family, including BCL2L11, are BH3-domain-only proteins [31]. Although the role of BCL2L11 has been reported in biological processes in cancer [32], the function of BCL2L11 in endometriosis remains largely unknown. The sequence showed that miR-132-3p could bind with BCL2L11 and BCL2L11 exerts its invasion-suppressive effect in endometriosis through promoting EMT, suggested that LINC00261 may influence the EMT process during the invasion of endometriosis.
The limitations of this research including the cellular location of LINC00261 remain to be done, which will guide the investigation into the inmost mechanism. Secondly, the role of LINC00261/miR-132-3p/BCL2L11 axis should be verified in vivo. And the association with clinical characteristics of patients should be confirmed in future studies with a large cohort of samples. Further research is still needed to make the function and mechanism of LINC00261 perfect.
In a word, our results provide the promising evidence that LINC00261 suppresses proliferation and invasion of endometriosis cells in vitro. Mechanically, LINC00261 functions as a competing endogenous RNA to regulate BCL2L11 expression by binding to miR-132-3p directly. The LINC00261/miR-132-3p/BCL2L11 regulatory networks may be a novel therapeutic target for endometriosis.
Acknowledgements
This study was granted by Wenzhou Municipal Science and Technology Bureau (Y20180271 & Y20140563), Health and Family Planning Commission of Zhejiang Province (2019317125), Population and Family Planning Science and Technology Project of Zhejiang Province (2014KYA262). We thank all of the laboratory members for their technical advice and helpful discussion.
Disclosure of conflict of interest
None.
References
- 1.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 2.Yu Y, Li L, Zheng Z, Chen S, Chen E, Hu Y. Long non-coding RNA linc00261 suppresses gastric cancer progression via promoting Slug degradation. J Cell Mol Med. 2017;21:955–967. doi: 10.1111/jcmm.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y, Wang YF, Su HF, Fang N, Zou C, Li WF, Fei ZH. Retraction Note to: Decreased expression of the long noncoding RNA LINC00261 indicate poor prognosis in gastric cancer and suppress gastric cancer metastasis by affecting the epithelial-mesenchymal transition. J Hematol Oncol. 2018;11:2. doi: 10.1186/s13045-017-0544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang ZK, Yang L, Wu LL, Mao H, Zhou YH, Zhang PF, Dai GH. Long non-coding RNA LINC00261 sensitizes human colon cancer cells to cisplatin therapy. Braz J Med Biol Res. 2017;51:e6793. doi: 10.1590/1414-431X20176793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang HF, Li W, Han YD. LINC00261 suppresses cell proliferation, invasion and Notch signaling pathway in hepatocellular carcinoma. Cancer Biomark. 2018;21:575–582. doi: 10.3233/CBM-170471. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Xue K, Guan Y, Jin Y, Liu S, Wang Y, Liu S, Wang L, Han L. Long noncoding RNA LINC00261 suppresses cell proliferation and invasion and promotes cell apoptosis in human choriocarcinoma. Oncol Res. 2017;25:733–742. doi: 10.3727/096504016X14772362173376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Xiao N, Xu SF. Decreased expression of long non-coding RNA LINC00261 is a prognostic marker for patients with non-small cell lung cancer: a preliminary study. Eur Rev Med Pharmacol Sci. 2017;21:5691–5695. doi: 10.26355/eurrev_201712_14014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang CM, Gao W, Wu YY, Zhao QL, Chen B, Liu QQ, Li WY, Wen SX, Wang BQ. [The expression of long non-coding RNA LINC00261 in laryngeal carcinoma tissue and their clinical significance] . Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;31:68–71. doi: 10.13201/j.issn.1001-1781.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Sha L, Huang L, Luo X, Bao J, Gao L, Pan Q, Guo M, Zheng F, Wang H. Long non-coding RNA LINC00261 inhibits cell growth and migration in endometriosis. J Obstet Gynaecol Res. 2017;43:1563–1569. doi: 10.1111/jog.13427. [DOI] [PubMed] [Google Scholar]
- 10.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper DK, Novitzky D, Wicomb WN. The pathophysiological effects of brain death on potential donor organs, with particular reference to the heart. Ann R Coll Surg Engl. 1989;71:261–266. [PMC free article] [PubMed] [Google Scholar]
- 12.Karginov FV, Conaco C, Xuan Z, Schmidt BH, Parker JS, Mandel G, Hannon GJ. A biochemical approach to identifying microRNA targets. Proc Natl Acad Sci U S A. 2007;104:19291–19296. doi: 10.1073/pnas.0709971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Y, Wang W, Guo H, Li H, Xiao Y, Zhang Y. miR-9-5p, miR-124-3p, and miR-132-3p regulate BCL2L11 in tuberous sclerosis complex angiomyolipoma. Lab Invest. 2018;98:856–870. doi: 10.1038/s41374-018-0051-6. [DOI] [PubMed] [Google Scholar]
