Abstract
Objective: To examine the effect of silencing SKP2 on chemosensitivity of human glioma cells U251 to temozolomide (TMZ). Methods: Adenoviruses harbouring shRNA targeting SKP2 (i.e. Ad-shSKP2) and non-targeting scrambled shRNA (i.e. Ad-shNC) were used to infect U251 cells. The transduced cells were then treated with TMZ. Cell viability after treatment was assayed using CCK8; while cell cycle and apoptosis were examined using flow cytometry. To study the effect of silencing SKP2 on autophagy in U251, we co-transduced the cells with Ad-mRFP-LC3 and Ad-shSKP2/Ad-shNC. The expression of autophagy marker LC3 after TMZ treatment was studied using microscopy and Western blotting assays. Results: The cytotoxicity of TMZ (i.e. 20-100 µM) was more significantly seen in Ad-shSKP2-transduced U251 cells than in the Ad-shNC-transduced U251 cells. The IC50 values in shSKP2-U251 were significantly lower than those of the shNC-U251 (P < 0.05). Both TMZ and Ad-shSKP2 alone increased apoptosis and promoted expression of LC3 in U251. Combined treatment of Ad-shSKP2 and TMZ further elevated apoptosis and LC3 expression. Conclusion: Silencing SKP2 in U251 cells increased chemosensitivity to TMZ that was accompanied with enhanced apoptosis and autophagy. Targeting SKP2 may be a potential approach to potentiate TMZ treatment in patients with glioma.
Keywords: Glioma, S-phase kinase-associated protein 2 (SKP2), temozolomide (TMZ), chemosensitivity, autophagy
Introduction
Glioma is a commonly seen as tumour of the central nervous system. In general, the prognosis of glioma is poor, showing an unacceptable high rate of recurrence after treatment. Surgical intervention alone cannot be curative, so in many cases, radiotherapy and chemotherapy are administered as adjuvant therapies. Temozolomide (TMZ), an oral chemotherapeutic agent that can be permeable across the blood-brain barrier, is the first-line treatment for glioma [1]. However, despite its clinical usefulness, TMZ shows limited efficacy in prolonging the survivals of patients with glioma [2]. Treatment modalities that can potentiate the therapeutic efficacy of TMZ are therefore believed to benefit patients’ outcomes [3].
S-phase kinase-associated protein 2 (SKP2) is a key mediator of ubiquitination. By targeting cell cycle control elements like p21 and p27 to protein degradation, SKP2 is involved in the normal regulation of cell cycle entry and G1/S transition [4,5]. However, deregulated proteolysis by SKP2 is implicated in cancer biogenesis [6]. SKP2 was also shown to reactivate the PI3K-AKT signalling pathway, which has already been inhibited by PI3K inhibitors, in breast cancer [7]. The oncogenic functions of SKP2 were also demonstrated in hepatocellular carcinoma [8], colorectal cancer [9], papillary thyroid cancer [10], and gastric cancer [11]. In human glioma, the expression of SKP2 was found up-regulated, of which the overexpressed level was associated with a poor prognosis of patients [12,13]. SilencingSKP2 suppressed the proliferation and invasive property of human glioma cells U251 in vitro [14]. Downregulation of SKP2 also resulted in growth arrest and apoptosis of T98G glioblastoma cells [15]. In view of these findings, the present study was aimed to further investigate the role of SKP2 in glioma by examining the silencing effect of SKP2 on cellular sensitivity to TMZ.
Materials and methods
Cell culture and reagents
Human glioma astrocytoma cells U251 were revived from the cell bank of our research institute and were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum in a humidified incubator (37°C, 5% CO2). Adenoviruses harbouring shRNA targeting SKP2 gene (i.e. Ad-shSKP2) and shRNA of scramble sequence (i.e. Ad-shNc) were prepared and validated in a previous study (12). Both shRNA plasmids contained GFP open reading frame, allowing the transduction efficacy to be studied by GFP fluorescence microscopy. Adenovirus expressing RFP-LC3 fusion protein (i.e. Ad-mRFP-LC3) was obtained from Hanbio (Shanghai, China). The use of this fusion protein construct permitted the detection of LC3 level by measuring RFP signal using microscopy. TMZ was purchased from MedChem Express (Monmouth Junction, NJ, USA), and was prepared as a 0.1 M stock solution with dimethyl sulfoxide (DMSO). SKP2 antibody was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). LC3 antibody was obtained from Cell Signaling Technology (Danvers, MA, USA).
