Abstract
Objective: The effects and enhancement of catalpol (CP) on specific immune therapy (SIT) were investigated with an established animal model associated with bronchial asthma. Materials and methods: A total of 50 adults BALB/c mice were randomly divided into five groups with 10 mice in each group. These groups are control group, model group, CP group, SIT group and CP/SIT joint group. The mice were sensitized and challenged with OVA and Bronchoalveolar lavage fluid (BALF) and peripheral blood were obtained, the cell counts and the levels of cytokines (IL-1, IL-4, IFN-γ) in BALF were detected using ELISA methods. Results: The total number of cells in BALF of the group treated with CP/SIT joint group was significantly lower than the other experimental groups. Furthermore the eosinophils of the mice were dramatically reduced comparing the other experimental groups, the cytokines IL-1 and IL-4 display the similar tendency, but the IFN-γ concentration for the experimental groups was higher than the model group. After the therapy using CP, SIT and a combination of CP and SIT, asthma-associated inflammation was inhibited, IL-4 level was decreased and IFN-γ level was increased. In addition, the expression of TLR-4 protein in peripheral blood was detected by Western Blot method. Conclusions: In summary, CP can enhance the treating effect of SIT and the joint treatment by CP/SIT might be a potential method for clinical treatment of asthma, the mechanism is related to the inhibition of TLR-4 signaling pathway.
Keywords: Catalpol, specific immune therapy, IL-1, IL-4, IFN-γ
Introduction
Bronchial asthma (or in short, asthma) is a common disease with a high incidence of occurrence, which has become a global social health problem and seriously threatened human health [1]. The primary pathological change of asthma has been considered eosinophil infiltration in airway, with associated clasmatoblast infiltration, lymphocyte infiltration and macrophage infiltration [2-4]. The main clinical features of asthma are airway hyperresponsiveness and airway dysventilation induced by airway inflammation [5,6]. Over the past two decades, chronic anti-inflammatory therapy has been able to effectively control the clinical symptoms of asthma by inhibiting airway inflammation [7]. However, the incidence and mortality of asthma have not efficiently reduced. Specific immunotherapy (SIT) is one of the effective etiological treatments for allergic asthma, especially for IgE-mediated allergic diseases. Currently, induction of peripheral antigen specific immune tolerance is considered to be one of the main mechanisms of SIT [8,9]. Immune tolerance refers to the specific immune nonresponsiveness or low responsiveness formed by the immune system after exposure to antigens. In addition, the mechanism of SIT is regulation of the balance of Th1/Th2. Recent studies have demonstrated that BCG vaccine and its derivatives can be used as non-specific immunomodulators to regulate immunity and Th1/Th2 balance [10,11]. A combination of SIT and BCG vaccine can further reduce the level of IL-4 secretion, increase the level of IFN-γ secretion, and regulate the immune response to Th1 cells as a dominant response [12,13].
Catalpol (CP) is one of the primary components in Radix Rehmanniae Preparata (Shudihuang). As a traditional Chinese herbal medicine, Radix Rehmanniae Preparata has been commonly used for neuroprotective, anti-aging and anti-tumor treatments [14-28]. The pharmacological effects of this Chinese medicine as well as its main ingredient CP have been widely investigated, including the pronounced anti-inflammatory properties to inhibit inflammatory responses induced by LPS and IFN-γ, reduce inflammatory production of cytokines, and protect against renal ischemia and reperfusion injury [14-28]. Therefore, such anti-inflammatory function of CP should be related to the treatment of asthma. Indeed, the anti-asthmatic effects of CP and its derivatives have been investigated by several research groups. In this study, a combined effect of CP and SIT on asthmatic mice was investigated by cell classification and counting in bronchoalveolar lavage fluid (BALF) and serum ovalbumin (OVA). The expression of TLR-4 protein in peripheral blood was detected to indicate that a combined treatment using CP and SIT can decrease the expression of TLR-4 protein and inflammatory factors in lung tissue of OVA-induced asthmatic mice. The mechanism is related to the inhibition of TLR-4 signaling pathway.