- 14.Nothnick WB. Endometriosis: bright future for a cloudy past? Sci Transl Med. 2015;7:271fs272. doi: 10.1126/scitranslmed.aaa5075. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Liu A, Feng X, Tian L, Bo W, Wang H, Hu Y. MiR-132 promotes the proliferation, invasion and migration of human pancreatic carcinoma by inhibition of the tumor suppressor gene PTEN. Prog Biophys Mol Biol. 2017 doi: 10.1016/j.pbiomolbio.2017.09.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Tahiri A, Leivonen SK, Luders T, Steinfeld I, Ragle Aure M, Geisler J, Makela R, Nord S, Riis ML, Yakhini Z, Kleivi Sahlberg K, Borresen-Dale AL, Perala M, Bukholm IR, Kristensen VN. Deregulation of cancer-related miRNAs is a common event in both benign and malignant human breast tumors. Carcinogenesis. 2014;35:76–85. doi: 10.1093/carcin/bgt333. [DOI] [PubMed] [Google Scholar]
- 18.Setty M, Helmy K, Khan AA, Silber J, Arvey A, Neezen F, Agius P, Huse JT, Holland EC, Leslie CS. Inferring transcriptional and microRNA-mediated regulatory programs in glioblastoma. Mol Syst Biol. 2012;8:605. doi: 10.1038/msb.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Formosa A, Lena AM, Markert EK, Cortelli S, Miano R, Mauriello A, Croce N, Vandesompele J, Mestdagh P, Finazzi-Agro E, Levine AJ, Melino G, Bernardini S, Candi E. DNA methylation silences miR-132 in prostate cancer. Oncogene. 2013;32:127–134. doi: 10.1038/onc.2012.14. [DOI] [PubMed] [Google Scholar]
- 20.Saraiva C, Esteves M, Bernardino L. MicroRNA: basic concepts and implications for regeneration and repair of neurodegenerative diseases. Biochem Pharmacol. 2017;141:118–131. doi: 10.1016/j.bcp.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Qian Y, Song J, Ouyang Y, Han Q, Chen W, Zhao X, Xie Y, Chen Y, Yuan W, Fan C. Advances in roles of miR-132 in the nervous system. Front Pharmacol. 2017;8:770. doi: 10.3389/fphar.2017.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Spiczak J, Morsbach F, Winklhofer S, Frauenfelder T, Leschka S, Flohr T, Maintz D, Seifarth H, Bunck AC, Stolzmann P, Alkadhi H. Coronary artery stent imaging with CT using an integrated electronics detector and iterative reconstructions: first in vitro experience. J Cardiovasc Comput Tomogr. 2013;7:215–222. doi: 10.1016/j.jcct.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Fukuoka M, Takahashi M, Fujita H, Chiyo T, Popiel HA, Watanabe S, Furuya H, Murata M, Wada K, Okada T, Nagai Y, Hohjoh H. Supplemental treatment for huntington’s disease with mir-132 that is deficient in huntington’s disease brain. Mol Ther Nucleic Acids. 2018;11:79–90. doi: 10.1016/j.omtn.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356:568–578. doi: 10.1016/j.canlet.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Bellon M, Lepelletier Y, Hermine O, Nicot C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood. 2009;113:4914–4917. doi: 10.1182/blood-2008-11-189845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng HY, Obrietan K. Revealing a role of microRNAs in the regulation of the biological clock. Cell Cycle. 2007;6:3034–3035. doi: 10.4161/cc.6.24.5106. [DOI] [PubMed] [Google Scholar]
- 27.Concannon CG, Tuffy LP, Weisova P, Bonner HP, Davila D, Bonner C, Devocelle MC, Strasser A, Ward MW, Prehn JH. AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J Cell Biol. 2010;189:83–94. doi: 10.1083/jcb.200909166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Peng T, Li L, Wang X, Duan R, Gao H, Guan W, Lu J, Teng J, Jia Y. MicroRNA-9 regulates neural apoptosis in methylmalonic acidemia via targeting BCL2L11. Int J Dev Neurosci. 2014;36:19–24. doi: 10.1016/j.ijdevneu.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Kilbride SM, Farrelly AM, Bonner C, Ward MW, Nyhan KC, Concannon CG, Wollheim CB, Byrne MM, Prehn JH. AMP-activated protein kinase mediates apoptosis in response to bioenergetic stress through activation of the pro-apoptotic Bcl-2 homology domain-3-only protein BMF. J Biol Chem. 2010;285:36199–36206. doi: 10.1074/jbc.M110.138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai Y, Grant S. BCL2L11/Bim as a dual-agent regulating autophagy and apoptosis in drug resistance. Autophagy. 2015;11:416–418. doi: 10.1080/15548627.2014.998892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansha M, Hussain A, Kofler A, Grubbauer C, Goetsch K, Ploner C, Kofler R. “Bam”, a novel glucocorticoid-induced BH3-only transcript from the BCL2L11/Bim locus, does not appear to be translated. Leuk Lymphoma. 2013;54:353–358. doi: 10.3109/10428194.2012.708928. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Duan J, Qu Y, Deng T, Liu R, Zhang L, Bai M, Li J, Ning T, Ge S, Wang X, Wang Z, Fan Q, Li H, Ying G, Huang D, Ba Y. Onco-miR-24 regulates cell growth and apoptosis by targeting BCL2L11 in gastric cancer. Protein Cell. 2016;7:141–151. doi: 10.1007/s13238-015-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]