U251 sensitivity towards TMZ
Single-cell suspension of U251 (1 × 105 cells/mL) was transduced with Ad-shSKP2 or Ad-shNc with a MOI of 20. Twelve hours after the transduction, U251 cells were treated with different concentrations of TMZ (10, 20, 40, 60, 80 and 100 µM) for 24, 48 and 72 hours. Cells treated with 1% DMSO served as the vehicle control. An empty well was also included for baseline measurement. Cell viability at the designated time points was determined using CCK8 assays, with the results read at an optical density of 450 nm. The sensitivity of U251 to TMZ was presented as the percentage of cell inhibition, which was calculated as (ODvehicle control-ODtest sample)/(ODvehicle control-ODbaseline) × 100%.
Cell cycle and apoptosis analysis
U251 cells were transduced with Ad-shSKP2 or Ad-shNC. After 24 hours, transduced cells were treated with 100 µM TMZ or 1% DMSO for 48 hours, and then fixed with ice-cold 70% ethanol at 4°C overnight. The fixed cells were washed with PBS, stained with propidium iodide (PI) solution (50 µg/mL PI, 100 µg/mL RNase A, 0.2% w/w Triton X-100) at 4°C for 30 minutes, and analysed by flow cytometry.
Autophagy analysis
U251 cells (1 × 105 cells/mL) were seeded onto glass slides placed in 24-well plates and were transduced with Ad-mRFP-LC3 with a MOI of 50. The transduced cells were then further transduced with Ad-shSKP2 or Ad-shNC with a MOI of 20. After 24 hours, the transduced cells were treated with 100 µM TMZ or 1% DMSO for 24 hours. The treated cells were fixed with 4% paraformaldehyde, washed with PBS, and stained with 5 µg/mL DAPI. Expression of LC3, which is an autophagy marker, was studied by determining mRFP signal.
Western blotting assays
Whole-cell lysate was prepared on ice using cell lysis buffer supplemented with protease inhibitors, and was subsequently resolved using SDS-PAGE. The resolved proteins were transferred onto PVDF membrane, which was then blocked with 5% non-fat milk at ambient temperature for 1 hour. The blocked membrane was incubated with antibody against SKP2 (1:500 dilutions), LC3 (1:1000 dilutions), and α-tubulin (1:1000 dilutions) at 4°C for overnight. After washing, the antibody-probed membrane was incubated with HRP-conjugated anti-rabbit (1:10000 dilutions) or anti-mouse (1:10000 dilutions) at room temperature for 2 hours. Signals were finally detected using ECL solution, with the image captured with the Bio-Rad imaging system.
Statistical analysis
All measurement data were presented as mean ± standard derivation (x̅ ± S.D.), and analysed using SPSS version 20. Differences between treatment groups were evaluated using t-test or one-way ANOVA whenever appropriate, with statistically significant difference indicated by P value < 0.05.
Results
Knockdown of SKP2 in U251
The transduction efficiency and SKP2 knockdown in U251 was assessed using GFP microscopy and Western blotting assays, respectively (Figure 1). Twenty-four hours after transduction, successful transduction of U251 with the adenovirus was confirmed by the GFP signal in both Ad-shSKP2 and Ad-shNC transduced U251 (Figure 1A). The protein level of SKP2 in Ad-shSKP2-transduced cells was found significantly reduced compared to that of the AdshNC transduced, as shown by the Western blot of whole-cell lysate collected at 48 hours after transduction (Figure 1B).
Figure 1.
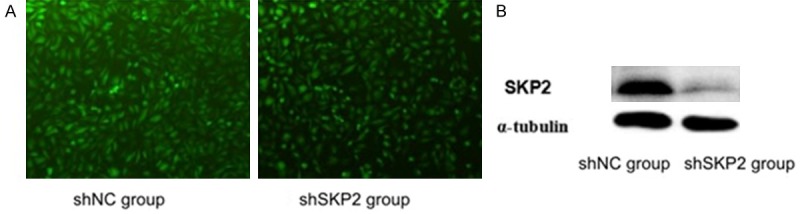
Transduction of human glioma cell U251 with Ad-shSKP2 (shSKP2 group) and Ad-shNC (shNC group). A. At 24 hours after transduction, the efficiency was examined by counting GFP-positive cell under microscope. B. At 48 hours after transduction, the down-regulation of SKP2 in shSKP2 group was examined using Western blotting assays. Shown is the representative set of data from 3 independent experiments.