Materials and methods
Experimental animals
Fifty SPF grade BALB/c mice (twenty-five males and twenty-five females) were obtained from Experimental Animal Center of Xiamen University. The mice were kept in a controlled environment at a temperature and a relative humidity of 24±2°C, and 52%±3%, respectively with 12 h-light-and-12 h-dark cycles. The care and treatment of the animals were approved by the Animal Care and Use Committees of Fuzhou General Hospital of Nanjing Military Area Command.
Chemicals and reagents
OVA (Grade II) was purchased from Sigma-Aldrich (Shanghai, China), aluminum hydroxide (99.9%) was purchased from Sinopharm (Beijing, China), catalpol injectable powder was purchased from Melone Pharmaceutical Co., Ltd (Dalian, China), ELISA reagent kits were purchased from Biosource Bioengineering Co., Ltd (St. Louis, MO, USA), Western blot kits were purchased from Beyotime Biotechnology (Shanghai, China) and TLR-4 antibody was purchased from Santa Cruz Biotechnology (Shanghai, China). All other chemicals or reagents were obtained from Sinopharm (Beijing, China) with a grade of analytical pure.
Establishment of asthma model
The mice were randomly divided into five groups (blank control group, asthma model group, CP group, SIT group and CP/SIT joint group) with 10 mice in each group. The asthma model was established by sensitization and challenge with OVA. Generally, the mice in the blank control group were treated by intraperitoneal injection using 0.5 mL of normal saline on Day 1, Day 7 and Day 14, followed by challenged with normal saline for 30 min every day from Day 15 to Day 28. The mice in the asthma model group were sensitized with 100 μg OVA and 2 mg aluminum hydroxide in 0.5 mL normal saline via intraperitoneal injection on Day 1, Day 7 and Day 14, followed by challenged with 50 g/L OVA in 0.5 mL normal saline for 30 min every day using an ultrasonic nebulizer from Day 15 to Day 28. The mice in the CP group received a dose (5 mg/kg in normal saline) of catalpol daily through intraperitoneal injection from Day 15 to Day 28 one hour before OVA-challenge after the sensitization on Day 1, Day 7 and Day 14 as for the model group. The mice in the SIT group received a dose (5 mg/kg in normal saline) of SIT therapeutic solution daily through intraperitoneal injection from Day 15 to Day 28 one hour before OVA-challenge after the sensitization on Day 1, Day 7 and Day 14 as for the model group. The mice in the CP/SIT joint group received a dose (5 mg/kg in normal saline) of mixed CP and SIT therapeutic solution daily through intraperitoneal injection from Day 15 to Day 28 one hour before OVA-challenge after the sensitization on Day 1, Day 7 and Day 14 as for the model group.
Bronchoalveolar lavage fluid and lung tissue preparation
After 24 hours of last OVA challenge, the mice in each group were anesthetized with 10% chloral hydrate (0.5 g/kg) via intraperitoneal injection. Peripheral blood was taken from postcava for about 1 mL and centrifuged for 10 minutes at 3500 r/min. Serum was collected and stored at -70°C. All mice were executed after blood taking, left lung was ligated and thoracic cavity was opened for tracheotomy intubation. The right lung was lavaged with normal saline three times with 1 mL for each time. The recovery rate of BLAF was more than 80%. BALF was obtained and the total number of cells was counted with 10 μL. After collecting BALF, the ligated left lung was intercepted and placed in 10% formalin solution.
Cell count and cytokines level determination
BALF cells were counted by direct counting method. The total number of cells was counted after dilution with 3% acetic acid solution, and eosinophils were counted after dilution with acetone-eosin diluent. The level of IgE in the BALF was determined using an IgE enzyme-linked immunosorbent assay (ELISA) kit. The levels of IL-1, IL-4, and IFN-γ in the BALF were determined using the corresponding ELISA kits according to the manufacturer’s instructions. The optical density (OD) values of cytokines in each sample were measured at a wavelength of 450 nm.