Effect of SKP2 KD on TMZ sensitivity of U251
The inhibition of cell viability of Ad-shSKP2 and Ad-shNC-transduced U251 by TMZ was examined using CCK8 assay (Figure 2). At all time points tested, at the lowest concentration of TMZ (i.e. 10 µM), there were no significant differences in cell viability inhibition between Ad-shSKP2 and Ad-shNC-transduced U251. At higher concentrations of TMZ (≥ 20 µM), TMZ treatment caused more significant death of Ad-shSKP2-U251 than Ad-shNC-U251. Treatment of Ad-shSKP2-U251 with TMZ for 24, 48, and 72 hours resulted in IC50 of 128 ± 6 μM, 119 ± 15 μM, and 75 ± 4 μM, respectively. Treatment of Ad-shNC-U251 with TMZ for 24, 48, and 72 hours resulted in IC50 of 173 ± 49 μM, 174 ± 26 μM, and 111 ± 10 μM, respectively. The IC50 values in shSKP2-U251 were significantly lower than those of the shNC-U251 (P < 0.05).
Figure 2.
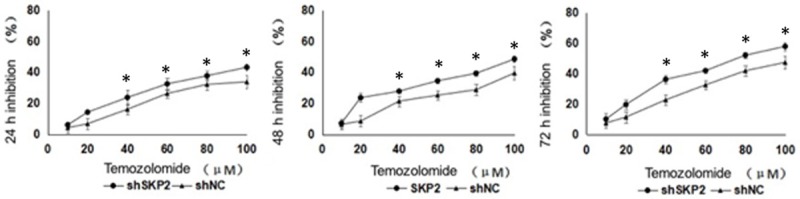
Inhibitory effect of TMZ on Ad-shSKP2 or Ad-shNC-transduced U251 at time points of 24, 48, and 72 hours. Asterisks indicate significant differences in cell viability (P < 0.05) between the two treatment groups. Shown is the representative set of data from 3 independent experiments.
Effect of SKP2 KD and TMZ on U251 cell cycle and apoptosis
Cell cycle and apoptosis of shRNA-treated U251, either alone or in combination with TMZ, was examined using flow cytometry (Table 1; Figure 3). Compared to Ad-shNC-U251, AdshSKP2-U251, TMZ-treated Ad-shNC-U251 and TMZ-treated Ad-shSKP2-U251 displayed significant increase in sub-G1 phase (P < 0.05), which was accompanied with substantial decrease in G0/G1 (P < 0.05). Notably, SKP2 KD combined with TMZ treatment resulted in a higher portion of sub-G1 than in SKP2 KD and TMZ treatment alone (P < 0.05).
Table 1.
Distribution of cell cycle phases of U251 receiving different treatments
| SUB | G0/G1 | S | G2/M | |
|---|---|---|---|---|
| shNC | 1.53 ± 1.05 | 65.63 ± 1.80 | 18.57 ± 2.12 | 14.27 ± 2.70 |
| shSKP2 | 13.73 ± 1.90a | 50.67 ± 1.42a | 14.63 ± 2.04 | 21.80 ± 1.80a |
| shNC+TMZ | 8.77 ± 1.33a,b | 51.63 ± 3.44a | 17.67 ± 1.11 | 21.93 ± 3.69a |
| shSKP2+TMZ | 19.23 ± 1.40a,b,c | 40.13 ± 4.69a,b,c | 16.63 ± 1.25 | 25.00 ± 3.47a |
P < 0.05 compared to shNC group;
P < 0.05 compared to shSKP2 group;
P < 0.05 compared to shNC+TMZ group.
Figure 3.
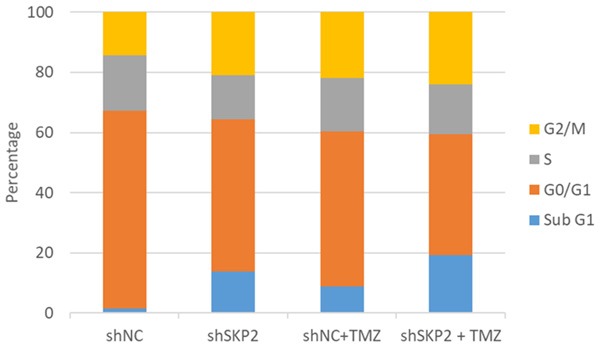
Effect of Ad-shSKP2 and TMZ on cell cycle and apoptosis was studied using flow cytometry. Shown is the representative set of data from 3 independent experiments.