TLR-4 protein detected by western blot
According to instruction of the detection kit, the protein concentration was detected and buffer was added after extraction of total protein from treated peripheral blood. The protein concentration of each group was adjusted to 5 μg/μL and heated in boiling water for 10 minutes. SDS-PAGE gel electrophoresis experiment was conducted, 5% skim milk powder with PBST was sealed for 1 h followed by incubated overnight with internal reference β-actin antibody (1:3000) and Rabbit anti human TLR-4 antibody (1:800) at 4°C. After washing, the membranes were incubated with the corresponding HRP-labeled secondary antibodies (1:5000). The target proteins were visualized using ECL detection system.
Statistical methods
The experimental data were analyzed by SPSS16.0 software. Normal test and homogeneity test of variance were carried out for data acquired from each group. If the data conformed to normal distribution with homogeneous variance, the data were tested by complete random design variance analysis and LSD method. The yielded results were expressed by x±s.
Results
Symptoms observation
The mice in experimental groups were sensitized via aerosol inhalation of OVA solution. After 10 minutes of sensitization, the mice started to show nasal irritation, increased and deep breathing, nodded breathing, occasional cough, irritability, forelimb contraction and elevation, etc. More excreta could be observed after taking the animals out of their living enviroment, suggesting that asthma has been successfully induced. After OVA challenge, the mice exhibited mental depression, hair disorder and reduced appetite. The mental and hair state of mice in each treatment group slightly recovered within 24 hours, the appetite was better than the model group. There was no obvious skin injury, ulcer or paralysis observed on the hind limbs of mice in each treatment group. All mice in the blank control group were in good condition.
IgE in BALF
The level of IgE in BALF was determined by the ELISA method. Figure 1 shows that the level of IgE in BLAF increased in the model group, it was significantly higher than that in the control group with P < 0.01. After CP or SIT treatment, the IgE level was remarkably decreased, and further decreased after joint CP/SIT treatment. The levels of all treatment groups were significantly lower than the model group with P < 0.01.
Figure 1.
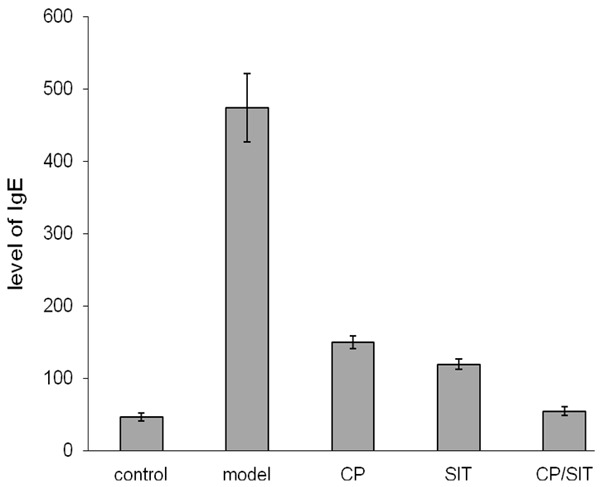
The level of IgE in BALF.
Cell counts in BALF
Figure 2 shows the total number of inflammatory cells and the percentages of eosinophils after sensitization and OVA-challenge of the mice. The total number of cells and the percentages of eosinophils in the model group were both significantly increased compared with the control group with P < 0.01, as shown in Figure 2. After treatment with different approaches, the total number of cells and the percentages of eosinophils in the CP and SIT groups were significantly decreased compared with the model group with P < 0.05. Moreover, the inflammatory cells and eosinophils in joint CP/SIT group were decreased compared with the CP group and the SIT group with P < 0.05.
Figure 2.
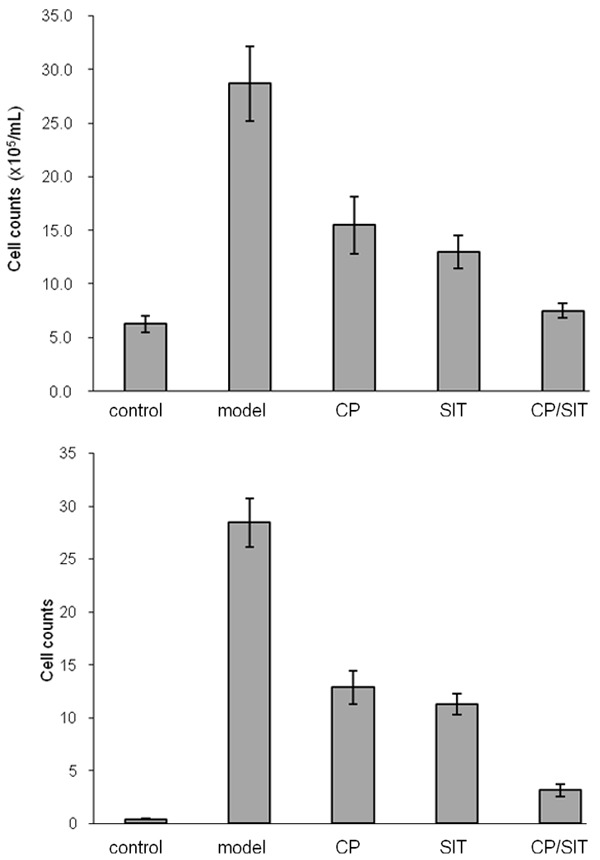
The total number of inflammatory cells (top) and the percentages of eosinophils (bottom) after sensitization and OVA-challenge.
Concentration of cytokines
Figure 3 shows the concentrations of different cytokines in the BALF from each group. The levels of IL-1 and IL-4 in the model group were significantly increased compared with the control group with P < 0.01, and the level of IFN-γ in the model group was significantly decreased compared with the control group with P < 0.01. After treatment with various approaches, the levels of IL-1 and IL-4 in the CP and SIT groups were significantly decreased compared with the model group with P < 0.05, and the levels of IFN-γ in the CP and SIT groups were significantly increased compared with the model group with P < 0.05. Furthermore, the levels of IL-1 and IL-4 in the joint CP/SIT group were significantly decreased compared with the CP or the SIT group with P < 0.05, and the level of IFN-γ in the joint CP/SIT group was significantly increased compared with the CP or the SIT group with P < 0.05.
Figure 3.
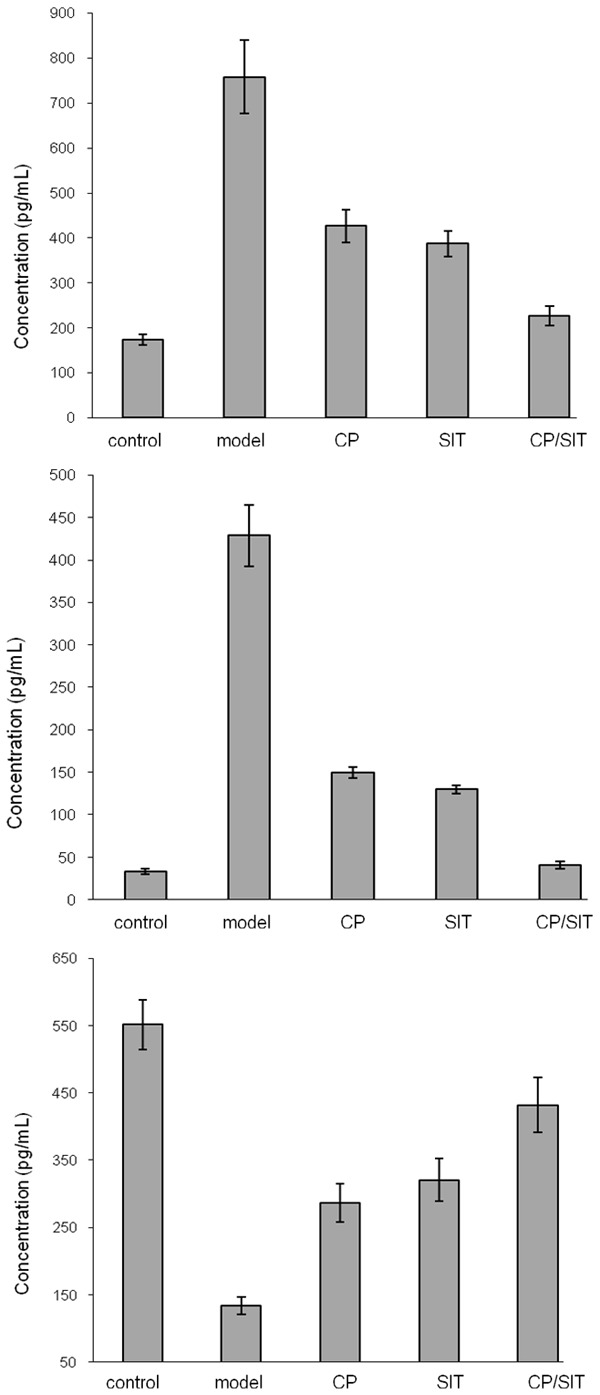
Concentrations of cytokines IL-1 (top), IL-4 (middle) and IFN-γ in BALF.
Expression of TLR-4 protein