Effect of SKP2 KD and TMZ on autophagy marker expression
The red signal of mRFP was seen in most Ad-mRFP-LC3-transduced U251 (Figure 4A). Compared to those co-transduced with AdshNC, U251 co-transduced with Ad-shSKP2 or treated with TMZ presented more mRFP-positive counts (P < 0.05). Ad-shSKP2 transduction combined with TMZ treatment further increased the number of mRFP-positive cells compared to other groups (P < 0.05) (Figure 4B). LC3-I is the cytosolic form of LC3. It is conjugated to phosphatidylethanolamine to form LC3-II. LC3-II is then recruited to autophagosomal membranes. Therefore, LC-II serves as the marker of autophagy. Western blot also revealed the combined treatment with Ad-shSKP2 and TMZ led to a substantial increase in LC3-II protein (Figure 4C).
Figure 4.
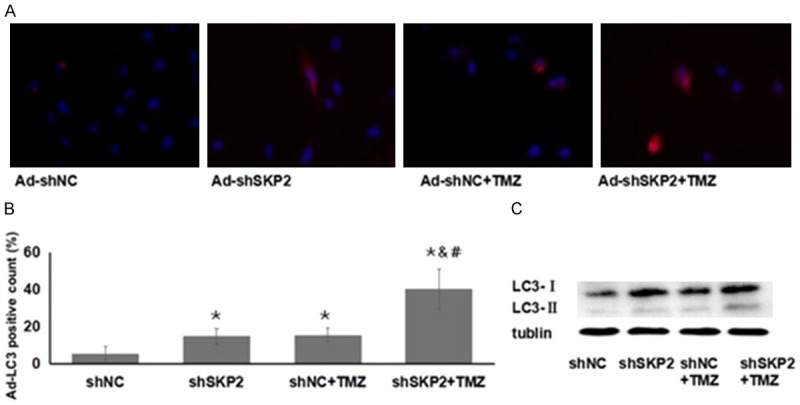
Effect of Ad-shSKP2 and TMZ on autophagy marker expression was examined. A. The red signal of LC3 of different treatment groups at 48 hour after transduction was examined under the microscope. Red, mRFP of LC3; Blue, DAPI; magnification, × 400. B. The percentage of L3-positive cells in each treatment group was determined and compared. *P < 0.05 compared to shNC group; #P < 0.05 compared to shNC+TMZ. C. The up-regulation of LC3-II by the combined treatment of shSKP2 and TMZ was examined using Western blotting assays. Shown is the representative set of data from 3 independent experiments.
Discussion
TMZ is an alkylating agent that kills cancer cells by inhibiting DNA synthesis through incorporation of base mismatches. However, cancer cells can overcome DNA methylation and hence become resistant to TMZ with their DNA repair machinery [16-18]. The present study demonstrated for the first time that silencing SKP2 could potentiate the cytotoxicity of TMZ on human glioma cell U251. The increase in TMZ sensitivity was attributed to apoptosis (as shown by increases in sub-G1 phase) and autophagy (as revealed by upregulations in LC3-II protein) induced by SKP2 silencing. Our findings might provide some clues into new treatment modalities that can enhance the therapeutic efficacy of TMZ in glioma treatment.
Many chemotherapeutic drugs including TMZ eradicate cancer cells by inducing apoptosis and autophagy [19]. However, in glioma, due to the presence of blood brain barrier, TMZ concentration may not reach the level sufficient for apoptosis induction. In addition, many PTEN mutant gliomas can overcome TMZ-induced apoptosis by activating AKT signalling [20]. Therapeutic approaches that sensitize glioma to chemotherapeutics are therefore urgently needed. Targeting SKP2 was shown to suppress glioma cells in vitro. The present study further showed that silencing SKP2 in glioma would enhance the sensitivity of cancer cells to TMZ becauseTMZ IC50 of Ad-shSKP2-transduced U251 was significantly lower than that of the Ad-shNC-transduced. Indeed, the role of SKP2 in drug sensitivity has been studied in different types of cancers. In paclitaxel-resistant prostate cancer, destabilization and degradation of SKP2 by a novel selenonucleoside was recently shown to recover the drug sensitivity in vitro and in vivo [21]. In tamoxifen-resistant breast cancer cell line MCF-7, the induction of apoptosis by a plant extract was attributed to the suppression of SKP2 [22]. The implication of SKP2 in chemoresistance in lung cancer was also reported [23].