The results obtained from Western blot are shown in Figure 4. The expression of TLR-4 in the model group was significantly enhanced compared with the control group with P < 0.01. After treatment, TLR-4 expression in the CP and SIT groups were significantly reduced compared with the model group with P < 0.05, TLR-4 expression in the joint CP/SIT group was significantly reduced compared with the CP or SIT group with P < 0.05.
Figure 4.
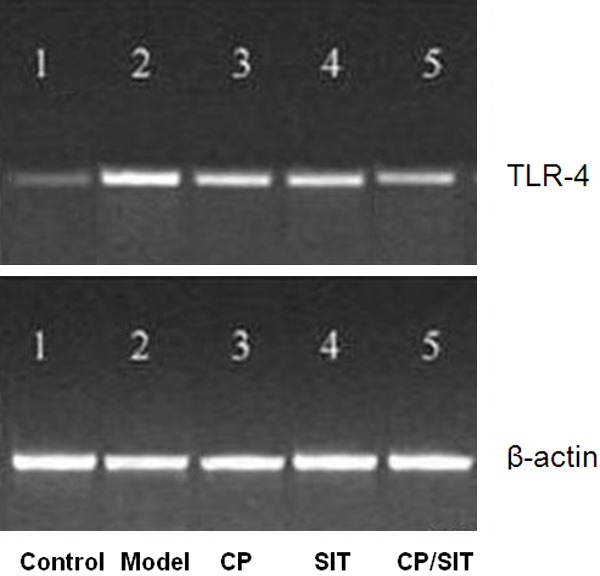
Expression of TLR-4 protein.
Discussion
In this study, OVA sensitization and challenge were used to construct an asthmatic mice model. During the process of OVA-challenge, it can be observed that the asthmatic mice exhibited dyspnea, irritability and other symptoms. The total number of cells and eosinophils in BALF increased significantly compared with the control group, suggesting that the model was successfully built. Compared with the control group, the levels of IFN-γ for characteristic Th1-type cytokine and IL-1 or IL-4 for characteristic Th2-type cytokine were decreased and increased, respectively. The Th1/Th2 ratio in the model group was decreased, Th2 cells showed a predominant response and Th1/Th2 ratio was imbalanced, which are consistent with the mechanisms of allergic diseases such as asthma.
During the development of asthma, allergens are distinguished and captured by antigen-presenting cells such as B cells, macrophages, dendritic cells, etc. via mucous membranes and immune response occurs. Allergens in blood and other parts of the body turn to selective Th2 lymphocyte response, while the number and activity of specific Th1 lymphocyte subsets decrease, resulting in the imbalance of Th1/Th2. It has been proved that SIT can target both antigen presenting cells and T cells to correct the imbalance of cytokines and reverse the imbalance of Th1/Th2. In this study, OVA-sensitized mice subjected to SIT and subsequently challenged also exhibited dyspnea and other symptoms, but the symptoms were slighter and the degree of infiltration of inflammatory cells in lung tissue slices was smaller than that in the model group, and the total number of cells and eosinophils in BALF were significantly reduced compared with the model group. SIT has obvious therapeutic effect on asthma. Moreover, compared with the model group, IFN-γ increased significantly and IL-1 and IL-4 decreased significantly, suggesting that SIT can up-regulate the function of Th1 cells and down-regulate the function of Th2 cells, thus the imbalance of Th1/Th2 was improved.
As an immunopotentiator, CP has been used clinically in patients with poor immunity and has achieved good adjuvant therapeutic effect. Moreover, the regulation of Th1/Th2 imbalance and the therapeutic effect of CP on asthma have been reported in some publications [29-32]. Therefore, in current study an attempt was made to explore the therapeutic effect of CP on asthma and to investigate the enhanced immunotherapeutic effect when CP is combined with SIT on treatment of asthma. The results for comparison between the CP group and the model group indicated that CP could also increase the level of Th1 response, inhibit the secretion of IL-1 or IL-4 and decrease the level of Th2 response by stimulating IFN-γ secretion. In terms of the stimulating symptoms, cell counts in BALF and serum cytokine detection for the mice in the joint CP/SIT therapeutic group, the CP/SIT combined treatment shows significant therapeutic effect, and the effect is more pronounced compared both therapeutic groups using single method, suggesting that CP can enhance SIT immunotherapy effect. The main manifestation is the enhancement of Th1 cell function between the combined and single applications.
Toll like receptor (TLR-4) is a kind of innate immune receptor and an important transmembrane protein in the body. It can recognize the specific molecular structure of antigen or its products, stimulate a series of signaling pathways such as TLR-4/NF-kB, activate antigen presenting cells, induce T lymphocyte differentiation, up-regulate the chemotactic factors, release the pro-inflammatory factors, collect various inflammatory cells to infiltrate and regulate the cycle of inflammatory cells, and promote the occurrence and aggravation of inflammation. In this study, it has been found that the transcription of TLR-4 gene and the expression of TLR-4 protein in serum of asthmatic mice after treament with CP, SIT and joint CP/SIT methods were significantly reduced, suggesting that the mechanism of CP, SIT and CP/SIT combination therapy might be related to the down-regulation of TLR-4 protein expression in serum besides the enhancement of Th1 cell function.
Conclusions
The effects of CP, SIT and joint CP/SIT treatments on asthma were investigated using an animal model associated with bronchial asthma. The asthma-associated inflammation was significantly inhibited by CP, SIT and combined CP/SIT therapeutic methods, IL-1 and IL-4 levels were decreased and IFN-γ level was increased. The CP/SIT combined treatment showed more pronounced therapeutic effect compared with single applications using CP or SIT alone. The mechanisms are related to the enhancement of Th1 cell function and the down-regulation of TLR-4 protein expression. In summary, CP can enhance the treating effect of SIT and the joint treatment by CP/SIT might be a potential method for clinical treatment of asthma.
Acknowledgements
This work is funded by Natural Science Foundation of Fujian Province, China: 1. Effects of RNA interference targeting VDR on NF-kB signal way and cell proliferation of smooth muscle cells (No. 2016J01474); 2. A novel experiment animal model for cerebral arteriovenous malformation in ALK1 deletion mouse (No. 2016J01587).
Disclosure of conflict of interest
None.