Induction of autophagy by TMZ has been accepted as a therapeutic approach for treatment of glioma [3]. This study clearly showed that an equivalent amount of TMZ resulted in higher autophagy marker LC3 expression in Ad-shSKP2-transduced than in Ad-shNC-transduced U251. SKP2 is a key signalling mediator of ubiquitination. In addition to its substantial roles in determining cell cycle and apoptosis, SKP2 is an important component of AMPK-SKP2-CARM1 signalling cascade for transcriptional regulation of autophagy. Down-regulation of SKP2 could elevate CARM1, ultimately leading to autophagy [24].
Whether autophagy sensitizes glioma to TMZ or vice versa remains to be studied. Previous published works showed that enhancing autophagy in glioma would promote cell chemosensitivity to TMZ [3,18,25]; however, there were studies illustrating that chemosensitivity to TMZ was increased by inhibiting autophagy [26,27]. Nevertheless, our data collectively revealed that silencing SKP2 could sensitize glioma cells to TMZ that was accompanied with an increase in autophagy marker expression. Studies will be conducted to decipher the underlying mechanism by which SKP2 regulates TMZ chemosensitivity in glioma.
Acknowledgements
This work was supported by Foundation of Hubei Educational Committee (B2015171).
Disclosure of conflict of interest
None.
References
- 1.Zhang J, Stevens MF, Bradshaw TD. Temozolomide: mechanisms of action, repair and resistance. Curr Mol Pharmacol. 2012;5:102–114. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Q, Han L, Dong Y, Tian J, Huang W, Liu Z, Jia X, Jiang T, Zhang J, Li X, Kang C, Ren H. JAK2/STAT3 targeted therapy suppresses tumor invasion via disruption of the EGFRvIII/JAK2/STAT3 axis and associated focal adhesion in EGFRvIII-expressing glioblastoma. Neuro Oncol. 2014;16:1229–1243. doi: 10.1093/neuonc/nou046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CM, Syu JP, Way TD, Huang LJ, Kuo SC, Lin CT, Lin CL. BC3EE2,9B, a synthetic carbazole derivative, upregulates autophagy and synergistically sensitizes human GBM8901 glioblastoma cells to temozolomide. Int J Mol Med. 2015;36:1244–1252. doi: 10.3892/ijmm.2015.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci U S A. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- 6.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clement E, Inuzuka H, Nihira NT, Wei W, Toker A. Skp2-dependent reactivation of AKT drives resistance to PI3K inhibitors. Sci Signal. 2018;11 doi: 10.1126/scisignal.aao3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei X, Li X, Yan W, Zhang X, Sun Y, Zhang F. SKP2 promotes hepatocellular carcinoma progression through nuclear AMPK-SKP2-CARM1 signaling transcriptionally regulating nutrient-deprived autophagy induction. Cell Physiol Biochem. 2018;47:2484–2497. doi: 10.1159/000491622. [DOI] [PubMed] [Google Scholar]
- 9.Shen L, Qu X, Li H, Xu C, Wei M, Wang Q, Ru Y, Liu B, Xu Y, Li K, Hu J, Wang L, Ma Y, Li M, Lai X, Gao L, Wu K, Yao L, Zheng J, Zhang J. NDRG2 facilitates colorectal cancer differentiation through the regulation of Skp2-p21/p27 axis. Oncogene. 2018;37:1759–1774. doi: 10.1038/s41388-017-0118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratheeshkumar P, Siraj AK, Divya SP, Parvathareddy SK, Begum R, Melosantos R, Al-Sobhi SS, Al-Dawish M, Al-Dayel F, Al-Kuraya KS. Downregulation of SKP2 in papillary thyroid cancer acts synergistically with TRAIL on inducing apoptosis via ROS. J Clin Endocrinol Metab. 2018;103:1530–1544. doi: 10.1210/jc.2017-02178. [DOI] [PubMed] [Google Scholar]
- 11.Wen Y, Wang K, Yang K. Inhibiting the role of Skp2 suppresses cell proliferation and tumorigenesis of human gastric cancer cells via the upregulation of p27kip1. Mol Med Rep. 2016;14:3917–3924. doi: 10.3892/mmr.2016.5676. [DOI] [PubMed] [Google Scholar]