References
- 1.Desai D, Brightling C. Cytokine and anti-cytokine therapy in asthma: ready for the clinic? Clin Exp Immunol. 2009;158:10–19. doi: 10.1111/j.1365-2249.2009.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 3.Venarske D, deShazo RD. Molecular mechanisms of allergic disease. South Med J. 2003;96:1049–1054. doi: 10.1097/01.SMJ.0000097887.04639.39. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56:985–1003. doi: 10.1258/0007142001903490. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brightling CE, Bradding P, Pavord ID, Wardlaw AJ. New insights into the role of the mast cell in asthma. Clin Exp Allergy. 2003;33:550–556. doi: 10.1046/j.1365-2222.2003.01636.x. [DOI] [PubMed] [Google Scholar]
- 7.Yssel H, Lecart S, Pene J. Regulatory T cells and allergic asthma. Microbes Infect. 2001;3:899–904. doi: 10.1016/s1286-4579(01)01450-2. [DOI] [PubMed] [Google Scholar]
- 8.Yuan X, Sun S, Wang S, Sun Y. Effects of astragaloside IV on IFN-gamma level and prolonged airway dysfunction in a murine model of chronic asthma. Planta Medica. 2011;77:328–333. doi: 10.1055/s-0030-1250408. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013;6:335–346. doi: 10.1038/mi.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durrant DM, Metzger DW. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol Invest. 2010;39:526–549. doi: 10.3109/08820131003615498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu X, Jiang D, Wang Y, Du Q, Cai J. Effects of astragaloside IV on eosinophil activation induced by house dust mite allergen. Mol Med Rep. 2012;6:115–120. doi: 10.3892/mmr.2012.869. [DOI] [PubMed] [Google Scholar]
- 12.Garcia G, Taille C, Laveneziana P, Bourdin A, Chanez P, Humbert M. Anti-interleukin-5 therapy in severe asthma. Eur Respir Rev. 2013;22:251–257. doi: 10.1183/09059180.00004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wegmann M. Targeting eosinophil biology in asthma therapy. Am J Respir Cell Mol Biol. 2011;45:667–674. doi: 10.1165/rcmb.2011-0013TR. [DOI] [PubMed] [Google Scholar]
- 14.Dong Z, Chen CX. Effect of catalpol on diabetic nephropathy in rats. Phytomedicine. 2013;20:1023–1029. doi: 10.1016/j.phymed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Liu Z, Peng Y, Zhang L, Ju P, Bi K, Chen X. Validated LC-MS method for simultaneous quantitation of catalpol and harpagide in rat plasma: application to a comparative pharmacokinetic study in normal and diabetic rats after oral administration of Zeng-Ye-Decoction. Biomed Chromatogr. 2013;27:1503–1510. doi: 10.1002/bmc.2949. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Li X, Jia LQ, Wang J, Zhang L, Hou D, Wang J, Ren L. Neuroprotective activities of catalpol against CaMKII-dependent apoptosis induced by LPS in PC12 cells. Br J Pharmacol. 2013;169:1140–1152. doi: 10.1111/bph.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi HJ, Jang HJ, Chung TW, Jeong SI, Cha J, Choi JY, Han CW, Jang YS, Joo HS, Ha KT. Catalpol suppresses advanced glycation end-products-induced inflammatory responses through inhibition of reactive oxygen species in human monocytic THP-1 cells. Fitoterapia. 2013;86:19–28. doi: 10.1016/j.fitote.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Bi J, Jiang B, Zorn A, Zhao RG, Liu P, An LJ. Catalpol inhibits LPS plus IFN-gamma-induced inflammatory response in astrocytes primary cultures. Toxicol In Vitro. 2013;27:543–550. doi: 10.1016/j.tiv.