- 12.Saigusa K, Hashimoto N, Tsuda H, Yokoi S, Maruno M, Yoshimine T, Aoyagi M, Ohno K, Imoto I, Inazawa J. Overexpressed Skp2 within 5p amplification detected by array-based comparative genomic hybridization is associated with poor prognosis of glioblastomas. Cancer Sci. 2005;96:676–683. doi: 10.1111/j.1349-7006.2005.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu SY, Wang F, Wei G, Wang B, Yang JY, Huang YZ, Zhang L, Zheng F, Guo LY, Wang JN, Tang JM. S-phase kinase-associated protein 2 knockdown blocks colorectal cancer growth via regulation of both p27 and p16 expression. Cancer Gene Ther. 2013;20:690–694. doi: 10.1038/cgt.2013.70. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Li C, Qu X, Lv W, Lin Y, Tang Z. Effect of S-phase kinase associated protein 2 gene silencing on the biological characteristics of human glioma U251 cells. Chin J Exp Surg. 2017;34:1274–1277. [Google Scholar]
- 15.Lee SH, McCormick F. Downregulation of Skp2 and p27/Kip1 synergistically induces apoptosis in T98G glioblastoma cells. J Mol Med (Berl) 2005;83:296–307. doi: 10.1007/s00109-004-0611-7. [DOI] [PubMed] [Google Scholar]
- 16.Agnihotri S, Gajadhar AS, Ternamian C, Gorlia T, Diefes KL, Mischel PS, Kelly J, McGown G, Thorncroft M, Carlson BL, Sarkaria JN, Margison GP, Aldape K, Hawkins C, Hegi M, Guha A. Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J Clin Invest. 2012;122:253–266. doi: 10.1172/JCI59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEllin B, Camacho CV, Mukherjee B, Hahm B, Tomimatsu N, Bachoo RM, Burma S. PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 2010;70:5457–5464. doi: 10.1158/0008-5472.CAN-09-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balvers RK, Lamfers ML, Kloezeman JJ, Kleijn A, Berghauser Pont LM, Dirven CM, Leenstra S. ABT-888 enhances cytotoxic effects of temozolomide independent of MGMT status in serum free cultured glioma cells. J Transl Med. 2015;13:74. doi: 10.1186/s12967-015-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho YY, Kim DJ, Lee HS, Jeong CH, Cho EJ, Kim MO, Byun S, Lee KY, Yao K, Carper A, Langfald A, Bode AM, Dong Z. Autophagy and cellular senescence mediated by Sox2 suppress malignancy of cancer cells. PLoS One. 2013;8:e57172. doi: 10.1371/journal.pone.0057172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 21.Byun WS, Jin M, Yu J, Kim WK, Song J, Chung HJ, Jeong LS, Lee SK. A novel selenonucleoside suppresses tumor growth by targeting Skp2 degradation in paclitaxel-resistant prostate cancer. Biochem Pharmacol. 2018;158:84–94. doi: 10.1016/j.bcp.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Lin YS, Lin YY, Yang YH, Lin CL, Kuan FC, Lu CN, Chang GH, Tsai MS, Hsu CM, Yeh RA, Yang PR, Lee IY, Shu LH, Cheng YC, Liu HT, Lee KD, Chang DC, Wu CY. Antrodia cinnamomea extract inhibits the proliferation of tamoxifen-resistant breast cancer cells through apoptosis and skp2/microRNAs pathway. BMC Complement Altern Med. 2018;18:152. doi: 10.1186/s12906-018-2204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Sun M, Guo J, Ma L, Jiang H, Gu L, Wen H, Liao S, Chen J, Zeng B, Li Y, Li Y, Yu X, Feng Y, Zhou Y. 3-O-(Z)-coumaroyloleanolic acid overcomes Cks1b-induced chemoresistance in lung cancer by inhibiting Hsp90 and MEK pathways. Biochem Pharmacol. 2017;135:35–49. doi: 10.1016/j.bcp.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Shin HJ, Kim H, Oh S, Lee JG, Kee M, Ko HJ, Kweon MN, Won KJ, Baek SH. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534:553–557. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZS, Wang J, Shen YB, Guo CC, Sai KE, Chen FR, Mei X, Han FU, Chen ZP. Dihydroartemisinin increases temozolomide efficacy in glioma cells by inducing autophagy. Oncol Lett. 2015;10:379–383. doi: 10.3892/ol.2015.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanotto-Filho A, Braganhol E, Klafke K, Figueiro F, Terra SR, Paludo FJ, Morrone M, Bristot IJ, Battastini AM, Forcelini CM, Bishop AJR, Gelain DP, Moreira JCF. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015;358:220–231. doi: 10.1016/j.canlet.2014.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Golden EB, Cho HY, Jahanian A, Hofman FM, Louie SG, Schonthal AH, Chen TC. Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy. Neurosurg Focus. 2014;37:E12. doi: 10.3171/2014.9.FOCUS14504. [DOI] [PubMed] [Google Scholar]