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Wan D, Xue L, Zhu H, Luo Y. Catalpol induces neuroprotection and prevents memory dysfunction through the cholinergic system and BDNF. Evid Based Complement Alternat Med. 2013;2013:134852. doi: 10.1155/2013/134852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Jin C, Li Y, Guan S, Han F, Zhang S. Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose. Food Chem Toxicol. 2013;58:50–55. doi: 10.1016/j.fct.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Cui Y, Ji L, Zhang W, Li R, Ma L, Xing W, Zhou H, Chen B, Yu J, Zhang H. Catalpol decreases peroxynitrite formation and consequently exerts cardioprotective effects against ischemia/reperfusion insult. Pharm Biol. 2013;51:463–473. doi: 10.3109/13880209.2012.740052. [DOI] [PubMed] [Google Scholar]
- 22.Jiang B, Du J, Liu J, Bao YM, An LJ. Catalpol attenuates the neurotoxicity induced by beta-amyloid (1-42) in cortical neuron-glia cultures. Brain Res. 2008;1188:139–147. doi: 10.1016/j.brainres.2007.07.105. [DOI] [PubMed] [Google Scholar]
- 23.Jin D, Cao M, Mu X, Yang G, Xue W, Huang Y, Chen H. Catalpol inhibited the proliferation of T24 human bladder cancer cells by inducing apoptosis through the blockade of Akt-mediated anti-apoptotic signaling. Cell Biochem Biophys. 2015;71:1349–1356. doi: 10.1007/s12013-014-0355-0. [DOI] [PubMed] [Google Scholar]
- 24.Tian YY, An LJ, Jiang L, Duan YL, Chen J, Jiang B. Catalpol protects dopaminergic neurons from LPS-induced neurotoxicity in mesencephalic neuron-glia cultures. Life Sci. 2006;80:193–199. doi: 10.1016/j.lfs.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Xu G, Ma S, Li F, Yuan M, Xu H, Huang K. Catalpol ameliorates high-fat-diet-induced insulin resistance and adipose tissue inflammation by suppressing the JNK and NF-kappaB pathways. Biochem Biophys Res Commun. 2015;467:853–858. doi: 10.1016/j.bbrc.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 26.Gao N, Tian JX, Shang YH, Zhao DY, Wu T. Catalpol suppresses proliferation and facilitates apoptosis of OVCAR-3 ovarian cancer cells through upregulating microRNA-200 and downregulating MMP-2 expression. Int J Mol Sci. 2014;15:19394–19405. doi: 10.3390/ijms151119394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang B, Shen RF, Bi J, Tian XS, Hinchliffe T, Xia Y. Catalpol: a potential therapeutic for neurodegenerative diseases. Curr Med Chem. 2015;22:1278–1291. doi: 10.2174/0929867322666150114151720. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Chen X, Wang H, Yan Q. Catalpol protects mice against renal ischemia/reperfusion injury via suppressing PI3K/Akt-eNOS signaling and inflammation. Int J Clin Exp Med. 2015;8:2038–2044. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Zhang Y, Xu M, Luan J, Piao S, Chi S, Wang H. Catalpol alleviates ovalbumin-induced asthma in mice: reduced eosinophil infiltration in the lung. Int Immunopharmacol. 2017;43:140–146. doi: 10.1016/j.intimp.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Lee HK, Oh SR, Ahn KS, Lee SK, Lee JK, Kwon OK, Kim DY, Joung H, Quang GH, Kim MJ, Park BY. Pharmaceutical composition comprising an extract of pseudolysimachion longifolium and the catalpol derivatives isolated therefrom having antiinflammatory, antiallergic and antiasthmatic activity, US8455541B2 (2013) [Google Scholar]
- 31.Li Y, Wang H, Yang X. Effects of catalpol on bronchial asthma and its relationship with cytokines. J Cell Biochem. 2018 doi: 10.1002/jcb.28170. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Park EJ, Lee HS, Oh SR, Lee HK, Lee HS. Pharmacokinetics of verproside after intravenous and oral administration in rats. Arch Pharm Res. 2009;32:559–564. doi: 10.1007/s12272-009-1412-x. [DOI] [PubMed] [Google Scholar]


